Abstract
Trypanosoma cruzi, the protozoan parasite that causes human Chagas' disease, induces a type I interferon (IFN) (IFN-α/β) response during acute experimental infection in mice and in isolated primary cell types. To examine the potential impact of the type I IFN response in shaping outcomes in experimental T. cruzi infection, groups of wild-type (WT) and type I IFN receptor-deficient (IFNAR−/−) 129sv/ev mice were infected with two different T. cruzi strains under lethal and sublethal conditions and several parameters were measured during the acute stage of infection. The results demonstrate that type I IFNs are not required for early host protection against T. cruzi. In contrast, under conditions of lethal T. cruzi challenge, WT mice succumbed to infection whereas IFNAR−/− mice were ultimately able to control parasite growth and survive. T. cruzi clearance in and survival of IFNAR−/− mice were accompanied by higher levels of IFN-γ production by isolated splenocytes in response to parasite antigen. The suppression of IFN-γ in splenocytes from WT mice was independent of IL-10 levels. While the impact of type I IFNs on the production of IFN-γ and other cytokines/chemokines remains to be fully determined in the context of T. cruzi infection, our data suggest that, under conditions of high parasite burden, type I IFNs negatively impact IFN-γ production, initiating a detrimental cycle that contributes to the ultimate failure to control infection. These findings are consistent with a growing theme in the microbial pathogenesis field in which type I IFNs can be detrimental to the host in a variety of nonviral pathogen infection models.
INTRODUCTION
Trypanosoma cruzi is the kinetoplastid protozoan parasite that causes Chagas' disease, a chronic and debilitating condition affecting several million individuals in South and Central America. T. cruzi can infect a wide variety of nucleated cell types, where it replicates as an obligate intracellular parasite in the host cell cytoplasm. The innate immune response mounted against T. cruzi is critical for controlling parasite replication and dissemination during the acute stage of infection and for priming the adaptive immune response to this pathogen (recently reviewed in reference 20). Toll-like receptor (TLR)-dependent recognition of T. cruzi ligands, primarily via TLR2 and TLR9 (3) and the signaling adaptor MyD88 (7), is necessary for infected hosts to mount a protective immune response against T. cruzi. Cytokines produced during acute infection, such as interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), as well as nitric oxide, play critical roles in controlling T. cruzi infection in the acute stage of infection (33). In addition, T. cruzi triggers a TLR-independent type I IFN response (9) in isolated cells and in murine models of acute infection (10, 12, 21, 22, 51, 52). Transcriptomic studies reveal changes in expression of a large number of IFN-inducible genes in a variety of primary mammalian cell types infected with T. cruzi in vitro and at the site of inoculation in vivo (10, 13, 52).
Type I IFNs (IFN-α/β) are a family of pleiotropic cytokines that signal through the IFN-α/β receptor (IFNAR) to regulate the expression of a large set of genes with cis-acting IFN-stimulated response elements in their promoter regions (26). Best characterized for their essential role in antiviral immunity (5), type I IFNs exert a range of immunomodulatory effects on cells of the innate and adaptive immune systems and, as such, have been increasingly recognized as modulators of the host response to nonviral pathogens (6, 15, 49). Unlike the universal role of IFN-γ (also known as type II IFN) in promoting innate resistance to bacteria and protozoa (56), the impact of type I IFNs on nonviral pathogen infection outcomes is variable: this class of cytokines can be neutral (11, 44) or protective (31, 54) but is often detrimental to the host (2, 25, 27, 28, 35, 38, 41). Type I IFN signaling is most often associated with heightened host sensitivity to bacterial infection under conditions of high pathogen load (2, 38), where the virulence capacity of an infecting isolate has been correlated with the level of IFN-α/β induced during infection (2, 27, 43). As such, the emerging literature supports the idea that type I IFNs can function as important host determinants of virulence in nonviral pathogen infection.
Despite observations that T. cruzi elicits a prominent type I IFN response early in infection (10, 13, 52), it is presently unclear if type I IFNs play a role in innate resistance to T. cruzi (12, 22, 51). The goal of the present study was to determine whether type I IFN-dependent signaling plays a role in innate host resistance to T. cruzi infection. To control for variables such as parasite strain and infecting dose, we determined the impact of type I IFN signaling on T. cruzi infection in the context of sublethal and lethal infections with two parasite strains. Our results demonstrate that type I IFNs are not required for innate immune protection against T. cruzi. Moreover, under conditions of lethal T. cruzi challenge, type I IFNs increase host susceptibility to T. cruzi infection.
MATERIALS AND METHODS
Parasite culture and mouse infection.
Tissue culture-derived T. cruzi trypomastigotes (Brazil or Y strain) were generated by weekly passage in confluent monolayers of LLcMK2 cells in Dulbecco's modified Eagle medium (DMEM) containing 2% fetal bovine serum (FBS) as described previously (47). Trypomastigotes harvested from culture supernatants were washed twice in sterile 0.9% saline prior to infections. Eight- to 10-week-old IFNAR−/− mice (129sv/ev) (34) (provided by Herbert Virgin, Washington University, St. Louis, MO) and wild-type (WT) mice (Charles River Laboratories) were inoculated with 100 μl of trypomastigotes in 0.9% saline by intradermal injection in the flank. Control mice were injected with the same volume of saline, and all mice were monitored daily for signs of illness. Microscopic examination of peripheral blood in a 5-μl sample obtained by tail venipuncture was carried out to monitor parasitemia over the course of infection. Survival curves were analyzed using a log rank test, while parasitemia curves were analyzed by paired Student's t tests.
Histology and immunohistochemical analysis.
