Abstract
Salmonella serotypes are a major cause of human morbidity and mortality worldwide. Over the past decades, a series of animal models have been developed to advance vaccine development, provide insights into immunity to infection, and study the pathogenesis of human Salmonella disease. The successive introduction of new animal models, each suited to interrogate previously neglected aspects of Salmonella disease, has ushered in important conceptual advances that continue to have a strong and sustained influence on the ideas driving research on Salmonella serotypes. This article reviews important milestones in the use of animal models to study human Salmonella disease and identify research needs to guide future work.
THE GLOBAL BURDEN OF SALMONELLA INFECTIONS
Most nontyphoidal Salmonella (NTS) serotypes are associated with gastroenteritis in immunocompetent individuals, in which the infection remains localized to the terminal ileum, colon, and mesenteric lymph node. Nontyphoidal Salmonella gastroenteritis is characterized by a short incubation period, averaging less than 1 day (26), followed by the development of diarrhea, fever, and intestinal inflammatory infiltrates that are dominated by neutrophils (102). The zoonotic pathogens Salmonella enterica serotype Typhimurium (S. Typhimurium) and S. enterica serotype Enteritidis (S. Enteritidis) are most frequently associated with this diarrheal disease (63). Although viral agents are generally a more common cause of diarrhea than bacterial agents, nontyphoidal Salmonella serotypes stand out by producing a more severe infection, which is associated with considerable mortality rates. As a result, nontyphoidal Salmonella serotypes are the leading cause of death from foodborne illness in the United States (48, 73). A recent estimate puts the global burden of NTS gastroenteritis at 93.8 million cases, resulting in 155,000 deaths annually (46).
In immunocompromised individuals, nontyphoidal Salmonella serotypes are associated with invasive disease, manifesting as a bloodstream infection known as NTS bacteremia. Individuals with NTS bacteremia present with fever, but symptoms of gastroenteritis are commonly absent (12, 18, 28). Thus, mortality from NTS bacteremia has to be considered separately from deaths attributed to NTS gastroenteritis. NTS bacteremia is a leading cause of hospital admission and death in sub-Saharan Africa (27). The serotypes most commonly associated with this bloodstream infection are S. Typhimurium and S. Enteritidis. A meta-analysis of 22 reports revealed that nontyphoidal Salmonella serotypes (18.7% of isolates) are second only to Streptococcus pneumoniae (23.3% of isolates) in causing bloodstream infections in Africa (67). NTS bacteremia is associated with different risk factors in adults and children. Risk factors for NTS bacteremia in African children include malnutrition, anemia, severe malaria, and human immunodeficiency virus (HIV) infection (10). These risk factors produce an estimated incidence of 175 to 388 child NTS bacteremia cases per 100,000 persons per year (5, 20, 75). In contrast, HIV infection is the predominant risk factor for NTS bacteremia in African adults (29) and is responsible for approximately 2,000 to 8,500 NTS bacteremia cases per 100,000 persons per year in HIV-positive cohorts (25, 89, 91). NTS bloodstream infections are associated with mortality rates in excess of 20% in pediatric and adult populations despite antibiotic therapy (2, 5, 10, 29, 30).
Salmonella enterica serotype Typhi (S. Typhi) is a strictly human-adapted pathogen associated with an invasive infection in immunocompetent individuals known as typhoid fever. Typhoid fever has an average incubation period of 2 weeks (59), which is followed by the development of nonspecific symptoms, commonly including fever and a slowed heart rate (bradycardia) (54). Unlike gastroenteritis, typhoid fever is not considered a diarrheal disease, because this symptom develops only in approximately one-third of patients. Pathological changes in the intestine are characterized by inflammatory infiltrates that are dominated by mononuclear cells, while neutrophils are scarce (38, 53, 55, 79). The pathogen persists in histiocytic granulomas, known as typhoid nodules, in internal organs, most frequently the bone marrow, the liver, and the spleen (54). Persistence in the gallbladder converts a small fraction (approximately 4%) of patients into chronic carriers, termed “typhoid Marys,” who can transmit the disease for the remainder of their lives. There are an estimated 16 million to 21.6 million cases of typhoid fever each year, with the highest incidence being reported from Southeast Asia. Typhoid fever causes considerable mortality worldwide, with estimates ranging from 216,510 to 600,000 deaths annually (6, 50).
In summary, the cumulative global death toll from NTS gastroenteritis, NTS bacteremia, and typhoid fever is considerable and highlights the need for research on vaccine development, immunity, and the pathogenesis of these diseases. The increasing prevalence of multidrug-resistant S. Typhi isolates that are fully fluoroquinolone resistant and the emergence of multidrug-resistant NTS serotypes with resistance to ciprofloxacin and expanded-spectrum cephalosporins threaten to limit treatment options (60). These data illustrate the need to develop new intervention strategies, which will require the use of adequate animal models. This article reviews how the use of animal models of infection (Table 1) has ushered in important conceptual advances that have improved our understanding of diseases caused by this important group of human pathogens.
Table 1.
