Abstract
Some enterotoxigenic Escherichia coli strains express the TibA adhesin/invasin, a multifunctional autotransporter that mediates the autoaggregation of bacteria, biofilm formation, adhesion to cultured epithelial cells, and invasion of these cells. To elucidate the structure-function relationship in TibA, we generated mutants by transposon-based linker scanning mutagenesis and by site-directed mutagenesis. Several insertion mutants had a defect in either adhesion or autoaggregation. Mutants with a defect in autoaggregation were found in the N-terminal half of the extracellular domain, while mutants with a defect in adhesion were found in the C-terminal half. The deletion of the putative N-terminal autoaggregation domain abolished the autoaggregation of the bacteria but did not affect adhesion. The deletion of a proline-rich region located at the C terminus of the extracellular domain abolished the adhesion properties of TibA but did not affect invasion. This finding suggests that adhesion and invasion may rely on distinct mechanisms. Thus, our results reveal that TibA possesses a modular organization, with the extracellular domain being separated into an autoaggregation module and an adhesion module.
INTRODUCTION
Many Escherichia coli strains cause gastrointestinal diseases. Enterotoxigenic E. coli (ETEC) is the most common cause of diarrhea mediated by E. coli (30). ETEC causes diarrhea by secreting one or more heat-stable or heat-labile enterotoxins. However, the first step in pathogenesis is the attachment to the intestinal epithelium. For this, ETEC possesses mainly fimbrial adhesins, but some ETEC strains also possess afimbrial adhesins (44). Furthermore, some ETEC strains, like strain H10407, are able to invade epithelial cells (10). Two different loci in this strain, tia (13) and tib (11, 27), were shown previously to mediate invasion (10). The tib locus codes for the expression of a 104-kDa surface protein, TibA, that promotes adhesion and invasion (11).
TibA is part of the autotransporter family of proteins (9, 17). Autotransporters are characterized by their organization and their secretion mechanism. They possess an N-terminal signal sequence that targets the preprotein to the Sec complex and is cleaved after translocation across the inner membrane (4, 34, 42). They have a C-terminal domain that forms a β-barrel inserted into the outer membrane and helps the translocation of the passenger domain across the outer membrane (25, 33). This passenger domain bears the function of the protein and is usually associated with virulence (16). Finally, at the C terminus of the passenger domain and leading to the β-barrel, there is a junction region that is important for folding and secretion (32, 36).
Recently, TibA was proposed to be a member of a group called the self-associating autotransporters (SAATs) (22). This group includes two other E. coli autotransporters, the adhesin involved in diffuse adherence (AIDA-I) (2) and the aggregation factor Ag43 (15). The three proteins have been grouped together because of their functional similarities. They can all mediate bacterial autoaggregation, biofilm formation, as well as adhesion and invasion of epithelial cells (1, 5, 11, 15, 20, 35, 40, 41). The three proteins also share a peculiar primary structure: the N terminus of the passenger domain of these three proteins is composed of repeats of the same 19-amino-acid consensus sequence. In addition, SAATs can be O glycosylated, and this glycosylation is important for the adhesion properties as well as for the stability and the conformation of the protein (3, 6, 23, 27, 39). In the case of TibA, glycosylation is achieved by TibC, a heptosyltransferase encoded right upstream of tibA (28). Despite their similarities, the proteins also have differences: (i) they have different numbers of repeats of the consensus sequence; (ii) they have differences in processing, since both AIDA-I and Ag43 are cleaved, while TibA is not (5, 7, 18, 43); and (iii) TibA possesses a unique proline-rich region between the junction domain and the β-barrel (28).
Structure-function relationship studies have been conducted on AIDA-I (8) and Ag43 (21). In both cases, the study revealed that the different functions were not all linked together and that distinct modules in the passenger domain could be associated with different functions. However, there are differences in the organizations of AIDA-I and Ag43. The N terminus of the passenger domain is responsible for adhesion in the case of AIDA-I and for autoaggregation in the case of Ag43. In AIDA-I, autoaggregation seems to be associated with the C terminus of the passenger domain. Furthermore, a second adhesion domain is present in the C terminus of the passenger domain of AIDA-I. In Ag43, the C terminus of the passenger domain is associated with biofilm formation. Thus, despite all the similarities, there are differences between SAAT proteins that are poorly understood. Therefore, a structure-function relationship in TibA could clarify the organization of this family of virulence factors.