Tissue from infected and control mice was harvested at day 13 (D13) and D27 postinfection and fixed in 10% buffered formalin for at least 24 h. Paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining were performed at the Rodent Histopathology Unit, Harvard Medical School. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) and immunostaining using anti-CD3, anti-B220, and anti-Mac20 were performed at the Haematopathology Unit at Brigham and Women's Hospital, Boston, MA.
Multianalyte profiles.
At various time points, 3 mice from each group (control and infected WT and IFNAR−/− mice) were euthanized and blood was collected in heparin-treated syringes. Plasma was collected after centrifugation at 13,000 × g for 2 min, frozen at −80°C, and submitted to Rules Based Medicine (Austin, TX) for screening of the RodentMAP antigen panel, which analyzed the plasma for the presence of 58 proteins. The minimum threshold for each analyte was set at the minimum detectable dose for that particular assay (defined as three standard deviations above the mean background). For each analyte the mean value ± standard error of the mean (SEM) was calculated for each experimental group. Two-tailed P values were determined using unpaired t tests with Welch's correction. Significant differences were defined as having P values <0.05.
Flow cytometry.
Spleens were isolated from control and infected WT or IFNAR−/− mice at day 27 postinoculation. Using standard methodology, single cell suspensions from individual spleens were prepared and red blood cells were lysed with Boyle's reagent and counted using a Coulter Counter (Z1; Beckman Coulter Inc., Fullerton, CA). Cells were incubated in 10% fetal calf serum (FCS)-phosphate-buffered saline (PBS) with 1 μg/ml of either fluorescein isothiocyanate (FITC)-conjugated anti-CD4 or anti-CD8 antibodies (BD Biosciences, San Jose, CA), washed with PBS, stained with the PE annexin V apoptosis detection kit in accordance with the manufacturer's instructions (BD Biosciences, San Jose, CA), and fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Cells were processed on a BD FACScan and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Splenocyte culture, ELISA, and ELISPOT.
Spleens were collected from individual mice, and splenocytes were prepared as described above. Splenocytes were plated in 24-well plates at 1.5 × 106 cells/ml (final volume, 1 ml) in RPMI 1640 containing 10% FBS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis, MO). Cells were stimulated with media (control), concanavalin A (ConA; 1 μg/ml), or T. cruzi antigen (shed/secreted antigens from trypomastigote/amastigote mixture; 10 μg/ml) for 60 h. Levels of IFN-γ and IL-10 in the culture supernatants were determined by enzyme-linked immunosorbent assays (ELISA) using the OptEIA mouse IFN-γ or IL-10 ELISA set according to the manufacturer's instructions (BD Biosciences, San Diego, CA). As a substrate, 3,3′,5,5′-tetramethylbenzidine (TMB)-hydrogen peroxide (H2O2) was used. Reactions were stopped by the addition of 5% phosphoric acid to all wells. The optical densities at 450 nm (OD450) were measured using a Spectra Max 190 ELISA plate reader (Molecular Devices, Sunnyvale, CA). Cytokine concentrations were calculated by using standard curves created with known amounts of recombinant cytokine. The relative number of IFN-γ-producing CD8+ T cells was determined using a standard IFN-γ-based enzyme-linked immunospot (ELISPOT) assay. Briefly, 150,000 splenocytes were seeded per well in HTS ELISPOT plates (Millipore, Bedford, MA) coated with anti-IFN-γ antibody (Mabtech, Mariemont, OH) and stimulated for 24 h with RPMI 1640, 1 μM control peptide (SIINFEKL), and a T. cruzi-specific peptide (TSKB20), generously provided by Rick Tarleton (University of Georgia). Cells were removed, and IFN-γ spots were detected using biotin-anti-IFN-γ (Mabtech, Mariemont, OH)-streptavidin-horseradish peroxidase (HRP) and AEC substrate (Vector Laboratories, Burlingame, CA). Plates were dried overnight, and spots were quantified using Immunospot Analyzer (Cellular Technology Ltd., Shaker Heights, OH).
RESULTS
IFNAR−/− mice survive lethal infection with T. cruzi.
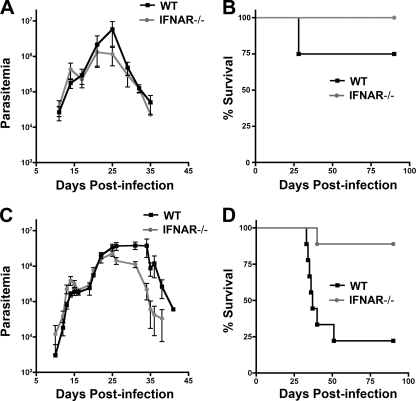
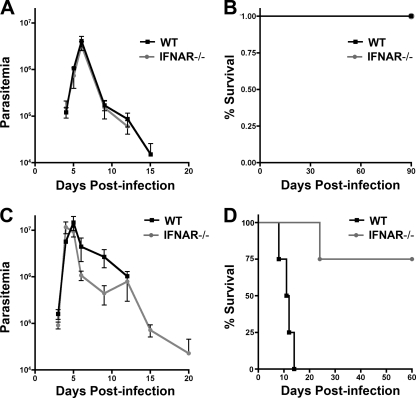
T. cruzi triggers a type I interferon response in a variety of primary cell types in vitro and in vivo in a parasite strain-dependent manner (10, 13). The role of type I IFNs in experimental T. cruzi infection is presently unclear given inconsistent outcomes observed in type I IFN receptor knockout (IFNAR−/−) mice (12, 22, 51). Given the phenotypic diversity exhibited by different T. cruzi strains (4, 16, 17), we posited that the variable outcomes reported previously might reflect differences in parasite strain and/or infecting dose. To more systematically approach the question of the role of type I IFNs in T. cruzi infection, susceptible 129sv/ev WT and IFNAR−/− mice were infected with two strains of T. cruzi differing in their virulence properties. T. cruzi Brazil strain is highly virulent in mice, causing detectable parasitemia with infecting doses as low as 10 parasites, and is lethal at doses ≥104 parasites (A.-D. C. Chessler and B. A. Burleigh, unpublished observations). In contrast, an infecting dose of 106 parasites is required to produce detectable parasitemia with T. cruzi Y strain and a dose of 108 parasites needed to cause lethal infection. WT and IFNAR−/− mice were infected with 103 (sublethal) or 104 (lethal) Brazil strain parasites, and both parasitemia and survival were monitored over time (Fig. 1). No significant differences in parasitemia or mortality were observed between infected WT and IFNAR−/− mice that received sublethal doses of T. cruzi Brazil (Fig. 1A and B) or Y strain (Fig. 2A and B). In contrast, a lethal challenge with either parasite strain resulted in death of WT mice, whereas the majority of IFNAR−/− mice survived this challenge (Fig. 1D and 2D). In both groups of mice infected with 104 Brazil strain parasites (Fig. 1C) the parasitemia curves were largely overlapping for the first 25 days of infection (P = 0.44). After day 25, the two groups of mice diverged. WT mice exhibited significantly higher parasitemia (P < 0.05) and succumbed to infection between days 30 and 50 (Fig. 1D). In general, IFNAR−/− mice survived lethal infection (Fig. 1D), cleared their parasitemia by ∼D45, and remained viable for >6 months (data not shown).