Animal models of human Salmonella disease
| Model | Advantages | Limitations | Reference(s) |
|---|---|---|---|
| Mouse typhoid | Natural infection; availability of host genetics and immunological reagents; model for fecal-oral transmission; low cost | Not suited to study gastroenteritis; S. Typhimurium does not cause typhoid fever in humans | 40, 87 |
| Mouse colitis | Availability of host genetics and immunological reagents; suited to study of intestinal inflammation; low cost | Requires disruption of the microbiota by pretreatment with antibiotics; colitis is accompanied by systemic infection | 4 |
| Humanized mouse | Provides a model to study S. Typhi | Requires extensive manipulation | 42, 78 |
| Guinea pig | Suited to study intestinal inflammation | Requires preconditioning through starvation and opium treatment; limited availability of host genetics and immunological reagents | 84 |
| Calf gastroenteritis | Natural infection that closely resembles human gastroenteritis | Limited availability of genetics and immunological reagents; requires specialized animal facilities | 86 |
| Rhesus macaque | Natural infection that closely resembles human gastroenteritis | Limited availability of genetics; requires specialized animal facilities; high cost | 36 |
THE OPIUM-TREATED GUINEA PIG MODEL
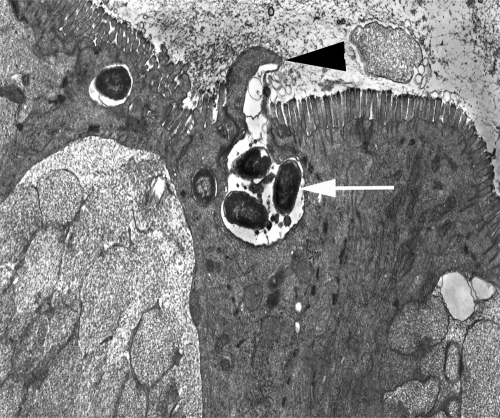
Intestinal lesions during gastroenteritis have historically been attributed to toxin production by bacteria. In the sixties, Samuel Formal and coworkers challenged this dogma by demonstrating that bacterial invasion of epithelial cells is a major factor in determining the pathogenicity of Shigella flexneri in guinea pigs preconditioned by treatment with opium (39). This work motivated Akio Takeuchi to perform electron microscopic studies of epithelial invasion by S. Typhimurium in guinea pigs preconditioned by starvation and treatment with opium (84). In this model, invasion of epithelial cells by S. Typhimurium was accompanied by a local degeneration of microvilli and the formation of membrane extrusions (Fig. 1). It took more than two decades for a genetic locus required for epithelial invasion by S. Typhimurium to be identified by Jorge Galán and Roy Curtiss III (24). Epithelial invasion by S. Typhimurium is mediated by a type III secretion system (T3SS-1) encoded by Salmonella pathogenicity island 1 (SPI-1) (51). The main function of T3SS-1 is to translocate proteins, termed effectors, into the host cell cytosol, where they trigger rearrangements in the actin cytoskeleton (23, 104). Stanley Falkow and coworkers demonstrated that these actin cytoskeletal rearrangements result in bacterial uptake through macropinocytosis, a process characterized by the formation of membrane extrusions, termed ruffles (22), which resemble those originally described by Akio Takeuchi.
Fig. 1.
S. Typhimurium invades the intestinal epithelium. A transmission electron micrograph of the ileal mucosa of a calf experimentally infected with S. Typhimurium is shown. Local degeneration of microvilli and formation of membrane extrusions at the apical surface (arrowhead) of an enterocyte are associated with internalization of bacteria (arrow). Akio Takeuchi recorded similar images in his pioneering studies of epithelial invasion by S. Typhimurium in the guinea pig (84).
THE MOUSE TYPHOID MODELS
In 1892, Friedrich Loeffler isolated a pathogen associated with an epidemic, typhoid fever-like disease in mice, which was termed Bacillus typhimurium (now S. Typhimurium) (44). Mice infected with S. Typhimurium do not develop gastroenteritis but instead contract a systemic disease characterized by bacterial multiplication in the liver and spleen, which results in hepatomegaly and splenomegaly (87). The small intestinal pathology is characterized by a predominantly mononuclear leukocyte infiltrate, with follicular hyperplasia, capillary thrombosis, hemorrhage, and ulcerations observed at areas of Peyer's patches in moribund animals (72). Some mouse lineages (e.g., C57BL/6 mice and BALB/c mice) are genetically susceptible to lethal S. Typhimurium infection due to a point mutation in the Slc11a1 gene that results in expression of a nonfunctional ferrous iron transporter, termed natural resistance-associated macrophage protein, in the phagosomal membranes of macrophages (7). S. Typhimurium also disseminates to the liver and spleen of genetically resistant mouse lineages (e.g., CBA mice and 129sv mice), which possess an intact Slc11a1 allele. However, genetically resistant mice typically control bacterial multiplication at systemic sites, thereby preventing the development of severe signs of disease.
The use of genetically susceptible animals in the mouse typhoid model was instrumental during the 1980s and early 1990s in establishing a number of new concepts, which had a sustained and powerful impact on the fields of Salmonella vaccine development, immunity, and pathogenesis research. For example, the discovery by Bruce Stocker and coworkers that a S. Typhimurium aroA mutant is attenuated and immunogenic in mice (35) led to the development of new live-attenuated S. Typhi vaccines for humans (82, 83). Pietro Mastroeni and coworkers used the mouse typhoid model to demonstrate that protective immunity to S. Typhimurium infection requires both antibodies and CD4+ T cells (47), a principle that still guides current efforts in vaccine development (1). Paul Gulig and Roy Curtiss III identified the Salmonella plasmid virulence (spv) operon during an in vivo screening for DNA regions that rescue virulence of a plasmid-cured S. Typhimurium strain in the mouse typhoid model (31). Finally, Michael Hensel, David Holden, and coworkers identified the second type III secretion system (T3SS-2) of S. Typhimurium while using the mouse typhoid model to devise a novel in vivo screening method, termed signature-tagged mutagenesis, for the identification of virulence genes (34). Subsequent work by the laboratory of Eduardo Groisman demonstrated that T3SS-2 enables S. Typhimurium to survive in host macrophages (58), a property previously shown by Fred Heffron and coworkers to be essential for virulence in the mouse typhoid model (21).
During the 1990s and the first decade of the 21st century, the mouse typhoid model continued to have a marked influence on the methods driving research on bacterial pathogenesis in general. For instance, John Mekalanos and coworkers developed a genetic in vivo expression technology (IVET) to identify bacterial genes specifically induced in tissue (77). The laboratories of Dirk Bumann and Brad Cookson used the mouse typhoid model to work out green fluorescent protein (GFP)-based approaches to follow bacterial gene expression during infection using flow cytometry (13, 17, 68). The mouse typhoid model remains an important platform for developing novel screening approaches for the in vivo identification of virulence genes, such as the array-based analysis of cistrons under selection (ABACUS) recently described by Helene Andrews-Polymenis, Michael McClelland, and coworkers (70). Finally, the laboratory of Ferric Fang led the way in combining knockout mice and bacterial genetics to investigate how virulence factors overcome host defenses in the mouse typhoid model (45, 90), and this “genetics-squared” approach is now widely used in innate immunity and bacterial pathogenesis research (61).