In this study, we gained information on the functionality of the TibA self-associating autotransporter. We have generated several insertion mutants in the extracellular portion of TibA as well as deletion mutants. We found that the N terminus of the passenger domain is associated with autoaggregation, while the C terminus of the passenger domain is associated with adhesion. We did not identify mutants that were affected in both adhesion and autoaggregation. However, mutations that affected invasion or biofilm formation also affected either adhesion or autoaggregation. This suggests that while adhesion and autoaggregation are distinct primary functions, invasion and biofilm formation may be secondary and related to these functions.
MATERIALS AND METHODS
Bacterial strain and plasmid.
E. coli K-12 strain C600 (New England BioLabs) (F− thr-1 leuB6 thi-1 lacY1 supE44 rfbD1 fhuA21) was used in this study. Plasmid pTgH allows the expression of TibA and its specific glycosyltransferase. Plasmid pTgH was derived from the pTRC99A vector (Pharmacia Biotech). TibA expressed by pTgH contains a protein tag consisting of six histidine residues at the N terminus of the passenger domain. The presence of the His tag did not affect the expression and the functionality of TibA (data not shown).
To construct 5-amino-acid insertion mutants, plasmid pTgH was randomly mutagenized by using the GPS-LS linker scanning system (New England BioLabs) according to the instructions of the manufacturer. The presence of an insertion in the passenger domain or in the junction region of TibA was assessed by PCR with the following primers: 5′-GTCTGGAATGAATCCACAG and 5′-GTTATCCAGCGTCAATGC. DNA sequencing was used to ascertain the location and integrity of the insertion sequence and to confirm that there were no secondary unwanted mutations. The ΔPP and the ΔN deletions were introduced into pTgH by oligonucleotide-directed mutagenesis performed with a QuikChange II site-directed mutagenesis kit (Stratagene) using primers 5′-CTGGTATCTGAAGGCTGATACTGGTACATCGTCGTCTCCAGTGCG and 5′-GGCGCATCATCATCATCATCACCAGTTTGTCTCCAGTGGCGGC, respectively, according to the instructions of the manufacturer.
Bacterial and cell culture growth conditions.
Bacteria containing the different plasmids were grown on Luria-Bertani (LB) agar plates or in liquid LB medium containing 100 μg·ml−1 ampicillin. Bacterial cultures were grown at 30°C, and growth was monitored by measuring the optical density at 600 nm (OD600). At an OD600 of 0.8, the cultures were induced with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG). This low concentration of IPTG was used to limit the toxicity associated with the overexpression of TibA and TibC. HEp-2 cells (ATCC CCL-23) were grown at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (Gibco) containing 10 mM sodium pyruvate (Sigma), 10% bovine growth serum (HyClone), 2.5 μg·ml−1 amphotericin B (Fungizone), and 100 μg·ml−1 penicillin-streptomycin (Gibco).
SDS-PAGE and immunoblotting.
Cultures (5 ml) were grown overnight, normalized, and centrifuged for 10 min at 12,000 × g in microcentrifuge tubes, and the pellets were resuspended in 50 μl of Tris-buffered saline (TBS). Whole-cell lysate samples were then diluted in 2× SDS-PAGE loading buffer containing β-mercaptoethanol and denatured by heating at 100°C for 10 min. The samples were then separated by SDS-PAGE on 10% acrylamide gels. The gels were either stained with Coomassie blue or transferred onto polyvinylidene fluoride membranes (Millipore). Immunodetection was performed with a serum raised against glycosylated AIDA-I diluted 1:60,000 in blocking buffer (5% skim milk, 50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Triton X-100). Alternatively, a His tag-specific antibody (Invitrogen) was used. Horseradish peroxidase-conjugated secondary antibodies (Sigma) were used according to the instructions of the manufacturer. Immune complexes were revealed by using a 3,3′,5,5′-tetramethylbenzidine solution for membranes (Sigma).
Functional assays.