Fig. 1.
IFNAR−/− mice are less susceptible than WT mice to lethal challenge with the Brazil strain of T. cruzi. WT and IFNAR−/− mice were infected with 103 (A and B) or 104 (C and D) Brazil strain T. cruzi parasites and parasitemia (A and C) and survival (B and D) were monitored over the course of infection. Representative results of two independent experiments are shown for the sublethal dose of Brazil strain (103 parasites) (A and B) and of four independent experiments shown for the lethal dose (104 parasites) (C and D). Parasitemia data were compared using a paired Student t test; survival curves were compared with a log rank test, and P values are reported in the text.
Fig. 2.
IFNAR−/− mice are less susceptible than WT mice to lethal challenge with the Y strain of T. cruzi. WT and IFNAR−/− mice were infected with 106 (A and B) or 108 (C and D) Y strain T. cruzi parasites, and parasitemia (A and C) and survival (B and D) were monitored over the course of infection. A representative of two independent experiments is shown here. Results for WT and IFNAR−/− mice are not statistically different, with the exception of panel D data, where the P value from the log rank test is reported.
Tissue pathologies in T. cruzi-infected WT and IFNAR−/− mice are similar.
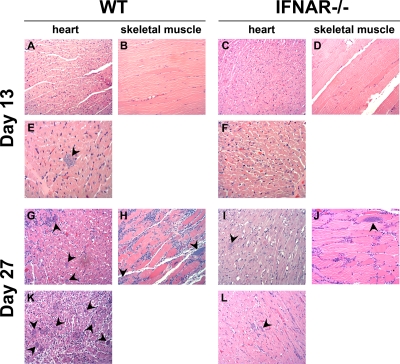
The observation that T. cruzi-infected IFNAR−/− mice are better able to control parasitemia when challenged with a lethal dose of parasites than WT mice is suggestive of a loss of immune control. To examine the histology of tissue infection under these divergent conditions, groups of 6 mice were infected with a lethal dose (104 parasites) of T. cruzi Brazil strain and sacrificed at D13, which represents the first peak of parasitemia following infection, or at D27, when obvious differences in blood parasitemia emerge (Fig. 1C and D). Examination of H&E-stained sections of tissue (heart, skeletal muscle, spleen, liver, and colon) from T. cruzi-infected mice reveals the presence of parasites in the hearts and skeletal muscle of both WT and IFNAR−/− mice (Fig. 3). For the majority of mice examined, no discernible differences in parasite burden or inflammation were seen at D27, when clear differences in parasitemia between the two groups of mice began to appear. However, in two independent experiments, one WT mouse per group (1/6) presented with a very high tissue parasite burden in the heart (e.g., Fig. 3K), and parasite nests were also detected in the colon (data not shown) at D27. Given the asynchronous nature of the survival curves (Fig. 1D) these “outliers” likely represent mice that were sacrificed closer to death than the remaining animals in the group. These observations lend support to the idea that WT mice eventually fail to control infection, with significantly higher parasitemias observed at time points >D25.
Fig. 3.
WT and IFNAR−/− mice have similar parasite burdens and pathologies in the heart and skeletal muscle following T. cruzi infection. WT and IFNAR−/− mice were infected with 104 Brazil strain T. cruzi parasites, and at 13 days (A to F) or 27 days (G to L) postinfection sections of paraffin-embedded heart or skeletal muscle tissue were stained with H&E. Representative images of the hearts and skeletal muscle at 13 days (A to D) show inflammation but minimal parasite burden in both WT and IFNAR−/− mice. Nonrepresentative images from the same tissue sections (E and F) show that some parasite nests are present in the heart at day 13. Representative images of the heart and skeletal muscle at 27 days (G to J) show significant inflammatory infiltrate and high parasite burden in the hearts and skeletal muscle in both WT and IFNAR−/− mice. A single WT mouse (out of 6) had a considerably higher parasite load and inflammation in the heart (K) than other WT and IFNAR−/− mice (G and I) and the matched IFNAR−/− control (L) at day 27.