In addition, the innovative use of the mouse typhoid model continues to provide important insights into the pathogenesis of infections caused by Salmonella serotypes. For example, the laboratories of Denise Monack and Corrella Detweiler pioneered the use of genetically resistant mouse lineages to elucidate mechanisms of chronic carriage in tissue (11, 41, 52, 56, 76). Genetically resistant mouse lineages were utilized to study mechanisms of intestinal persistence (19, 37, 93) and to develop an animal model for S. Typhimurium transmission (40). Finally, the laboratories of John Gunn and Brett Finlay demonstrated that the mouse typhoid model provides an opportunity to investigate colonization of the gallbladder (16, 49), a reservoir important for transmission of typhoid fever.
One limitation of the mouse typhoid model is that S. Typhimurium causes gastroenteritis rather than typhoid fever in humans. Since the mouse typhoid model is not suited to study the pathogenesis of NTS gastroenteritis, this disease manifestation remained largely unexplored until alternative animal models became more widely used in the late 1990s and the first decade of the 21st century.
THE CALF GASTROENTERITIS MODEL
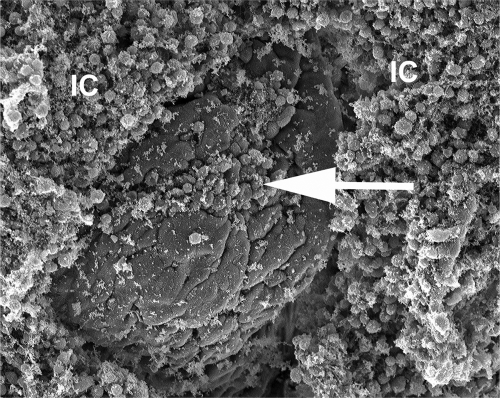
S. Typhimurium is a natural cause of gastroenteritis in calves, and infection is associated with signs of disease that parallel the symptoms observed in humans. The most severe pathological lesions accompanying this localized infection are restricted to the intestinal mucosa and mesenteric lymph nodes (86, 100). Animals develop intestinal inflammation characterized by a severe diffuse infiltrate composed predominantly of neutrophils (86, 100). Neutrophil recruitment is associated with necrosis of the upper mucosa (57) and migration of neutrophils into the intestinal lumen (Fig. 2). In severe cases, necrosis of the upper mucosa leads to formation of a pseudomembrane, a gross pathological change observed in the terminal ileum and the cranial portions of the colon (Fig. 3) (86).
Fig. 2.
Neutrophils migrate into the intestinal lumen in response to S. Typhimurium infection. A scanning electron micrograph of the ileal mucosa of a calf experimentally infected with S. Typhimurium is shown. The image shows epithelial erosion at the tip of an intestinal villus (arrow) and marked accumulation of inflammatory cells (IC) on the luminal surface of the intestinal mucosa.
Fig. 3.
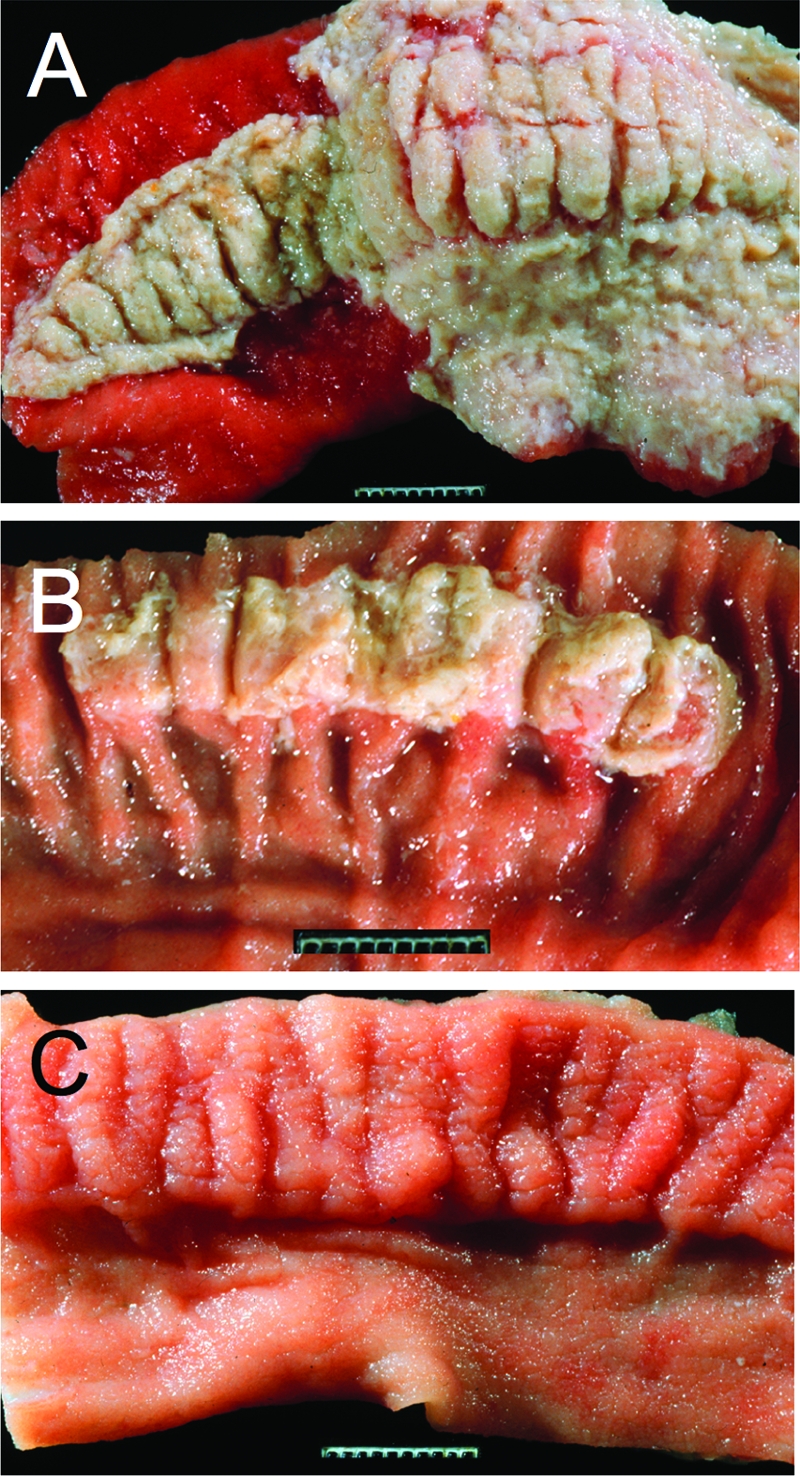
T3SS-1 and T3SS-2 contribute to intestinal inflammation in the calf gastroenteritis model. (A to C) Gross pathological appearance of the luminal surface of the terminal ileum collected 2 days after oral infection of calves with the S. Typhimurium wild-type (A), a T3SS-2-deficient mutant (B), or a T3SS-1-deficient mutant (C). Compared to the severe lesions caused by infection with the wild type (severe acute fibrinopurulent necrotizing enteritis with segmental or continuous pseudomembrane formation [A]), intestinal inflammation is reduced in calves infected with a T3SS-2-deficient mutant (moderate to marked subacute fibrinopurulent enteritis often confined to Peyer's patches [B]) and absent in calves infected with a T3SS-1-deficient mutant (normal Peyer's patch and ileal mucosa [C]). (Reprinted from reference 86.)