Autoaggregation, biofilm formation, adhesion, and invasion assays were performed as previously described (8). E. coli C600 cultures harboring vector pTRC99A, plasmid pTgH, and plasmid pTgH containing one insertion or a deletion in TibA were induced with 10 μM IPTG at an OD600 of 0.8 and grown overnight. In the autoaggregation assay, the cultures grown overnight were normalized in 5 ml of LB broth to an OD600 of approximately 1.5 in culture tubes and left standing at 4°C. Samples (100 μl) were taken 1 cm below the surface at the beginning of the assay and after 120 min, and the OD600 values of the samples were measured. OD600 values at 120 min were compared to the OD600 at the beginning of the assay, and results are shown as percentages of the initial OD600. For TibAΔPP, samples were collected every 30 min. In the biofilm formation assay, the cultures grown overnight were normalized in M9 minimal medium and grown, without IPTG, for 24 h at 30°C in plastic microtiter plates. Biofilms were stained with crystal violet. After washes, the dye was then solubilized with a mixture of ethanol and acetone (80:20), and the absorption at 595 nm of the solution was measured. In the adhesion and invasion assays, the cultures grown overnight were inoculated onto monolayers of confluent HEp-2 cells in a 24-well plate (approximately 2.5 × 105 cells) using 106 CFU per well. After 3 h, the cells were washed with phosphate-buffered saline (PBS), and the adhering bacteria were recovered with 100 μl of Triton X-100 (1%), plated, and counted. The total numbers of bacteria present were also determined and did not vary significantly between mutants and the wild type (WT) (data not shown). In the invasion assay, fresh medium containing 100 μg·ml−1 gentamicin was added after the 3 h, and the preparations were incubated for an additional 2 h before recovery and plating.
All functional assays were performed at least three times in duplicate or triplicate. For each assay, the results obtained with each mutant were compared to those obtained with His-TibA by performing an analysis of variance (ANOVA) and Dunnett posttests by using Prism 4.0 software (Graphpad Software).
RESULTS
Generation of insertion mutants in TibA.
To analyze structure-function relationships in TibA, we used a Tn7-derived transposon system to generate a library of mutants in TibA. The same system was used in a previously reported structure-function relationship study of AIDA-I (8). The insertion and excision of this transposon cause a 5-amino-acid insertion in the protein. The mutagenesis was performed with a plasmid that allows the expression of the glycosyltransferase TibC and the autotransporter TibA under the control of an IPTG-inducible promoter. TibA also possesses a His tag at the N terminus of the passenger domain.
We screened for mutants in the repeats of TibA as well as in the N-terminal half of the junction. The procedure yielded 64 insertion mutants. Of these, 29 different mutants were expressed at level similar to that of His-TibA, as assessed by immunoblotting of whole-cell lysates with an antibody raised against the His tag or with an antibody raised against glycosylated AIDA-I (data not shown). Our anti-AIDA-I antibody recognizes AIDA-I only when glycosylated (3) and cross-reacts with glycosylated TibA (29). Therefore, we concluded that these 29 mutants were still glycosylated and that these insertions resulted in the expression of stable proteins. We could not detect protein expression for the remaining mutants (data not shown). The mutagenesis procedure creates a stop codon in one of the three possible reading frames of the insertion. Therefore, for the majority of these mutants, the lack of expression is due to truncation at the insertion site. Some insertions may also have yielded unstable proteins.
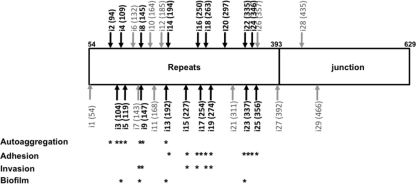
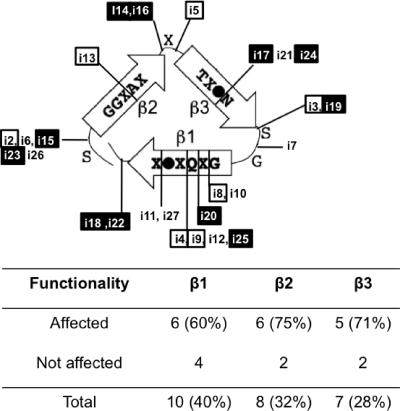
Insertions were spread throughout the repeats, but there were only a few insertions in the junction (Fig. 1 and Table 1). The junction region is important for the folding of the passenger domain of autotransporters (32, 36). Therefore, most of the insertions in the junction region may have yielded proteins with a folding problem, which would have led to the degradation of the protein. The same observation was made with AIDA-I, another SAAT; the repeats were permissive for insertions, but outside the repeats, only a few stable insertions were observed (8). Passenger domains of autotransporters are generally rich in β-strands, and most of the known structures for the passenger domain have a β-helix backbone (31). A model for TibA was proposed previously where each 19-amino-acid repeat is composed of three β-strands and forms one coil of the β-helix (19). Placement of the insertions on this model (Fig. 2) shows that the insertions occurred in the loops as well as in the three strands. In the latter case, it is possible that the sequences of the 5-amino-acid linker could insert, providing an inward-facing hydrophobic residue that maintains the integrity of the hydrophobic core. All three strands contained insertions that affected functionality, suggesting that the functions of TibA are not localized on a particular side of the β-helix.