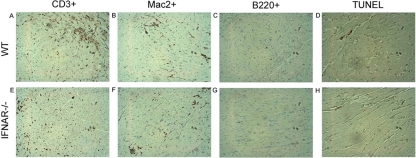
Immunohistochemical staining of cell surface markers to identify T cells (CD3+), B cells (B220+), and macrophages (Mac20+) in hearts isolated from infected WT and IFNAR−/− mice at D27 revealed no discernible differences in the steady-state levels of these cell types in infected tissue (representative images shown in Fig. 4). In Listeria monocytogenes infection, type I IFNs may enhance the susceptibility of lymphocytes undergoing listeriolysin O (LLO)-dependent apoptosis and thereby increase host susceptibility to this bacterial pathogen (8, 38). TUNEL staining carried out to identify apoptotic cells in T. cruzi-infected and uninfected heart tissue at D27 revealed no detectable differences between WT and IFNAR−/− mice, with very little apoptosis observed in general (Fig. 4D and H; data not shown). Furthermore, by profiling splenocyte CD4+ and CD8+ T-cell populations on D27 postinfection by flow cytometry for relative population size and number of apoptotic cells, we found that ratios of CD4+ to CD8+ cells in WT and IFNAR−/− uninfected and infected populations were not statistically different (Table 1). No differences in the proportions of CD4+ annexin V+ or CD8+ annexin V+ cells between WT and IFNAR−/− mice were observed. Thus, despite dramatically different fates following lethal T. cruzi challenge, no obvious differences between WT and IFNAR−/− mice were detected when infected tissues were examined for inflammation, parasite burden, cellular infiltrate, or apoptotic cells.
Fig. 4.
T. cruzi-infected WT and IFNAR−/− mice have similar levels of immune cell infiltration and apoptosis in the heart. WT (A to D) and IFNAR−/− (E to H) mice were infected with 104 Brazil strain T. cruzi parasites, and at 27 days postinfection sections of paraffin-embedded heart tissue were stained with anti-CD3 (A, E), anti-Mac2 (B, F), anti-B220 (C, G), or TUNEL (D, H). Brown staining demonstrates that similar numbers of T cells (A, E) macrophages (B, F), and B cells (C, D) are recruited to WT and IFNAR−/− mouse hearts following T. cruzi infection. Minimal apoptotic cells (D, H) were present in the hearts of WT and IFNAR−/− mice.
Table 1.
Determination of apoptosis in T-cell subsetsa
| Gated population | Infected | % of annexin V+ cells in: |
P | |
|---|---|---|---|---|
| WT mice | IFNAR−/− mice | |||
| CD4 | No | 12.07 ± 1.73 | 12.96 ± 1.25 | 0.5934 |
| Yes | 22.53 ± 1.83 | 25.08 ± 3.83 | 0.2748 | |
| CD8 | No | 10.3 ± 1.14 | 14.03 ± 0.79 | 0.0639 |
| Yes | 15.65 ± 6.03 | 20.33 ± 5.06 | 0.4044 | |
Flow cytometry was performed on freshly prepared splenocytes from WT and IFNAR−/− mice at D27 postinfection. Nonnecrotic (7-aminoactinomycin D-negative) lymphocytes were gated, and the percentages of annexin V-positive CD4 and CD8 cells were analyzed. Values represent the averages ± standard deviations (SD) for 2 to 4 mice.
Plasma cytokine profiles in T. cruzi-infected WT and IFNAR−/− mice are similar.
To determine whether phenotypic differences between the two groups of infected mice (e.g., Fig. 1C and D) were associated with differences in plasma protein levels, samples collected from mock- or T. cruzi-infected WT and IFNAR−/− mice were subjected to multianalyte analysis in which the relative abundances of 58 plasma proteins were measured. Plasma collected at D0 revealed no significant differences in baseline plasma protein levels between WT and IFNAR−/− mice (not shown). Significant changes in the levels of 26/58 plasma proteins were observed in T. cruzi-infected WT mice compared to mock-infected controls at D13 postinfection (Table 2), including elevated levels of IFN-γ (7.5-fold), IL-10 (10.8-fold), IL-6 (3.9-fold), TNF-α (2.4-fold), monocyte chemoattractant protein 1 (MCP-1) (36.3-fold), MCP-3 (18.3-fold), and MCP-5 (11-fold). Of the 26 analytes that were significantly elevated as a result of infection, only 8 were found to be significantly different between T. cruzi-infected WT and IFNAR−/− mice at D13 (Table 3), where WT mice exhibited higher levels of IL-10 and MCP-1, -3, and -5 than IFNAR−/− mice. Strikingly, however, at D27, significant differences in the levels of circulating plasma proteins between WT and IFNAR−/− mice were no longer observed (see Table S1 in the supplemental material).
Table 2.