Analysis of S. Typhimurium mutants in the calf model in the late 1990s provided important first insights into the role virulence factors play during gastroenteritis, which exposed some parallels to, but also some differences from, their respective roles in the mouse typhoid model. Oral-challenge studies revealed that T3SS-1 is essential for the ability of S. Typhimurium to cause intestinal inflammation and diarrhea (86, 92, 103), while inactivation of T3SS-2 reduces the severity of intestinal lesions in calves (86) (Fig. 3). In contrast to its important role during systemic infection of mice, the spv operon plays only a small role during the localized infection caused by S. Typhimurium in the bovine host (86).
The development of a bovine ligated-ileal loop model provided the opportunity to study the development of intestinal inflammation at defined early time points after S. Typhimurium infection (71, 74, 101, 103). The use of this model revealed that flagella contribute to neutrophil recruitment in the intestinal mucosa during S. Typhimurium infection (74). The role of flagella in eliciting inflammatory responses is in part indirect, by promoting bacterial invasion, and in part direct, by serving as a pathogen-associated molecular pattern (PAMP) that stimulates innate pathways of inflammation (97). Importantly, a role for flagella in virulence had not been apparent from work in the mouse typhoid model (43, 74).
Limitations of the calf model include the scarcity of reagents available to manipulate the host and limited availability of animal facilities to perform the research. Research on S. Typhimurium-induced gastroenteritis therefore experienced a second boost when a mouse colitis model gained popularity in the first decade of the 21st century.
THE MOUSE COLITIS MODEL
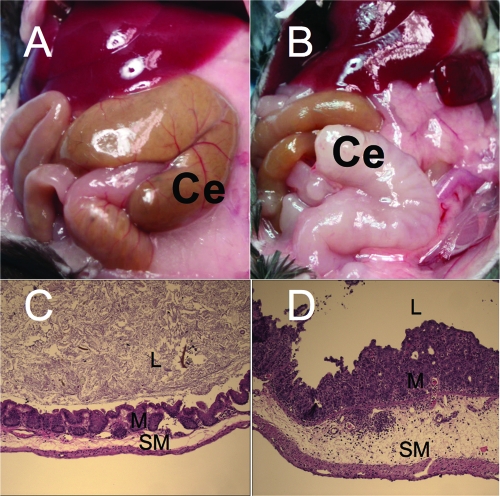
Work performed by Marjorie Bohnhoff and coworkers in the 1950s and 1960s established that mice preconditioned by treatment with streptomycin exhibit a greatly increased susceptibility to oral S. Enteritidis and S. Typhimurium infections (8, 9). Subsequently, streptomycin pretreatment was shown to be associated with enhanced growth of S. Typhimurium in the murine cecum (62). In 2003, these initial observations were followed up by Wolf-Dietrich Hardt and coworkers, who demonstrated that S. Typhimurium infection of streptomycin-pretreated mice results in the development of intestinal inflammatory infiltrates that are dominated by neutrophils (4). The most severe pathological lesions in the intestines of streptomycin-pretreated mice are restricted to the cecum (Fig. 4). Streptomycin-pretreated mice can thus be used to model the orchestration and the consequences of intestinal neutrophil recruitment during S. Typhimurium-induced gastroenteritis (mouse colitis model).
Fig. 4.
Inflammation of the cecum in the mouse colitis model. (A) Normal appearance of the cecum (Ce) in a mock-infected control animal. (B) Shrunken and edematous cecum (Ce) from a S. Typhimurium-infected mouse. (C and D) Histopathological appearance of the murine cecum of a mock-infected mouse (C) or a mouse infected with the S. Typhimurium wild type (D) 72 h after infection. Note that S. Typhimurium infection (D) is associated with severe diffuse neutrophil infiltrate in the mucosa (M) and submucosa (SM) and severe edema in the submucosa. L, lumen. (The images in panels C and D were reprinted from reference 32.)
The initial use of the mouse colitis model confirmed observations from the calf model that flagella, T3SS-1, and T3SS-2 contribute to intestinal inflammation during S. Typhimurium infection (4, 15, 80). A subsequent important conceptual advance was the finding that intestinal inflammation enables S. Typhimurium to outgrow the resident intestinal microbiota in the intestinal lumen (3, 81). Recently, the mouse colitis model was used to demonstrate that inflammation promotes a luminal outgrowth of S. Typhimurium, because the respiratory burst of neutrophils, which transmigrate into the intestinal lumen (Fig. 2), oxidizes an endogenous sulfur compound, thiosulfate, to generate tetrathionate (98). Tetrathionate serves as a respiratory electron acceptor for S. Typhimurium (33), thereby empowering the pathogen to use respiration to outgrow the resident microbiota, which rely on fermentation for energy production during growth in the anaerobic environment of the gut (98). Finally, luminal outgrowth of S. Typhimurium was shown by Denise Monack and coworkers to enhance transmission of the pathogen (40).