Fig. 1.
Positions of the insertion mutants in the passenger domain and the junction region of TibA. The effects of the insertions on functionality are summarized. Mutants that are affected functionally are shown in black, and mutants that are not are shown in gray.
Table 1.
Positions and sequences of the insertion mutants
| Mutant | Position of the insertion | Inserted sequence |
|---|---|---|
| i1 | D58 | MFKHD |
| i2 | S94 | CLNTS |
| i3 | N104 | CLNIN |
| i4 | Q109 | LFKHQ |
| i5 | S119 | CLNTS |
| i6 | S132 | CLNTS |
| i7 | N143 | CLNNN |
| i8 | G145 | RLFKQ |
| i9 | Q147 | CLNRQ |
| i10 | G164 | VFKQG |
| i11 | V168 | MFKHV |
| i12 | Q185 | LFKQQ |
| i13 | I192 | CLNSI |
| i14 | K194 | CLNIK |
| i15 | S227 | CLNTS |
| i16 | T250 | CLNTT |
| i17 | I254 | LFKHI |
| i18 | S263 | CLNIS |
| i19 | Y274 | CLNIY |
| i20 | M297 | CLNSM |
| i21 | Q311 | MFKHQ |
| i22 | S335 | CLNIS |
| i23 | A337 | VFKHA |
| i24 | L346 | VFKQL |
| i25 | L352 | CLNNL |
| i26 | D356 | CLNID |
| i27 | V392 | VFKHV |
| i28 | L435 | LFKQL |
| i29 | T466 | CLNST |
Fig. 2.
Localization of the insertion mutants in the coil model of TibA. One repeat of the consensus sequence is predicted to form three β-strands corresponding to one coil of a β-helix. A filled circle represents an isoleucine, a leucine, or a valine residue, while “X” represents any residue. Insertion mutants are positioned in this model. Mutants with a defect in autoaggregation (□) and mutants with a defect in adhesion (▪) are highlighted. The number as well as the percentage of insertions in each strand are shown below.
Functionality of the insertion mutants.
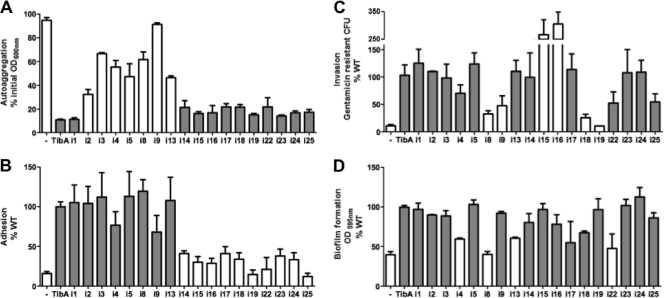
To determine the effect of the insertions on the functionality of TibA, we tested each mutant for its ability to mediate autoaggregation, to form biofilms, to adhere to cultured epithelial cells, and to invade these cells. Figure 3 shows the results for each mutant that had a defect in at least one of the functions. We included one mutant (i1) that had the same functionality as that of His-TibA as a control. Other mutants that are not shown were as functional as His-TibA in the four assays.
Fig. 3.
Functionality of the insertion mutants (A) Autoaggregation assay. E coli C600 cells bearing an empty vector (−) or plasmids allowing the expression of His-TibA or the different insertion mutants were normalized to an OD600 of 1.5 and left standing. The OD600 was measured at the beginning of the assay and after 2 h. Results are shown as percentages of the initial OD600. (B) Adhesion assay. Bacteria were inoculated onto monolayers of confluent Hep-2 cells. After 3 h, the adhering bacteria were recovered, plated, and counted. Results represent the percentages of adhered bacteria compared to His-TibA. (C) Invasion assay. After adhesion, extracellular bacteria were killed by the addition of gentamicin, and invaded bacteria were recovered, plated, and counted. Results represent the percentages of gentamicin-resistant bacteria compared to His-TibA. (D) Biofilm formation assay. Biofilms were stained with crystal violet, and the amount of fixed dye is represented as a percentage compared to His-TibA. ANOVA and Dunnett posttests were used to identify significant differences from the WT (white bars [P < 0.05]).
Of the 29 mutants, 17 mutants had a defect in either adhesion or autoaggregation, but no mutants were affected in both phenotypes. All mutants defective for one or more functions were found in the repeats. From these 17 mutants, 10 (i14 to i25) had a defect in adhesion, and the seven others (i2 to i13) had a defect in autoaggregation. Strikingly, the separation between autoaggregation-defective mutants and adhesion-defective mutants is very clear and lies between mutants i13 and i14. These results suggest that the N-terminal portion of the repeats of TibA is responsible for autoaggregation, while the C-terminal part is responsible for adhesion.