T. cruzi infection alters plasma cytokine levelsa
| Analyteb | Avg concn ± SEM at day: |
||
|---|---|---|---|
| 0 | 13 | 27 | |
| CD40 | 96.33 ± 5.2 pg/ml | 312.7 ± 9.0 pg/ml | 802.0 ± 134.4 pg/ml |
| CD40 ligand | 201.7 ± 59.4 pg/ml | 1,363 ± 138.6 pg/ml | 15,230 ± 13,080 pg/ml |
| Haptoglobin | 24.67 ± 2.0 μg/ml | 129.3 ± 22.7 μg/ml | 196.0 ± 22.9 μg/ml |
| IFN-γ | 68 ± 0.0 pg/ml | 505.3 ± 46.3 pg/ml | 93.00 ± 17.2 pg/ml |
| IL-10 | 109 ± 0.0 pg/ml | 1,187 ± 29.63 pg/ml | 877.0 ± 63.57 pg/ml |
| IL-18 | 5.67 ± 0.43 ng/ml | 13.67 ± 1.45 ng/ml | 9.73 ± 1.33 ng/ml |
| IL-1α | 119.0 ± 16.77 pg/ml | 261.0 ± 17.44 pg/ml | 267.7 ± 73.00 pg/ml |
| IL-6 | 14 ± 0.0 pg/ml | 55.67 ± 6.96 pg/ml | 54.00 ± 18.48 pg/ml |
| MCP-1 | 74.67 ± 5.78 pg/ml | 2,713 ± 101.4 pg/ml | 1,134 ± 403.9 pg/ml |
| MCP-3 | 247.3 ± 25.15 pg/ml | 4,527 ± 288.7 pg/ml | 2,813 ± 615.4 pg/ml |
| MCP-5 | 51.33 ± 5.33 pg/ml | 580.0 ± 43.49 pg/ml | 274.7 ± 82.66 pg/ml |
| MΦ-derived chemokine | 1,071 ± 91.62 pg/ml | 1,623 ± 191.9 pg/ml | 2,403 ± 163.3 pg/ml |
| MIP-1β | 78 ± 0.0 pg/ml | 575.7 ± 27.69 pg/ml | 1,076 ± 402.9 pg/ml |
| MIP-1γ | 27.33 ± 2.33 ng/ml | 46.33 ± 3.93 ng/ml | 51.67 ± 9.82 ng/ml |
| MIP-2 | 11.67 ± 1.20 pg/ml | 102.3 ± 11.72 pg/ml | 142.3 ± 39.57 pg/ml |
| MIP-3β | 1.467 ± 0.07 ng/ml | 3.733 ± 0.30 ng/ml | 4.40 ± 1.02 ng/ml |
| MMP-9 | 103.0 ± 17.95 ng/ml | 141.7 ± 18.52 ng/ml | 205.7 ± 19.03 ng/ml |
| Myeloperoxidase | 71.67 ± 8.65 ng/ml | 202.7 ± 14.19 ng/ml | 242.0 ± 8.51 ng/ml |
| Myoglobin | >8,648 ± 0.0 ng/ml | 1,457 ± 72.19 ng/ml | 2,284 ± 977.3 ng/ml |
| Oncostatin M | 0.13 ± 0.00 ng/ml | 0.37 ± 0.01 ng/ml | 0.45 ± 0.18 ng/ml |
| Stem cell factor | 428.0 ± 35.4 pg/ml | 852.7 ± 52.16 pg/ml | 676.0 ± 154.1 pg/ml |
| TIMP-1 | 2.43 ± 0.27 ng/ml | 8.43 ± 0.55 ng/ml | 16.07 ± 4.93 ng/ml |
| TNF-α | 0.14 ± 0.0 ng/ml | 0.34 ± 0.01 ng/ml | 1.10 ± 0.38 ng/ml |
| VCAM-1 | 1,250 ± 75.06 ng/ml | 3,523 ± 291.7 ng/ml | 4,420 ± 231.6 ng/ml |
| VEGF | 349.3 ± 38.8 pg/ml | 582.7 ± 18.28 pg/ml | 574.3 ± 135.8 pg/ml |
| von Willebrand factor | 165.3 ± 3.67 ng/ml | 154.3 ± 8.69 ng/ml | 427.3 ± 19.63 ng/ml |
Multianalyte profiling was used to measure levels of 58 proteins in plasma from T. cruzi-infected WT mice at day 0 (baseline), day 13, and day 27 postinfection. Twenty-six proteins are up- or downregulated compared to baseline at day 13 and/or day 27 postinfection. Those levels that are significantly different (P < 0.05) from baseline values are in boldface.
MΦ, macrophage; MIP-1β, macrophage inflammatory protein 1β, MMP-9, matrix metalloproteinase 9; TIMP-1, tissue inhibitor of metalloproteinase 1; VCAM-1, vascular cellular adhesion molecule 1; VEGF, vascular endothelial growth factor.
Table 3.
WT and IFNAR−/− mice differ in the levels of plasma cytokines induced in response to T. cruzi infectiona
| Analyte | Avg concn (pg/ml) ± SEM for: |
P | |
|---|---|---|---|
| WT mice | IFNAR−/− mice | ||
| MCP-1 | 2,713 ± 101.4 | 836.3 ± 171.9 | 0.0025 |
| IL-10 | 1,187 ± 29.6 | 859.0 ± 31.8 | 0.0048 |
| MCP-5 | 580.0 ± 43.5 | 214.0 ± 40.5 | 0.0086 |
| VEGF | 582.7 ± 18.3 | 425.7 ± 3.7 | 0.0138 |
| MCP-3 | 4,527 ± 288.7 | 1,880 ± 127.7 | 0.0139 |
| MIP-1β | 575.7 ± 27.6 | 325.3 ± 44.7 | 0.0176 |
| Stem cell factor | 852.7 ± 52.1 | 542.7 ± 51.3 | 0.0241 |
| Leukemia inhibitory factor | 766.7 ± 20.5 | 652.3 ± 23.4 | 0.0348 |
Multianalyte profiling was used to measure plasma levels for 58 proteins in WT and IFNAR−/− mice 13 days after T. cruzi infection. Values are presented for the 8 proteins that are significantly different (P < 0.05) between WT and IFNAR−/− mice (n = 3 mice).
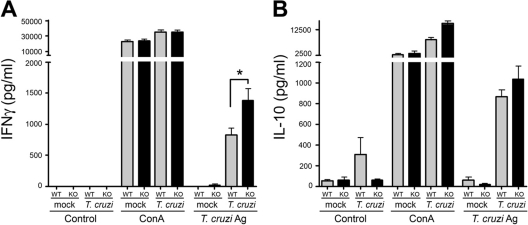
Splenocytes from IFNAR−/− mice produce higher levels of IFN-γ than those from WT mice.