Collectively, these new insights connect previous observations to provide a coherent picture of S. Typhimurium-induced gastroenteritis: S. Typhimurium uses its virulence factors to trigger inflammation by using flagella and T3SS-1 to invade the intestinal epithelium, followed by T3SS-2-mediated survival in tissue macrophages. The ensuing inflammatory response generates a new respiratory electron acceptor that supports outgrowth of S. Typhimurium in the gut lumen, thereby promoting its transmission by the fecal-oral route.
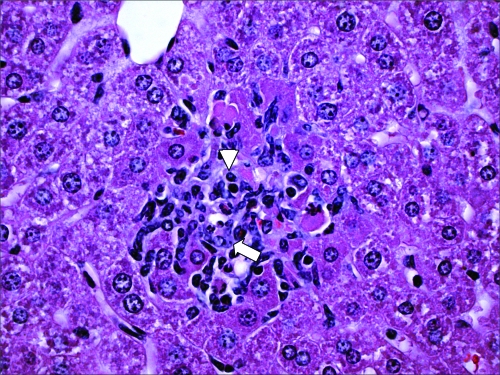
One caveat of the mouse colitis model is that development of acute cecal inflammation requires the use of an antibiotic to disrupt the microbiota. Furthermore, S. Typhimurium disseminates systemically in both genetically resistant and genetically susceptible mouse lineages (Fig. 5), thus making it difficult to study mucosal barrier functions in the mouse colitis model.
Fig. 5.
S. Typhimurium disseminates systemically in the mouse colitis model. A microgranuloma in the liver of a streptomycin-pretreated mouse infected with S. Typhimurium is shown. The image shows focal accumulation of lymphocytes (arrowhead) and epithelioid macrophages (arrow) in a section of the liver.
COINFECTION MODELS
While recent years have seen significant advances in our understanding of NTS gastroenteritis, the pathogenesis of NTS bacteremia remains understudied. The relative paucity of studies on this topic is compounded by the emergence of NTS bacteremia as a leading cause of death in sub-Saharan Africa (27, 67). Risk factors for NTS bacteremia include an underlying malaria infection or HIV disease. Initial studies suggest that animal models are well suited to interrogate the pathogenesis of these coinfections.
To study the underlying susceptibility of children with severe malaria to NTS bacteremia, a mouse coinfection model was developed in resistant CBA mice, using the rodent malaria parasite Plasmodium yoelii subsp. nigeriensis and S. Typhimurium (69). Using this model, the investigators demonstrated that increased systemic loads of S. Typhimurium during coinfection were caused by both hemolytic anemia and malaria parasite-mediated immunosuppression. A reduction in circulating interleukin-12 (IL-12) found in the coinfection model suggests that impaired inflammatory responses in patients with severe malaria may be one factor that predisposes them to NTS bacteremia.
Ligated ileal loops in rhesus macaques infected with simian immunodeficiency virus (SIV) and S. Typhimurium have recently been used to model NTS bacteremia in HIV-infected individuals (66) (Fig. 6). Initial characterization of this model revealed that SIV-mediated depletion in the ileal mucosa of IL-17-producing CD4+ T cells (Th17 cells) selectively blunted expression of IL-17, IL-22, IL-26, CCL-20, and lipocalin-2 in response to S. Typhimurium infection and was associated with increased bacterial dissemination to the mesenteric lymph nodes. Furthermore, IL-17 deficiency resulted in increased systemic dissemination of S. Typhimurium in the mouse colitis model. These data suggest that Th17 depletion is one factor that predisposes HIV-infected individuals to NTS bacteremia.
Fig. 6.
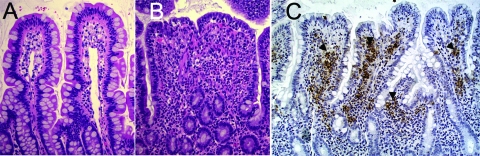
The rhesus macaque (Macaca mulatta) model. (A) Image of the uninfected ileal mucosa. (B) Section of the intestinal mucosa 8 h after infection of a ligated ileal loop with S. Typhimurium. Note the blunting of intestinal villi and the marked infiltration of inflammatory cells, predominately neutrophils. (C) Immunohistochemical labeling (brown precipitate) of S. Typhimurium in the intestinal mucosa of a rhesus macaque (Macaca mulatta) 8 h after inoculation of a ligated ileal loop with S. Typhimurium. Note the marked immunolabeling (arrowheads) of S. Typhimurium in the lamina propria, which illustrates the invasive nature of the infection.
ADAPTATION OF MODELS TO STUDY VIRULENCE OF HUMAN-ADAPTED S. TYPHI
Although the mouse typhoid model has been used extensively to study S. Typhimurium infection, the pathogenesis of S. Typhi infections remains poorly understood. This is in part due to a limitation of the mouse typhoid model, namely, the fact that S. Typhimurium causes gastroenteritis rather than typhoid fever in humans. In other words, some of the genes that enable S. Typhi to cause typhoid fever in humans are not present in S. Typhimurium. The second reason S. Typhi pathogenesis is understudied is the fact that this pathogen is strictly human adapted, which has long prevented the use of animal models to study its virulence in vivo. The recent utilization of innovative new approaches to overcome these limitations holds promise for improving our understanding of S. Typhi pathogenesis.
One approach to study S. Typhi-specific virulence factors in vivo is their introduction into the mouse-pathogenic S. Typhimurium. This approach revealed that introduction of the S. Typhi-specific viaB locus into S. Typhimurium attenuates inflammation and neutrophil recruitment in bovine ligated ileal loops (65) and the mouse colitis model (32) by preventing complement receptor 3 (CR3)-mediated clearance (95) and reducing Toll-like receptor 4 (TLR4) stimulation (94). These stealth properties of the virulence (Vi) capsule-encoding viaB locus might help to explain the long incubation period of typhoid fever and the scarcity of neutrophils in intestinal infiltrates in typhoid fever patients (64, 88). Separate studies showed that the TviA regulatory protein encoded within the viaB locus rapidly represses flagellum expression and induces Vi capsule expression when the pathogen enters intestinal tissue (85, 99). The TviA-mediated repression of flagellum expression during the transition through the intestinal epithelium might help S. Typhi to evade detection of flagellin by the innate immune system through TLR5- or IL-1β-converting enzyme-protease activating factor (IPAF) (96, 97). Collectively, these data suggest that repression of PAMPs during entry into tissue enables S. Typhi to evade detection by the innate immune surveillance system through a stealth strategy, which might promote bacterial dissemination (64, 88). Consistent with this idea, introduction of tviA into the S. Typhimurium genome increases bacterial dissemination to internal organs in a chick model of infection (99). Since S. Typhimurium infection causes little clinical disease in chickens, this species is not discussed here as a model for human disease and the interested reader is referred to a recent review article on this subject (14). In summary, studies of S. Typhi-specific virulence factors in animal models are starting to reveal mechanistic differences in host-pathogen interactions that occur during a localized infection (S. Typhimurium-induced gastroenteritis) and a systemic infection (typhoid fever).