Some of these mutants also had a defect in invasion or in biofilm formation. However, we did not find mutants that were affected only in invasion or only in biofilm formation. Four mutants (i8, i9, i18, and i19) had reduced invasion. It was not possible to associate invasion with a particular region or function (adhesion or autoaggregation), since invasion-defective mutants were found in the N-terminal part associated with autoaggregation (i8 and i9) as well as in the C-terminal part associated with adhesion (i18 and i19). Interestingly, some mutants deficient for adhesion could still promote invasion (i14 to i17 and i22 to i25). Adhesion-deficient mutants that are still able to invade were also isolated previously for AIDA-I (8). This finding suggests that adhesion and invasion are not necessarily related to each other. Biofilm formation and autoaggregation are not strictly linked, since many autoaggregation-defective mutants still make biofilms. However, it seems that it is possible that biofilm formation is to some degree associated with autoaggregation, since 3 of the 4 biofilm-defective mutants (i4, i8, i13, and i22) were also affected in autoaggregation. Since biofilm formation assays are performed with minimal medium, we ascertained that variations in protein expression levels or growth rates did not account for the defect of these mutants under these conditions (data not shown).
Taken together, our results suggest that the repeats of TibA contain an N-terminal autoaggregation domain and a C-terminal adhesion domain. While it was clear for adhesion and autoaggregation, it is not possible to associate biofilm formation and invasion with a particular region of the repeats. Also, it seems that the functionality of the protein is limited to the repeats and does not extend to the junction region.
Effect of an N-terminal deletion of the repeats.
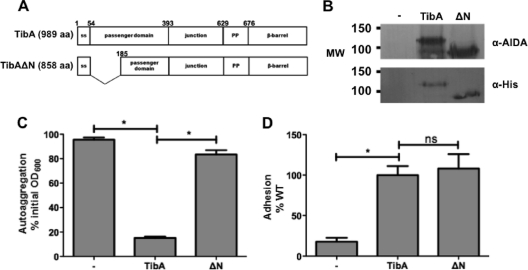
All mutants with a defect in autoaggregation were found in the N-terminal portion of the repeats. In order to confirm that this region contains the autoaggregation domain, we deleted amino acids A55 to Q185 to create TibAΔN (Fig. 4A). The mutant was correctly expressed and glycosylated, as shown by the total protein extract probed with antibodies against the His tag or recognizing glycosylation (anti-AIDA-I) (Fig. 4B). We then assessed the autoaggregation and adhesion properties of TibAΔN (Fig. 4C and D). While the deletion of the N-terminal half of the passenger domain did not affect the adhesion properties of TibA, TibAΔN was not able to mediate autoaggregation anymore. This finding confirms that the domain responsible for autoaggregation is found in the N-terminal part of the repeats of TibA, while the adhesion domain is in the C-terminal part of the repeats.
Fig. 4.
Effect of the deletion of the N-terminal portion of the passenger domain of TibA. (A) Schematic representation of TibA and the N-terminal deletion mutant TibAΔN. aa, amino acids. (B) Whole-cell lysates of bacteria bearing an empty vector (−), a plasmid allowing the expression of His-TibA, or the N-terminal deletion mutant (ΔN) were probed with an antibody raised against glycosylated AIDA-I (top) or against the His tag (bottom). MW, molecular weight (in thousands). (C and D) Autoaggregation (C) and adhesion (D) assays were performed as described in the legend of Fig. 3. ANOVA and Dunnett posttests were used to distinguish significant (* [P < 0.05]) from nonsignificant (ns) differences.
Role of the proline-rich region.
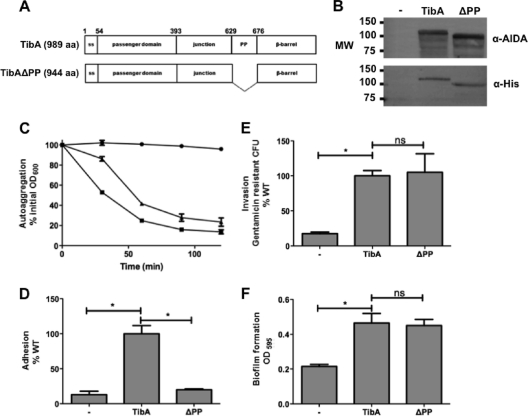
One difference between TibA and other SAATs is the presence of a proline-rich region between the junction region and the membrane-embedded domain. To determine the role of this domain in TibA, we deleted it to create TibAΔPP (Fig. 5A). The mutant was correctly expressed and glycosylated (Fig. 5B).