Splenocytes harvested from T. cruzi-infected WT and IFNAR−/− mice at D27 postinfection were stimulated with T. cruzi antigen, ConA, or medium for 60 h, and IFN-γ and IL-10 were measured in the culture supernatants by ELISA. While splenocytes from WT and IFNAR−/− mice exhibited comparable responses to ConA stimulation (Fig. 5A and B), significantly less IFN-γ was produced in WT splenocyte cultures than in IFNAR−/− cultures in response to T. cruzi antigen (Fig. 5A), without a concomitant increase in IL-10 levels (Fig. 5B). We next sought to determine if a deficit of T. cruzi-specific effector CD8+ cells could account for the decreased IFN-γ production by WT splenocytes during antigen recall response. Isolated splenocytes from infected WT and IFNAR−/− mice were stimulated with the immunodominant T-cell peptide TSKB20, which specifically activates roughly one-third of the CD8+ population in T. cruzi-infected mice (30), as well as an ovalbumin (OVA) control peptide. The numbers of IFN-γ-producing cells in splenocyte populations isolated from infected IFNAR−/− and WT mice were similar, as determined in ELISPOT assays (data not shown). Together with the observation that T-cell populations are not inherently different in infected WT and IFNAR−/− mice (Table 1), these data point to a dampening of the quality of the antigen recall response within WT splenocyte populations, rather than a decrease in the quantity of effector cells present.
Fig. 5.
IFN-γ and IL-10 production in response to T. cruzi antigens (Ag). Splenocytes from individual animals were prepared and stimulated as described in Materials and Methods. Levels of IFN-γ (A) and IL-10 (B) were determined by ELISA in culture supernatant at 60 h poststimulation. Splenocytes from infected WT mice produced significantly less IFN-γ in response to T. cruzi antigens than splenocytes from infected IFNAR−/− mice. KO, knockout.
DISCUSSION
Transcriptomic studies have demonstrated that Trypanosoma cruzi infection triggers a prominent type I IFN response in a variety of primary cell types in vitro and at the site of intradermal inoculation of mice (10, 13). The goal of the present study was to determine whether type I IFN-dependent signaling plays a role in innate host protection against T. cruzi. From a comparison of the courses of acute T. cruzi infection of WT and IFNAR−/− mice, controlling for variables such as parasite strain and infecting dose, two clear findings have emerged. First, type I IFNs do not play a substantive role in host protection against T. cruzi during the acute phase of infection. Second, IFNAR−/− mice exhibit a strong advantage over WT mice in their ability to survive T. cruzi infection, which emerges under conditions of lethal parasite challenge. These findings clarify the discordant conclusions of earlier studies, which reported different outcomes for the involvement of type I IFNs in experimental acute T. cruzi infection (12, 22, 51). Specifically, in the study in which type I IFN-dependent signaling was associated with increased host susceptibility to T. cruzi infection, a lethal dose of parasites was used (51). The two studies reporting no deleterious effects of type I IFN signaling on the host were carried out under conditions of sublethal infection (12, 22). It should be noted that the mice in our study were infected intradermally, which more closely resembles natural infection, while mice in the other studies were infected intraperitoneally (12, 22, 51). Although it is possible that the macrophage-rich environment of the peritoneum could influence type I IFN-dependent responses, which might be reduced in an intradermal infection, we have not observed significant differences in parasitemia or survival in WT mice infected with T. cruzi via the intraperitoneal or intradermal route (Chessler and Burleigh, unpublished). Overall, a side-by-side comparison of lethal and sublethal T. cruzi infection conditions, with two different parasite strains, provides evidence that type I IFNs are not protective under the experimental infection conditions used in this study and that, importantly, this cytokine response becomes detrimental under conditions of higher parasite burden. These findings are consistent with those reported for several nonviral pathogens, including the intracellular bacterial pathogens Mycobacterium tuberculosis, Listeria monocytogenes, and Chlamydia muridarum and the extracellular protozoan parasite Trypanosoma brucei (2, 6, 25, 35, 38, 41, 53), where sensitivity to type I IFNs is associated with increased host susceptibility to infection, particularly under conditions of high pathogen burden.
The mechanistic basis for the detrimental effects associated with type I IFNs in the context of nonviral pathogen infection is not well understood and is likely to differ within the framework of a specific host-pathogen interaction. As a group, type I IFNs exhibit pleiotropic properties, exerting both stimulatory and repressive effects on cells of the innate and adaptive immune systems (18). For example, type I IFNs act as potent activators of NK cells to generate the initial wave of IFN-γ (24, 37) and have been shown to be important for the generation of antigen-specific effector CD8+ and memory T cells (23). On the other hand, elevated levels of type I IFNs can dampen protective immune responses by inhibiting IFN-γ expression by both NK and T cells in a STAT1-dependent manner (14, 36) and by downregulating IFN-γ receptor (42) or chemokine expression (46) during pathogen infection. Failure of mice to control experimental infection with Chlamydia muridarum or Listeria monocytogenes has been correlated with type I IFN-dependent apoptosis of macrophage and lymphocyte populations (32, 38, 41). We detected no differences in abundance or relative apoptosis in splenocyte CD4+ and CD8+ T-cell populations isolated from groups of T. cruzi-infected WT and IFNAR−/− mice. However, splenocytes isolated from WT mice exhibited a diminished capacity to produce IFN-γ in antigen recall experiments compared to IFNAR−/− splenocytes, with no accompanying change in absolute numbers of IFN-γ-producing CD8+ cells or larger-scale variability in the profile of the T-cell compartment. These findings are consistent with those reported in a recent study (29) showing that parasite-specific CD8+ T-cell responses and immunity are not dependent upon type I IFN signaling. Given that IFN-γ is crucial for defense against T. cruzi, where NK cells and CD4+ and CD8+ T cells are important sources of IFN-γ in T. cruzi-infected mice (1, 45, 48, 50), the diminished ability of splenocyte populations from infected WT mice to produce this Th1 cytokine is consistent with increased susceptibility to infection compared to IFNAR−/− mice. In this manner, our findings are similar to those reported for experimental T. brucei infection of Ubp43−/− mice, which exhibit increased sensitivity to type I IFNs, where a significant reduction in IFN-γ production was cited as a likely cause for the increased susceptibility of Ubp43−/− mice to infection with this extracellular parasite (25). Similarly, induction of type I IFNs by virulent strains of M. tuberculosis leads to increased expression of negative regulators of the Jak-Stat pathway, which is predicted to dampen IFN-γ-mediated signaling and host protection against this pathogen (19, 28). In these studies, the dampening of the Th1 response associated with increased type I IFN expression was accompanied by decreased IL-12, IL-6, and TNF-α expression in the lungs of infected mice (27). A similar correlation with proinflammatory cytokine expression was not observed in our study with T. cruzi infection.
