Abstract
Previously, we demonstrated unique protein expression patterns in 20-week-Schistosoma mansoni-infected CBA/J mice with moderate splenomegaly syndrome (MSS) or hypersplemomegaly syndrome (HSS). To better understand the development of severe pathology, we compared the two-dimensional differential in-gel electrophoresis (2D-DIGE) proteomic signatures of livers from uninfected mice and mice infected for 6, 8, 12, or 20 weeks and found significant changes in collagen isoforms, interleukin-2 (IL-2), cytokeratin 18, hydroxyproline, S. mansoni phosphoenolpyruvate carboxykinase, major urinary protein isoforms, and peroxiredoxin 6. Cytokeratin 18, hydroxyproline, and connective tissue growth factor (CTGF) were chosen for analysis in mouse and human sera using targeted biochemical assays. Consistent with the liver analysis, cytokeratin 18, CTGF, and hydroxyproline were significantly elevated in sera from mice with HSS compared to those from uninfected mice or mice with MSS. Moreover, cytokeratin 18 and CTGF were found to be markers for subjects with hepatosplenic and intestinal schistosomiasis, respectively, while serum hydroxyproline was a strong indicator of fibrosis for severe HS. These findings indicate that schistosome-associated changes to the liver can be detected in the serum and reveal the potential for cytokeratin 18 to be used as a diagnostic marker for early detection of hepatosplenic schistosomiasis.
INTRODUCTION
Schistosomiasis is endemic in 74 countries, affecting 207 million people and with a further 700 million people at the risk of infection (32). Schistosoma mansoni infects almost 83 million people, causing a range of pathologies from intestinal disease (INT) to hepatosplenic schistosomiasis (HS) (10), with approximately 8.5 million people suffering from S. mansoni-associated hepatosplenic disease. Subjects with HS present with hepatic fibrosis, portal hypertension, hepatomegaly, and splenomegaly resulting from unregulated immune responses to parasite eggs embedded in the liver (3). The eggs induce inflammatory granulomatous responses that cause this severe pathology (24), which is marked by accumulation of extracellular matrix proteins, especially collagen. The ensuing hepatic fibrosis causes liver dysfunction and ultimately liver failure. Ultrasound is a noninvasive imaging technique for schistosomiasis morbidity, but it underestimates liver fibrosis, requires expensive equipment and trained personnel, and is ineffective at detecting early liver pathology (9). Identification of serum biomarkers to detect developing fibrosis in persons with schistosomiasis could promote earlier detection of hepatosplenic disease and better patient management.
We previously demonstrated unique liver protein abundance patterns in CBA/J mice with 20-week S. mansoni infections that displayed either hypersplenomegaly syndrome (HSS) or moderate splenomegaly syndrome (MSS) (19). In this model of chronic schistosomiasis, 20% of mice spontaneously present with HSS, which resembles human HS, and 80% of mice show MSS symptoms that are similar to the less severe human INT. The MSS and HSS liver proteomic signatures depicted a distinct pattern of proteins, including increased keratin D (cytokeratin 18) and decreased major urinary protein (MUP) in mice with HSS. Other proteins, such as collagen isoforms and S. mansoni phosphoenolpyruvate carboxykinase (PEPCK), were markers of infection (19). Using two-dimensional differential in-gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry, we sought to identify potential biomarkers for HS. To this end, we compared liver proteomic signatures from uninfected mice and from mice with 6-, 8-, 12, and 20-week infections to identify candidate markers and validated these candidates in mouse and human sera.
MATERIALS AND METHODS
Mouse liver sample and serum collection.
Male CBA/J mice were obtained from Jackson Laboratory and were maintained at the American Association for Accreditation of Laboratory Animal Care, Centers for Disease Control and Prevention (Atlanta, GA), in accordance with institutional guidelines and federal regulations. Mice were infected by subcutaneous injection as described previously (19). Portions of the right lobe of the liver and serum samples were collected from uninfected mice (n = 5) or mice with 6-week (n = 10), 8-week (n = 10), 12-week (n = 10), or 20-week (n = 10) infections. Samples were collected at the same time of the day to avoid bias due to collection time and were snap-frozen at −80°C (19). The 20-week-infected mice were classified as having MSS or HSS based on the percent spleen-to-body weight ratio (%SBW) and the gross pathological appearance of the liver and spleen (17). The percent liver-to-body weight ratio (%LBW) was calculated as a measure of hepatomegaly.