Fig. 5.
Construction and expression of TibA lacking the proline-rich region. (A) Schematic representation of TibA and the proline-rich region deletion mutant TibAΔPP. (B) Whole-cell lysates of bacteria bearing an empty vector (−), a plasmid allowing the expression of His-TibA, or the proline-rich region deletion mutant (ΔPP) were probed with an antibody raised against glycosylated AIDA-I (top) or against the His tag (bottom). MW, molecular weight (in thousands). (C to F) Autoaggregation (C), adhesion (D), invasion (E), and biofilm formation (F) assays were performed, as described in the legend of Fig. 3, on bacteria bearing an empty vector (− [•]) or plasmids allowing the expression of TibA (WT [▪]) or TibA deleted for the proline-rich region (ΔPP [▴]). ANOVA and Dunnett posttests were used to identify significant differences (* [P < 0.05]) and nonsignificant differences (ns).
We then tested the functionality of the mutant in autoaggregation, adhesion, invasion, and biofilm formation (Fig. 5C to F). TibAΔPP was not able to mediate adhesion to cultured epithelial cells but was still able to mediate autoaggregation, biofilm formation, and the invasion of cultured epithelial cells. TibAΔPP is another example of a mutant that is defective in adhesion but is as invasive as His-TibA. Again, this suggests that adhesion and invasion are not necessarily related to each other. The main conclusion of these results is that the proline-rich region is involved in adhesion.
DISCUSSION
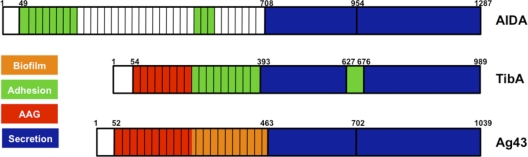
In this study, we created different mutations in the self-associating autotransporter TibA to gain information on the functionality of this protein. We first generated 29 insertion mutants in the passenger domain and in the junction region of TibA. Analysis of the functionality of these mutants revealed that the N-terminal portion of the repeats is responsible for autoaggregation, while the C-terminal part is associated with adhesion. The autoaggregation domain in Ag43 is also found in the N-terminal portion of the repeats (21), while in AIDA-I, the N terminus of the mature protein contains an adhesion domain (8). A region responsible for adhesion was not sought for Ag43. The organization of TibA seems to be closer to the organization of Ag43. Both proteins also have a similar length compared to that of AIDA-I, which possesses a passenger domain that is twice the size of those of TibA and Ag43 (Fig. 6).
Fig. 6.
Functional organization of SAATs. Functional domains found by structure-function studies of AIDA-I (8), TibA (this study), and Ag43 (21) are positioned on a schematic representation of each SAAT. Domains responsible for secretion (blue), autoaggregation (AAG) (red), adhesion (green), and biofilm formation (orange) are represented. No specific domain for invasion was found in these studies.
Adhesion domains are often located at the N terminus of adhesins, like AIDA-I, (8) or the trimeric autotransporter YadA from Yersinia enterocolitica (38). Having the adhesion domain farther away from the cell surface may facilitate the binding of the adhesin to its receptor. In the case of TibA, the adhesion domain would be closer to the membrane. However, TibA also possesses a proline-rich domain between the extracellular and the membrane-embedded portions. This domain could serve as a spacer so that the passenger domain is farther away from the membrane. Consistent with this hypothesis, the deletion of this proline-rich region completely abrogates the ability of TibA to mediate adhesion to epithelial cells. TonB is another example of an E. coli protein that harbors a proline-rich region. In the case of TonB, this proline-rich region was shown previously to adopt an elongated and rigid conformation (12, 24), and its role seems to be limited to providing a physical extension to TonB (26).
In the present study, it is interesting that we did not observe mutants that were affected only in invasion or biofilm formation. All mutants affected in these functionalities were also affected in either autoaggregation or adhesion. Similar observations were made in previous studies with AIDA-I (8, 14) and Ag43 (21, 35). This suggests that adhesion and autoaggregation are the main functions of SAATs, whereas biofilm formation and invasion are secondary to these two functions.