Overall, our findings support a broader theme in the innate immunity literature, i.e., that type I IFNs play an important immunomodulatory role in nonviral pathogen infection (6, 15). Under particular conditions, sensitivity of the host to this class of cytokine appears to erode the functionality of critical components of the innate and/or adaptive immune response, highlighting the necessity to balance type I IFN production to maintain immune control of pathogen growth. Results from the present study lend additional support to the idea that variables such as the particular host-pathogen system, strain, infecting dose, or route of infection influence the net effect of type I IFNs on host infection with nonviral pathogens (6, 15). Furthermore, with regard to the controversy in the T. cruzi field regarding the role of type I IFNs in experimental infection of mice, our results provide a clear explanation for the differential outcomes reported previously. In addition to pathogen-related factors affecting type I IFN activity, human genetic variants that affect type I IFN signaling have been associated with altered susceptibility to, and response to treatment for, both viral and autoimmune diseases (39, 40). Very little is currently known about the function of type I IFNs in human Chagas' disease. Considering results from the present study, we would predict that type I IFNs would not be required for innate control of T. cruzi infection in humans. However, under conditions of high parasite burden, as seen in acute T. cruzi infections following oral transmission (55) or potentially within the microenvironment of focal tissue infection or in cases of viral coinfection, type I IFNs may negatively impact the capacity to control infection. Thus, understanding the signaling events induced by type I IFN in the context of human T. cruzi infection and how these events influence susceptibility to infection and parasite persistence may aide in the development of novel therapies for Chagas' disease.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant AI047960 (B.A.B.).
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Abrahamsohn I. A., Coffman R. L. 1996. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 84:231–244 [DOI] [PubMed] [Google Scholar]
- 2. Auerbuch V., Brockstedt D. G., Meyer-Morse N., O'Riordan M., Portnoy D. A. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200:527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bafica A., et al. 2006. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 177:3515–3519 [DOI] [PubMed] [Google Scholar]
- 4. Bertoli M., Ando M. H., De Ornelas Toledo M. J., De Araujo S. M., Gomes M. L. 2006. Infectivity for mice of Trypanosoma cruzi I and II strains isolated from different hosts. Parasitol. Res. 99:7–13 [DOI] [PubMed] [Google Scholar]
- 5. Biron C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383–390 [DOI] [PubMed] [Google Scholar]
- 6. Bogdan C., Mattner J., Schleicher U. 2004. The role of type I interferons in non-viral infections. Immunol. Rev. 202:33–48 [DOI] [PubMed] [Google Scholar]
- 7. Campos M. A., et al. 2004. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J. Immunol. 172:1711–1718 [DOI] [PubMed] [Google Scholar]
- 8. Carrero J. A., Calderon B., Unanue E. R. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chessler A. D., Ferreira L. R., Chang T. H., Fitzgerald K. A., Burleigh B. A. 2008. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J. Immunol. 181:7917–7924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chessler A. D., Unnikrishnan M., Bei A. K., Daily J. P., Burleigh B. A. 2009. Trypanosoma cruzi triggers an early type I IFN response in vivo at the site of intradermal infection. J. Immunol. 182:2288–2296 [DOI] [PubMed] [Google Scholar]
- 11. Cooper A. M., Pearl J. E., Brooks J. V., Ehlers S., Orme I. M. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68:6879–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa V. M., et al. 2006. Type I IFNs stimulate nitric oxide production and resistance to Trypanosoma cruzi infection. J. Immunol. 177:3193–3200 [DOI] [PubMed] [Google Scholar]
- 13. Costales J. A., Daily J. P., Burleigh B. A. 2009. Cytokine-dependent and -independent gene expression changes and cell cycle block revealed in Trypanosoma cruzi-infected host cells by comparative mRNA profiling. BMC Genomics 10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cousens L. P., Orange J. S., Su H. C., Biron C. A. 1997. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. U. S. A. 94:634–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Decker T., Muller M., Stockinger S. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5:675–687 [DOI] [PubMed] [Google Scholar]
- 16. de Diego J. A., Penin P., del Rey J., Mayer R., Gamallo C. 1991. A comparative pathological study of three strains of Trypanosoma cruzi in an experimental model. Histol. Histopathol. 6:199–206 [PubMed] [Google Scholar]
- 17. de Freitas J. M., et al. 2006. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PLoS Pathog. 2:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Sastre A., Biron C. A. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879–882 [DOI] [PubMed] [Google Scholar]
- 19. Imai K., Kurita-Ochiai T., Ochiai K. 2003. Mycobacterium bovis bacillus Calmette-Guerin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol. Med. Microbiol. 39:173–180 [DOI] [PubMed] [Google Scholar]
- 20. Junqueira C., et al. 2010. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev. Mol. Med. 12:e29. [DOI] [PubMed] [Google Scholar]
- 21. Kierszenbaum F., Sonnenfeld G. 1982. Characterization of the antiviral activity produced during Trypanosoma cruzi infection and protective effects of exogenous interferon against experimental Chagas' disease. J. Parasitol. 68:194–198 [PubMed] [Google Scholar]