Human samples.
Human serum samples were collected from car washers above 18 years of age with S. mansoni infections in Kisumu, Kenya. On approval by the participants, sera were tested for antibodies to HIV-1. The modified Kato Katz technique was used to quantify S. mansoni eggs in fecal specimens, which were expressed as eggs per g (21). Assessment of schistosomiasis morbidity was performed using a portable Aloka SSD-620 ultrasound machine with a 3.5-MHz convex probe (Aloka Co., Ltd., Tokyo, Japan). Subjects' schistosome-induced liver pathology was evaluated and assigned an image pattern (IP) according to the Niamey classification (23). Normal liver texture (smooth liver surface) was designated IPA, IPB was indicative of subjects with a small degree of fibrosis but no definitive pathology, IPC showed “ring echoes” that appeared as pipe stems in a perpendicular scan, IPD was characterized by a “ruff” around the main portal vein and bifurcation, and IPE showed a “ruff” extending into the liver parenchyma with patches of fibrosis. In our study, subjects with IPA were classified as having INT, while individuals demonstrating IPC, IPD, or IPE were classified as having HS. Twenty-three INT serum samples and 14 HS serum samples, matched for infection intensity (i.e., eggs per g), were selected for the study. Sera from 13 age- and sex-matched uninfected individuals were included as controls. The institutional review boards of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, and the Kenya Medical Research Institute approved the human subject protocols.
2D-DIGE with liver lysates.
Each murine liver sample was homogenized with standard lysis buffer, and the liver protein concentration was determined using a 2D-Quant kit (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The liver lysates were subjected to 2D-DIGE using the Minimal CyDye kit (GE Healthcare) as per the manufacturer's recommended protocol. The internal standard, a pool of 15 liver lysates (5 each for uninfected, MSS, and HSS) was labeled using Cy2; individual samples were labeled with Cy3 and Cy5. The labeled samples were focused on pI 4 to 7 and pI 6 to 11 IPG DryStrips and electrophoresed as previously described (19). The Cy2, Cy3, and Cy5 images were scanned sequentially as described previously (6). 2D-DIGE gels were analyzed for differential protein patterns using DeCyder 2-D 6.5 software (GE Healthcare). The biological variation analysis module was used for matching 69 gel images for each pI range and the difference in protein spots between gels expressed as the average volume ratio between the six study groups (uninfected mice, 6-week-infected mice, 8-week-infected mice, 12-week-infected mice, 20-week-infected mice with MSS, or 20-week-infected mice with HSS), and individual protein spot volumes were also extracted for further analyses. To minimize false-positive discovery of protein spots, the false-discovery rate feature was applied. The data were analyzed according to the guidelines for a target power of 0.8, as described previously (19).
Protein identification using mass spectrometry.
The excised liver proteins of interest from the preparative gels were subjected to tryptic digestion using an Ettan Digester (GE Healthcare). The dried tryptic digests were analyzed by MALDI-TOF (Voyager-DE Pro MALDI-TOF mass spectrometer; Applied Biosystems, Foster City, CA). Spectra were processed using Data Explorer software (v 5.1; Applied Biosystems) to generate monoisotopic peptide masses which were used to identify proteins as described previously (19).
Western blotting.
For cytokeratin 18, mouse and human serum samples were separated by one-dimensional electrophoresis using Bio-Rad Laboratories Criterion precast gels (Bio-Rad Laboratories, Inc., Hercules, CA), and the proteins were transferred onto an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Sigma Aldrich). Membranes were probed with cytokeratin 18 goat polyclonal antibody (1:600 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by Cy5-labeled anti-goat antibody (1:2,000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA). Fluorescence intensity of the bands was visualized using a Fujifilm FLA-5100 scanner (Fuji Photo Film Co.), and band volumes were analyzed using ImageQuant 5.2v (GE Healthcare) software.
CTGF and hydroxyproline assays.
A human serum connective tissue growth factor (CTGF) enzyme-linked immunosorbent assay (ELISA) development kit (Leinco Technologies, Inc., MO) was used to measure CTGF in duplicate 100-μl serum samples as per the manufacturer's guidelines.