In order to invade eukaryotic cells, bacteria need to first adhere to the cells. Therefore, a mutant that is deficient for adhesion is also likely to be deficient for invasion. However, in this study, we isolated several mutants that were not able to adhere to epithelial cells but could still promote invasion (i16, i17, and TibAΔPP, for instance). All these mutants are still able to promote autoaggregation. Therefore, it is tempting to hypothesize that there is a link between invasion and the two main functions of TibA, adhesion and autoaggregation. For instance, the invasion mediated by TibA could be the invasion of bacterial aggregates, as can be seen with Bartonella henselae (37). More work is needed to test this hypothesis.
In conclusion, our study suggests that SAATs evolve by the acquisition and/or modification of individual modules that bring new functionalities, either independently or in conjunction with existing modules. In this light, TibA would bear an autoaggregation module, an adhesion module, and a spacer module, along with the junction region and membrane-embedded domain, which could be construed as a secretion module (Fig. 6). Other SAATs would stem from a different combination of equivalent modules. This modular aspect is a complexified version of the passenger-translocator organization that is characteristic of all autotransporters but could represent an evolutionary mechanism that is peculiarly well suited for these proteins.
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes for Health Research grant 84578, funds from the Groupe de Recherche et d'Etudes sur les Maladies Infectieuses du Porc, Canada Research Chair and Canada Foundation for Innovation grant 201414, and a graduate fellowship to J.-P.C. from the Fonds de Recherche sur la Nature et les Technologies du Québec (FQRNT 126554).
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Benz I., Schmidt M. A. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539–1546 [DOI] [PubMed] [Google Scholar]
- 2. Benz I., Schmidt M. A. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benz I., Schmidt M. A. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol. Microbiol. 40:1403–1413 [DOI] [PubMed] [Google Scholar]
- 4. Brandon L. D., et al. 2003. IcsA, a polarly localized autotransporter with an atypical signal peptide, uses the Sec apparatus for secretion, although the Sec apparatus is circumferentially distributed. Mol. Microbiol. 50:45–60 [DOI] [PubMed] [Google Scholar]
- 5. Charbonneau M. E., Berthiaume F., Mourez M. 2006. Proteolytic processing is not essential for multiple functions of the Escherichia coli autotransporter adhesin involved in diffuse adherence (AIDA-I). J. Bacteriol. 188:8504–8512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charbonneau M. E., et al. 2007. O-linked glycosylation ensures the normal conformation of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 189:8880–8889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charbonneau M. E., Janvore J., Mourez M. 2009. Autoprocessing of the Escherichia coli AIDA-I autotransporter: a new mechanism involving acidic residues in the junction region. J. Biol. Chem. 284:17340–17351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charbonneau M. E., Mourez M. 2007. Functional organization of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 189:9020–9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dautin N., Bernstein H. D. 2007. Protein secretion in Gram-negative bacteria via the autotransporter pathway. Annu. Rev. Microbiol. 61:89–112 [DOI] [PubMed] [Google Scholar]
- 10. Elsinghorst E. A., Kopecko D. J. 1992. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect. Immun. 60:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsinghorst E. A., Weitz J. A. 1994. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect. Immun. 62:3463–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans J. S., Levine B. A., Trayer I. P., Dorman C. J., Higgins C. F. 1986. Sequence-imposed structural constraints in the TonB protein of E. coli. FEBS Lett. 208:211–216 [DOI] [PubMed] [Google Scholar]
- 13. Fleckenstein J. M., Kopecko D. J., Warren R. L., Elsinghorst E. A. 1996. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 64:2256–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girard V., et al. 2010. Conformation change in a self-recognizing autotransporter modulates bacterial cell-cell interaction. J. Biol. Chem. 285:10616–10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henderson I. R., Meehan M., Owen P. 1997. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol. Lett. 149:115–120 [DOI] [PubMed] [Google Scholar]
- 16. Henderson I. R., Nataro J. P. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henderson I. R., Owen P. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and oxyR. J. Bacteriol. 181:2132–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kajava A. V., Steven A. C. 2006. The turn of the screw: variations of the abundant beta-solenoid motif in passenger domains of type V secretory proteins. J. Struct. Biol. 155:306–315 [DOI] [PubMed] [Google Scholar]