A second approach for studying the strictly human-adapted S. Typhi is the development of humanized in vivo models. Ferric Fang and coworkers recently pioneered the use of a humanized mouse model to study S. Typhi pathogenesis (42). Nonobese diabetic immunodeficient (scid) mice that lack the γ chain of the IL-2 receptor were engrafted with human hematopoietic stem cells (hu-SRC-SCID mice) and were shown to support growth of S. Typhi after intraperitoneal infection. Similar results were reported by Jorge Galán's group, who used immunodeficient (Rag2) mice lacking the IL-2 receptor γ chain engrafted with human fetal-liver hematopoietic stem and progenitor cells (78). The initial characterization of these models demonstrated that humanized mice can be used for the in vivo characterization of S. Typhi mutants (42, 78). Further use of humanized mice promises to provide rare direct insights into S. Typhi pathogenesis in vivo.
CONCLUSIONS
Animal models have been instrumental in establishing a number of conceptual advances in our understanding of human Salmonella disease. These advances were made possible by approaches that exploited the unique strengths of each animal model. However, each animal model also has shortcomings that limit its usefulness for studying certain disease manifestations associated with Salmonella serotypes. Current limitations of animal models have contributed to the relative paucity of knowledge about NTS bacteremia, a leading cause of death in sub-Saharan Africa. Furthermore, the pathogenesis of the strictly human-adapted pathogen S. Typhi remains understudied. Innovative new approaches and models to help fill these key gaps in knowledge are promising developments that should guide future research efforts. Before these new animal models can become widely used, a number of additional limitations, such as high cost, the unavailability of adequate animal facilities, and difficulty in manipulating the host, need to be overcome. Due to these restrictions, the development of future top models will likely continue to rely predominantly on mice.
ACKNOWLEDGMENTS
We thank Melita Gordon, Jennie Musto, and John Crump for insightful discussions on the global epidemiology of Salmonella serotypes and Sebastian E. Winter for helpful comments to improve the manuscript.
Work in A.J.B.'s laboratory is supported by Public Health Service grants AI040124, AI044170, AI073120, AI076246, and AI088122.
Biographies
Renée Tsolis is an Associate Professor at the University of California at Davis. She received a Ph.D. for her work in the laboratory of Fred Heffron at the Oregon Health & Science University, Portland, OR, on iron acquisition during infection with Salmonella species. She began studying the pathogenesis of Brucella abortus infection during her postdoctoral training with Thomas Ficht at Texas A&M University. Her current work is focused on understanding interactions of Brucella and Salmonella species with the host immune system.
Mariana Xavier received her doctorate in veterinary medicine in 2008 from the Universidade Federal de Minas Gerais, Brazil. She continued her education with Dr. Renato Santos, working on the pathogenesis and diagnosis of Brucella ovis infection in sheep, and received her master's degree in animal pathology in 2009. During this period, she developed her interest in understanding the cellular mechanisms of pathology in bacterial infections. Dr. Xavier is now pursuing her Ph.D. in immunology and pathology in a joint program between the Universidade Federal de Minas Gerais and the University of California, Davis. Under the direction of Dr. Renée Tsolis, she is currently investigating mechanisms of immune modulation during malaria and Salmonella coinfection as well as granuloma formation during Brucella infection.
Renato L. Santos graduated from veterinary school and earned a master's degree at the Universidade Federal de Minas Gerais (UFMG; Belo Horizonte, Brazil). He earned his Ph.D. in veterinary pathology at Texas A&M University. He has been a visiting Associate Professor at the University of California at Davis, and he is currently an Associate Professor of Veterinary Pathology at UFMG. His research interests are primarily salmonellosis, brucellosis, and leishmaniasis. He is a Researcher of the Brazilian National Council for Scientific and Technological Development (CNPq) and a Fellow of the John Simon Guggenheim Foundation. He currently holds the positions of Provost for Research at UFMG and President of the Brazilian Association for Veterinary Pathology (ABPV).