Liver and serum hydroxyproline levels were measured following acid digestion to hydrolyze proteins to their component amino acids, including hydroxyproline derived from collagen and any collagen degradation products. Liver samples were hydrolyzed in 6 M HCl for 18 h at 110°C (20), and mouse or human serum samples (100 μl) were hydrolyzed with 6 M HCl for 3 h at 120°C in tightly sealed glass tubes (29). After cooling, the samples were reacted with freshly prepared oxidant solution (chloramine-T/citrate buffer, pH 6.0) and Ehrlich's reagent (20). The absorbance of the end product was measured at 570 nm (Molecular Devices). Hydroxyproline levels were determined by comparison with a standard curve and expressed as μg/g liver tissue and μg/ml serum.
Statistical analysis.
Individual protein spot volumes, Western blot band volume data, and human serum sample data were analyzed using GraphPad Prism (v4.0; GraphPad, San Diego, CA). For statistical analysis, spot volumes from 2D-DIGE data were log ratio transformed for compositional data as described by Aitchison (1), and multiple regression analyses were performed using SPSS (v16.0.1; SPSS Inc., Chicago, IL).
RESULTS
Linear regression correlations using liver 2D-DIGE spot volume data.
To determine whether changes in liver protein abundance correlated with the progression of infection and pathology in S. mansoni-infected CBA/J mice, we analyzed the changes in liver protein expression from uninfected mice and mice infected for 6, 8, 12, and 20 (MSS and HSS) weeks. A total of 4,900 protein spots were detected in the pI ranges 4 to 7 and 6 to 11. The abundance of 76 protein spots between the six study groups changed significantly, with one-way analysis of variance (ANOVA) of ≤0.01, a 2-fold change in average volume ratio, and application of false-discovery rate using the DeCyder software. MALDI-TOF mass fingerprinting elucidated the identities of 45 protein spots, which are listed in Table S1 and illustrated in Fig. S1 in the supplemental material.
Protein spot volumes from each mouse were plotted against %SBW, and simple linear regression correlations were performed. This approach helped analyze the effect of each protein on the pathogenesis of splenomegaly, since 20-week classification of the mice as having MSS or HSS was based on the %SBW and gross pathological changes in the liver. Interleukin-2 (IL-2), cytokeratin 18, MUP, peroxiredoxin 6, and S. mansoni PEPCK were chosen for further evaluation (Fig. 1 and 2). Additionally, because fibrosis is one of the major pathological consequences of HSS, we included liver hydroxyproline as a potential marker for HSS (Fig. 1 and 2).
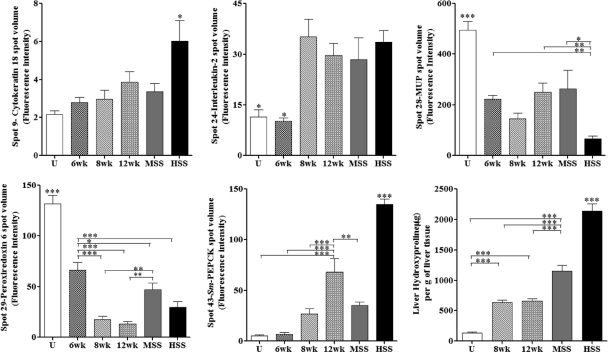
Fig. 1.
Liver candidate markers in different study groups in a mouse model, showing comparisons of mouse liver cytokeratin 18, IL-2, MUP, S. mansoni PEPCK, peroxiredoxin 6, and liver hydroxyproline for uninfected (U), 6-week-infected (6wk), 8-week-infected (8wk), 12-week-infected (12wk), and 20-week-infected (MSS and HSS) mice. Data shown are means ± standard errors of the means (SEM). Overall ANOVA, P ≤ 0.01. Individual groups were compared using the Newman-Keuls multiple-comparison test: ***, P ≤ 0.001; **, P ≤ 0.01; and * P ≤ 0.05 (compared to all other study groups and as indicated).