- 22. Koga R., et al. 2006. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J. Immunol. 177:7059–7066 [DOI] [PubMed] [Google Scholar]
- 23. Kolumam G. A., Thomas S., Thompson L. J., Sprent J., Murali-Krishna K. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee C. K., et al. 2000. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 165:3571–3577 [DOI] [PubMed] [Google Scholar]
- 25. Lopez R., Demick K. P., Mansfield J. M., Paulnock D. M. 2008. Type I IFNs play a role in early resistance, but subsequent susceptibility, to the African trypanosomes. J. Immunol. 181:4908–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malmgaard L., Salazar-Mather T. P., Lewis C. A., Biron C. A. 2002. Promotion of alpha/beta interferon induction during in vivo viral infection through alpha/beta interferon receptor/STAT1 system-dependent and -independent pathways. J. Virol. 76:4520–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manca C., et al. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. U. S. A. 98:5752–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manca C., et al. 2005. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25:694–701 [DOI] [PubMed] [Google Scholar]
- 29. Martin D. L., Murali-Krishna K., Tarleton R. L. 2010. Generation of Trypanosoma cruzi-specific CD8+ T-cell immunity is unaffected by the absence of type I interferon signaling. Infect. Immun. 78:3154–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin D. L., et al. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattner J., et al. 2004. Protection against progressive leishmaniasis by IFN-beta. J. Immunol. 172:7574–7582 [DOI] [PubMed] [Google Scholar]
- 32. Merrick J. C., Edelson B. T., Bhardwaj V., Swanson P. E., Unanue E. R. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151:785–792 [PMC free article] [PubMed] [Google Scholar]
- 33. Michailowsky V., et al. 2001. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am. J. Pathol. 159:1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muller U., et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 35. Nagarajan U. M., et al. 2008. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 76:4642–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen K. B., et al. 2000. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 1:70–76 [DOI] [PubMed] [Google Scholar]
- 37. Nguyen K. B., et al. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279–4287 [DOI] [PubMed] [Google Scholar]
- 38. O'Connell R. M., et al. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Doherty C., Villoslada P., Vandenbroeck K. 2007. Pharmacogenomics of type I interferon therapy: a survey of response-modifying genes. Cytokine Growth Factor Rev. 18:211–222 [DOI] [PubMed] [Google Scholar]
- 40. Pascual V., Farkas L., Banchereau J. 2006. Systemic lupus erythematosus: all roads lead to type I interferons. Curr. Opin. Immunol. 18:676–682 [DOI] [PubMed] [Google Scholar]
- 41. Qiu H., et al. 2008. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 181:2092–2102 [DOI] [PubMed] [Google Scholar]
- 42. Rayamajhi M., Humann J., Penheiter K., Andreasen K., Lenz L. L. 2010. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J. Exp. Med. 207:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reutterer B., et al. 2008. Type I IFN are host modulators of strain-specific Listeria monocytogenes virulence. Cell. Microbiol. 10:1116–1129 [DOI] [PubMed] [Google Scholar]
- 44. Rothfuchs A. G., et al. 2006. STAT1 regulates IFN-alpha beta- and IFN-gamma-dependent control of infection with Chlamydia pneumoniae by nonhemopoietic cells. J. Immunol. 176:6982–6990 [DOI] [PubMed] [Google Scholar]
- 45. Russo M., Starobinas N., Marcondes M. C., Minoprio P., Honteyberie-Joskowicz M. 1996. The influence of T cell subsets on Trypanosoma cruzi multiplication in different organs. Immunol. Lett. 49:163–168 [DOI] [PubMed] [Google Scholar]
- 46. Shahangian A., et al. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 119:1910–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tardieux I., Nathanson M. H., Andrews N. W. 1994. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J. Exp. Med. 179:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tarleton R. L., Sun J., Zhang L., Postan M. 1994. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect. Immun. 62:1820–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor G. A. 2007. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell. Microbiol. 9:1099–1107 [DOI] [PubMed] [Google Scholar]
- 50. Torrico F., et al. 1991. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 146:3626–3632 [PubMed] [Google Scholar]
- 51. Une C., Andersson J., Orn A. 2003. Role of IFN-alpha/beta and IL-12 in the activation of natural killer cells and interferon-gamma production during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 134:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vaena de Avalos S., Blader I. J., Fisher M., Boothroyd J. C., Burleigh B. A. 2002. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J. Biol. Chem. 277:639–644 [DOI] [PubMed] [Google Scholar]
- 53. van den Broek M. F., Muller U., Huang S., Zinkernagel R. M., Aguet M. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5–18 [DOI] [PubMed] [Google Scholar]
- 54. Vigario A. M., et al. 2007. Recombinant human IFN-alpha inhibits cerebral malaria and reduces parasite burden in mice. J. Immunol. 178:6416–6425 [DOI] [PubMed] [Google Scholar]
- 55. Yoshida N. 2008. Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity. Parasitol. Int. 57:105–109 [DOI] [PubMed] [Google Scholar]
- 56. Zhang S. Y., et al. 2008. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol. Rev. 226:29–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.