Andreas J. Bäumler worked on mechanisms of iron acquisition in Yersinia enterocolitica during his graduate studies with Dr. Klaus Hantke at the University of Tübingen, Germany. He developed an interest in the interaction of Salmonella serotypes with the intestinal mucosa during his postdoctoral training with Dr. Fred Heffron at the Oregon Health & Science University in Portland, OR. After joining the faculty at Texas A&M University Health Science Center in College Station, TX, in 1996, he initiated his ongoing studies on the pathogenesis of NTS gastroenteritis using bovine and murine models. He further expanded his research after moving to the University of California, Davis, CA, in 2005 by developing animal models for NTS bacteremia and S. Typhi infection. He serves as a permanent member of the NIH Host Interactions of Bacterial Pathogens (HIBP) Study Section and as an Editor for Infection and Immunity and is a Fellow of the American Academy of Microbiology.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Andrews-Polymenis H. L., Bäumler A. J., McCormick B. A., Fang F. C. 2010. Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect. Immun. 78:2356–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arthur G., et al. 2001. Trends in bloodstream infections among human immunodeficiency virus-infected adults admitted to a hospital in Nairobi, Kenya, during the last decade. Clin. Infect. Dis. 33:248–256 [DOI] [PubMed] [Google Scholar]
- 3. Barman M., et al. 2008. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 76:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barthel M., et al. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkley J. A., et al. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39–47 [DOI] [PubMed] [Google Scholar]
- 6. Bhan M. K., Bahl R., Bhatnagar S. 2005. Typhoid and paratyphoid fever. Lancet 366:749–762 [DOI] [PubMed] [Google Scholar]
- 7. Blackwell J. M., et al. 2001. SLC11A1 (formerly NRAMP1) and disease resistance. Cell. Microbiol. 3:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohnhoff M., Drake B. L., Miller C. P. 1954. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc. Soc. Exp. Biol. Med. 86:132–137 [DOI] [PubMed] [Google Scholar]
- 9. Bohnhoff M., Miller C. P. 1962. Enhanced susceptibility to Salmonella infection in streptomycin-treated mice. J. Infect. Dis. 111:117–127 [DOI] [PubMed] [Google Scholar]
- 10. Brent A. J., et al. 2006. Salmonella bacteremia in Kenyan children. Pediatr. Infect. Dis. J. 25:230–236 [DOI] [PubMed] [Google Scholar]
- 11. Brown D. E., McCoy M. W., Pilonieta M. C., Nix R. N., Detweiler C. S. 2010. Chronic murine typhoid fever is a natural model of secondary hemophagocytic lymphohistiocytosis. PLoS One 5:e9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown M., Eykyn S. J. 2000. Non-typhoidal Salmonella bacteraemia without gastroenteritis: a marker of underlying immunosuppression. Review of cases at St. Thomas' Hospital 1970-1999. J. Infect. 41:256–259 [DOI] [PubMed] [Google Scholar]
- 13. Bumann D. 2002. Examination of Salmonella gene expression in an infected mammalian host using the green fluorescent protein and two-colour flow cytometry. Mol. Microbiol. 43:1269–1283 [DOI] [PubMed] [Google Scholar]
- 14. Chappell L., et al. 2009. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 128:53–59 [DOI] [PubMed] [Google Scholar]
- 15. Coburn B., Li Y., Owen D., Vallance B. A., Finlay B. B. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crawford R. W., et al. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. U. S. A. 107:4353–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cummings L. A., Wilkerson W. D., Bergsbaken T., Cookson B. T. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61:795–809 [DOI] [PubMed] [Google Scholar]
- 18. De Wit S., Taelman H., Van de Perre P., Rouvroy D., Clumeck N. 1988. Salmonella bacteremia in African patients with human immunodeficiency virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 7:45–47 [DOI] [PubMed] [Google Scholar]
- 19. Dorsey C. W., Laarakker M. C., Humphries A. D., Weening E. H., Bäumler A. J. 2005. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 57:196–211 [DOI] [PubMed] [Google Scholar]
- 20. Enwere G., et al. 2006. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2-29 months in The Gambia. Pediatr. Infect. Dis. J. 25:700–705 [DOI] [PubMed] [Google Scholar]
- 21. Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. U. S. A. 83:5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis C. L., Ryan T. A., Jones B. D., Smith S. J., Falkow S. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639–642 [DOI] [PubMed] [Google Scholar]
- 23. Fu Y., Galan J. E. 1998. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol. 27:359–368 [DOI] [PubMed] [Google Scholar]
- 24. Galán J. E., Curtiss R., III 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 86:6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilks C. F. 1998. Acute bacterial infections and HIV disease. Br. Med. Bull. 54:383–393 [DOI] [PubMed] [Google Scholar]
- 26. Glynn J. R., Palmer S. R. 1992. Incubation period, severity of disease, and infecting dose: evidence from a Salmonella outbreak. Am. J. Epidemiol. 136:1369–1377 [DOI] [PubMed] [Google Scholar]
- 27. Gordon M. A. 2008. Salmonella infections in immunocompromised adults. J. Infect. 56:413–422 [DOI] [PubMed] [Google Scholar]
- 28. Gordon M. A., et al. 2002. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633–1641 [DOI] [PubMed] [Google Scholar]
- 29. Gordon M. A., et al. 2001. Bacteraemia and mortality among adult medical admissions in Malawi—predominance of non-typhi salmonellae and Streptococcus pneumoniae. J. Infect. 42:44–49 [DOI] [PubMed] [Google Scholar]
- 30. Graham S. M., Walsh A. L., Molyneux E. M., Phiri A. J., Molyneux M. E. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310–314 [DOI] [PubMed] [Google Scholar]
- 31. Gulig P. A., Curtiss R., III 1988. Cloning and transposon insertion mutagenesis of virulence genes of the 100-kilobase plasmid of Salmonella typhimurium. Infect. Immun. 56:3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haneda T., et al. 2009. The capsule-encoding viaB locus reduces intestinal inflammation by a Salmonella pathogenicity island 1-independent mechanism. Infect. Immun. 77:2932–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hensel M., Hinsley A. P., Nikolaus T., Sawers G., Berks B. C. 1999. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol. Microbiol. 32:275–287 [DOI] [PubMed] [Google Scholar]
- 34. Hensel M., et al. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400–403 [DOI] [PubMed] [Google Scholar]
- 35. Hoiseth S. K., Stocker B. A. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 36. Kent T. H., Formal S. B., Labrec E. H. 1966. Salmonella gastroenteritis in rhesus monkeys. Arch. Pathol. 82:272–279 [PubMed] [Google Scholar]
- 37. Kingsley R. A., et al. 2003. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype typhimurium: identification of intestinal colonization and persistence determinants. Infect. Immun. 71:629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kraus M. D., Amatya B., Kimula Y. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12:949–955 [PubMed] [Google Scholar]
- 39. Labrec E. H., Schneider H., Magnani T. J., Formal S. B. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 88:1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawley T. D., et al. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 76:403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lawley T. D., et al. 2006. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Libby S. J., et al. 2010. Humanized nonobese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella Typhi infection. Proc. Natl. Acad. Sci. U. S. A. 107:15589–15594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lockman H. A., Curtiss R., III 1992. Virulence of non-type 1-fimbriated and nonfimbriated nonflagellated Salmonella typhimurium mutants in murine typhoid fever. Infect. Immun. 60:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loeffler F. 1892. Ueber Epidemieen unter den im hygienishcen Institute zu Greifswald gehaltenen Mäusen und über die Bekämpfung der Feldmausplage. Zentbl. Bakteriol. Parasitenkd. 11:129–141 [Google Scholar]