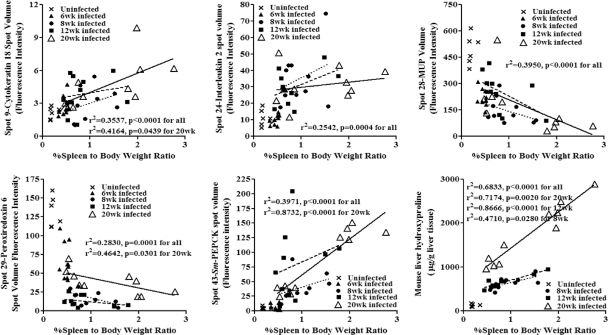
Fig. 2.
Linear regression correlations of spot volume intensity for liver candidate markers cytokeratin 18, IL-2, MUP, S. mansoni PEPCK, peroxiredoxin 6, and liver hydroxyproline content with percent spleen-to-body weight ratio from uninfected (U), 6-week-infected (6wk), 8-week-infected (8wk), 12-week-infected (12wk), and 20-week-infected mice. Linear regression lines: dotted, 8-week-infected mice; dashed, 12-week-infected mice; solid, 20-week-infected mice.
Cytokeratin 18, MUP, and hydroxyproline are markers of murine schistosomiasis pathology.
Three spots identified as liver cytokeratin 18 had increased abundance in mice with HSS compared to those with MSS, control mice, or 6- to 12-week-infected animals. In particular, the volume for spot 9 suggested that mice with HSS had significantly higher levels of this isoform of cytokeratin D than the other five study groups (Fig. 1). Cytokeratin 8 (spot 12), which occurs as paired filaments with cytokeratin 18 in liver, also increased in mice with HSS. While cytokeratin 18 levels correlated with increased pathology, two spots representing MUP demonstrated significantly decreased levels in mice with HSS compared to the other five study groups (Fig. 1). The significant inverse correlation between MUP levels and splenomegaly was independent of the duration of infection (P < 0.0001) (Fig. 2).
A marked feature of schistosomiasis is liver fibrosis. Previously, we found an increased abundance of collagen isoforms for mice with MSS and HSS compared to uninfected mice (19). Collagen contains the amino acid hydroxyproline, which is formed by hydroxylation of proline in preformed collagen and therefore can be used as a measure of collagen-associated fibrosis. Liver hydroxyproline was greater in mice with HSS than in those with MSS or 8-week and 12-week infected mice and was also greater in all infected mice than in uninfected controls. The linear regression analysis for the liver hydroxyproline and %SBW showed that hydroxyproline was significantly associated with splenomegaly independent of time of infection (P < 0.0001) (Fig. 2).
IL-2, peroxiredoxin 6, and S. mansoni PEPCK are markers of schistosome infection.
Changes in the abundances of IL-2, peroxiredoxin 6, and S. mansoni PEPCK were found to correlate significantly with S. mansoni infection. The spot volumes for the cytokine IL-2 were significantly higher for 8-week- and 12-week-infected mice and for mice with MSS and HSS than for uninfected and 6-week-infected mice (Fig. 1). In contrast, the spot volumes for peroxiredoxin 6 significantly decreased for 6-week, 8-week, and 12-week-infected mice and for mice with MSS and HSS compared to uninfected mice (Fig. 1), with a dramatic decrease in abundance at 8 and 12 weeks after infection.
S. mansoni PEPCK, a parasite protein, showed increased abundance during disease progression (Fig. 1). In addition to the MALDI mass fingerprint identification of S. mansoni PEPCK (7), we confirmed identification of the protein by tandem mass spectrometry of tryptic digests of the excised proteins and analysis using an AB SCIEX TOF/TOF 5800 system (Applied Biosystems, Foster City, CA). The identified protein had higher mass and was more alkaline than predicted for S. mansoni PEPCK, suggesting extensive modification of the protein. There was a significant association of the protein with %SBW during schistosomiasis independent of the time of infection, suggesting a relationship between the number of eggs in the liver and degree of splenomegaly. However, given the high levels in livers of all infected animals, S. mansoni PEPCK may serve as a valuable infection-specific marker.
Multiple regression analysis using mouse liver 2D-DIGE data.