- 20. Kjaergaard K., Schembri M. A., Ramos C., Molin S., Klemm P. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695–702 [DOI] [PubMed] [Google Scholar]
- 21. Klemm P., Hjerrild L., Gjermansen M., Schembri M. A. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51:283–296 [DOI] [PubMed] [Google Scholar]
- 22. Klemm P., Vejborg R. M., Sherlock O. 2006. Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol. 296:187–195 [DOI] [PubMed] [Google Scholar]
- 23. Knudsen S. K., Stensballe A., Franzmann M., Westergaard U. B., Otzen D. E. 2008. Effect of glycosylation on the extracellular domain of the Ag43 bacterial autotransporter: enhanced stability and reduced cellular aggregation. Biochem. J. 412:563–577 [DOI] [PubMed] [Google Scholar]
- 24. Kohler S. D., Weber A., Howard S. P., Welte W., Drescher M. 2010. The proline-rich domain of TonB possesses an extended polyproline II-like conformation of sufficient length to span the periplasm of Gram-negative bacteria. Protein Sci. 19:625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konieczny M. P. J., et al. 2001. Modular organization of the AIDA autotransporter translocator: the N-terminal beta1-domain is surface-exposed and stabilizes the transmembrane beta2-domain. Antonie Van Leeuwenhoek 80:19–34 [DOI] [PubMed] [Google Scholar]
- 26. Larsen R. A., Wood G. E., Postle K. 1993. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol. Microbiol. 10:943–953 [DOI] [PubMed] [Google Scholar]
- 27. Lindenthal C., Elsinghorst E. A. 2001. Enterotoxigenic Escherichia coli TibA glycoprotein adheres to human intestine epithelial cells. Infect. Immun. 69:52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindenthal C., Elsinghorst E. A. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moormann C., Benz I., Schmidt M. A. 2002. Functional substitution of the TibC protein of enterotoxigenic Escherichia coli strains for the autotransporter adhesin heptosyltransferase of the AIDA system. Infect. Immun. 70:2264–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nataro J. P., Kaper J. B. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishimura K., Tajima N., Yoon Y. H., Park S. Y., Tame J. R. 2010. Autotransporter passenger proteins: virulence factors with common structural themes. J. Mol. Med. 88:451–458 [DOI] [PubMed] [Google Scholar]
- 32. Oliver D. C., Huang G., Nodel E., Pleasance S., Fernandez R. C. 2003. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol. Microbiol. 47:1367–1383 [DOI] [PubMed] [Google Scholar]
- 33. Oomen C. J., et al. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterson J. H., Szabady R. L., Bernstein H. D. 2006. An unusual signal peptide extension inhibits the binding of bacterial presecretory proteins to the signal recognition particle, trigger factor, and the SecYEG complex. J. Biol. Chem. 281:9038–9048 [DOI] [PubMed] [Google Scholar]
- 35. Reidl S., Lehmann A., Schiller R., Khan A. S., Dobrindt U. 2009. Impact of O-glycosylation on the molecular and cellular adhesion properties of the Escherichia coli autotransporter protein Ag43. Int. J. Med. Microbiol. 299:389–401 [DOI] [PubMed] [Google Scholar]
- 36. Renn J. P., Clark P. L. 2008. A conserved stable core structure in the passenger domain beta-helix of autotransporter virulence proteins. Biopolymers 89:420–427 [DOI] [PubMed] [Google Scholar]
- 37. Rhomberg T. A., Truttmann M. C., Guye P., Ellner Y., Dehio C. 2009. A translocated protein of Bartonella henselae interferes with endocytic uptake of individual bacteria and triggers uptake of large bacterial aggregates via the invasome. Cell. Microbiol. 11:927–945 [DOI] [PubMed] [Google Scholar]
- 38. Roggenkamp A., Ruckdeschel K., Leitritz L., Schmitt R., Heesemann J. 1996. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect. Immun. 64:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherlock O., Dobrindt U., Jensen J. B., Vejborg R. M., Klemm P. 2006. Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein. J. Bacteriol. 188:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sherlock O., Schembri M. A., Reisner A., Klemm P. 2004. Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation. J. Bacteriol. 186:8058–8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherlock O., Vejborg R. M., Klemm P. 2005. The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation. Infect. Immun. 73:1954–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sijbrandi R., et al. 2003. Signal recognition particle (SRP)-mediated targeting and Sec-dependent translocation of an extracellular Escherichia coli protein. J. Biol. Chem. 278:4654–4659 [DOI] [PubMed] [Google Scholar]
- 43. Suhr M., Benz I., Schmidt M. A. 1996. Processing of the AIDA-I precursor: removal of AIDAc and evidence for the outer membrane anchoring as a beta-barrel structure. Mol. Microbiol. 22:31–42 [DOI] [PubMed] [Google Scholar]
- 44. Turner S. M., Scott-Tucker A., Cooper L. M., Henderson I. R. 2006. Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 263:10–20 [DOI] [PubMed] [Google Scholar]