- 45. Lundberg B. E., Wolf R. E., Jr., Dinauer M. C., Xu Y., Fang F. C. 1999. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect. Immun. 67:436–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Majowicz S. E., et al. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889 [DOI] [PubMed] [Google Scholar]
- 47. Mastroeni P., Villarreal-Ramos B., Hormaeche C. E. 1993. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 61:3981–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mead P. S., et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Menendez A., et al. 2009. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J. Infect. Dis. 200:1703–1713 [DOI] [PubMed] [Google Scholar]
- 50. Merican I. 1997. Typhoid fever: present and future. Med. J. Malaysia 52:299–308 [PubMed] [Google Scholar]
- 51. Mills D. M., Bajaj V., Lee C. A. 1995. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749–759 [DOI] [PubMed] [Google Scholar]
- 52. Monack D. M., Bouley D. M., Falkow S. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J. Exp. Med. 199:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mukawi T. J. 1978. Histopathological study of typhoid perforation of the small intestines. Southeast Asian J. Trop. Med. Public Health 9:252–255 [PubMed] [Google Scholar]
- 54. Nasrallah S. M., Nassar V. H. 1978. Enteric fever: a clinicopathologic study of 104 cases. Am. J. Gastroenterol. 69:63–69 [PubMed] [Google Scholar]
- 55. Nguyen Q. C., et al. 2004. A clinical, microbiological, and pathological study of intestinal perforation associated with typhoid fever. Clin. Infect. Dis. 39:61–67 [DOI] [PubMed] [Google Scholar]
- 56. Nix R. N., Altschuler S. E., Henson P. M., Detweiler C. S. 2007. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog. 3:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nunes J. S., et al. 2010. Morphologic and cytokine profile characterization of Salmonella enterica serovar typhimurium infection in calves with bovine leukocyte adhesion deficiency. Vet. Pathol. 47:322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ochman H., Soncini F. C., Solomon F., Groisman E. A. 1996. Identification of a pathogenicity island for Salmonella survival in host cells. Proc. Natl. Acad. Sci. U. S. A. 93:7800–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olsen S. J., et al. 2003. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiol. Infect. 130:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parry C. M., Threlfall E. J. 2008. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 21:531–538 [DOI] [PubMed] [Google Scholar]
- 61. Persson J., Vance R. E. 2007. Genetics-squared: combining host and pathogen genetics in the analysis of innate immunity and bacterial virulence. Immunogenetics 59:761–778 [DOI] [PubMed] [Google Scholar]
- 62. Que J. U., Hentges D. J. 1985. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect. Immun. 48:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rabsch W., Tschape H., Bäumler A. J. 2001. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 3:237–247 [DOI] [PubMed] [Google Scholar]
- 64. Raffatellu M., et al. 2006. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 74:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raffatellu M., et al. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raffatellu M., et al. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reddy E. A., Shaw A. V., Crump J. A. 2010. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 10:417–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rollenhagen C., Bumann D. 2006. Salmonella enterica highly expressed genes are disease specific. Infect. Immun. 74:1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roux C. M., et al. 2010. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect. Immun. 78:1520–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Santiviago C. A., et al. 2009. Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 5:e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Santos R. L., et al. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 69:4610–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Santos R. L., et al. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3:1335–1344 [DOI] [PubMed] [Google Scholar]
- 73. Scallan E., et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schmitt C. K., et al. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sigaúque B., et al. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr. Infect. Dis. J. 28:108–113 [DOI] [PubMed] [Google Scholar]
- 76. Silva-Herzog E., Detweiler C. S. 2010. Salmonella enterica replication in hemophagocytic macrophages requires two type three secretion systems. Infect. Immun. 78:3369–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Slauch J. M., Mahan M. J., Mekalanos J. J. 1994. In vivo expression technology for selection of bacterial genes specifically induced in host tissues. Methods Enzymol. 235:481–492 [DOI] [PubMed] [Google Scholar]
- 78. Song J., et al. 2010. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe 8:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sprinz H., Gangarosa E. J., Williams M., Hornick R. B., Woodward T. E. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am. J. Dig. Dis. 11:615–624 [DOI] [PubMed] [Google Scholar]
- 80. Stecher B., et al. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:4138–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stecher B., et al. 2007. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5:2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tacket C. O., et al. 1992. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine 10:443–446 [DOI] [PubMed] [Google Scholar]
- 83. Tacket C. O., et al. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 65:452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takeuchi A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109–136 [PMC free article] [PubMed] [Google Scholar]
- 85. Tran Q. T., et al. 2010. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect. Immun. 78:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tsolis R. M., Adams L. G., Ficht T. A., Bäumler A. J. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tsolis R. M., et al. 1999. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473:261–274 [PubMed] [Google Scholar]
- 88. Tsolis R. M., Young G. M., Solnick J. V., Bäumler A. J. 2008. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 6:883–892 [DOI] [PubMed] [Google Scholar]
- 89. van Oosterhout J. J., et al. 2005. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J. Acquir. Immune Defic. Syndr. 39:626–631 [PubMed] [Google Scholar]
- 90. Vazquez-Torres A., et al. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655–1658 [DOI] [PubMed] [Google Scholar]
- 91. Watera C., et al. 2004. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 18:1210–1213 [DOI] [PubMed] [Google Scholar]
- 92. Watson P. R., Galyov E. E., Paulin S. M., Jones P. W., Wallis T. S. 1998. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66:1432–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weening E. H., et al. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wilson R. P., et al. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol. 10:876–890 [DOI] [PubMed] [Google Scholar]
- 95. Wilson R. P., et al. 2011. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun. 79:830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Winter S. E., Raffatellu M., Wilson R. P., Rüssmann H., Bäumler A. J. 2008. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 10:247–261 [DOI] [PubMed] [Google Scholar]
- 97. Winter S. E., et al. 2009. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype typhimurium infection. Infect. Immun. 77:1904–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Winter S. E., et al. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Winter S. E., et al. 2010. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 6:e1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wray C., Sojka W. J. 1978. Experimental Salmonella typhimurium infection in calves. Res. Vet. Sci. 25:139–143 [PubMed] [Google Scholar]
- 101. Zhang S., et al. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang S., et al. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang S., et al. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhou D., Mooseker M. S., Galan J. E. 1999. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc. Natl. Acad. Sci. U. S. A. 96:10176–10181 [DOI] [PMC free article] [PubMed] [Google Scholar]