To test the multiple protein spot volumes simultaneously and investigate the concurrent effects of different proteins on disease pathology represented by %SBW (splenomegaly) or %LBW (hepatomegaly), we used multiple regression analysis in our study. In this analysis, the standardized β value indicates the extent of effect of the independent variable on the dependent variable in the presence of other independent variables. The coefficient of determination (r2) shows the linear relationship between the two measures and indicates how much of the variation in the dependent variable can be predicted by the independent variable. The protein spot volumes were logarithmically transformed to normalize the data as per the guidelines for compositional data (1) and for hydroxyproline values to satisfy the assumption of normality. All the protein spot volumes (independent variables) that were significantly related to the disease progression using linear regression were used for multiple regression analysis. The spot volumes were entered by the forward stepwise method into a multiple regression analysis to identify the determinants of splenomegaly and hepatomegaly (dependent variables).
Using stepwise multiple regression analysis of IL-2, cytokeratin 18, peroxiredoxin 6, MUP, S. mansoni PEPCK spot volumes, and hydroxyproline levels, we found that at 8 and 12 weeks postinfection, liver hydroxyproline was the strongest predictor of splenomegaly, followed by cytokeratin 18 at 12 weeks postinfection (Table 1). However, at 20 weeks of infection, 85% of the variation in splenomegaly could be explained by MUP expression (standardized β = −0.535). Like splenomegaly, hepatomegaly was predicted strongly by liver hydroxyproline at 8 and 12 weeks postinfection. For 20-week infections, S. mansoni PEPCK was the strongest variable, predicting 54% of the variation in hepatomegaly. When the spot volume data at 8, 12, and 20 weeks postinfection were analyzed independent of the effect of hydroxyproline levels, none showed any protein predictors except for 20-week infections, which showed that MUP was negatively correlated with splenomegaly (85%; standardized β = −0.922) and hepatomegaly (45%; standardized β = −0.673) (Table 1).
Table 1.
Predictors of splenomegaly and hepatomegaly
| Condition | Analysis (n) | Predictor | r2 | Standardized β coefficient | P value |
|---|---|---|---|---|---|
| Splenomegaly | 8-wk infection (10) | Hydroxyproline | 0.514 | +0.717 | 0.020 |
| 12-wk infection (10) | Hydroxyproline | 0.825 | +0.908 | 0.000 | |
| Cytokeratin 18 | 0.120 | +0.437 | 0.006 | ||
| 20-wk infection (10) | MUP | 0.849 | −0.535 | 0.006 | |
| Hydroxyproline | 0.099 | +0.497 | 0.008 | ||
| 20-wk infection independent of hydroxyproline (10) | MUP | 0.849 | −0.922 | 0.000 | |
| Independent of time of infection (35) | Hydroxyproline | 0.757 | +0.657 | 0.000 | |
| Peroxiredoxin 6 | 0.051 | −0.295 | 0.000 | ||
| MUP | 0.045 | −0.277 | 0.004 | ||
| Independent of time of infection and hydroxyproline (35) | IL-2 | 0.477 | +0.294 | 0.032 | |
| MUP | 0.111 | −0.503 | 0.000 | ||
| Peroxiredoxin 6 | 0.063 | −0.330 | 0.008 | ||
| Hepatomegaly | 8-wk infection (10) | Hydroxyproline | 0.478 | +0.692 | 0.027 |
| 12-wk infection (10) | Hydroxyproline | 0.822 | +0.799 | 0.000 | |
| 20-wk infection (10) | S. mansoni PEPCK | 0.541 | +0.736 | 0.015 | |
| 20-wk infection independent of hydroxyproline (10) | MUP | 0.452 | −0.673 | 0.033 | |
| Independent of time of infection (35) | Hydroxyproline | 0.455 | +0.623 | 0.000 | |
| Peroxiredoxin 6 | 0.095 | −0.313 | 0.012 | ||
| Independent of time of infection and hydroxyproline (35) | IL-2 | 0.430 | +0.656 | 0.000 |
When data were analyzed independent of the time of infection, liver hydroxyproline was the strongest predictor of splenomegaly (r2 = 0.757; standardized β = 0.657; P = 0.000) and hepatomegaly (r2 = 0.455, standardized β = 0.623; P = 0.000), followed by peroxiredoxin 6 (Table 1). As hydroxyproline was such a strong predictor for both splenomegaly and hepatomegaly, we analyzed the data independently of hydroxyproline levels and time of infection in order to identify other protein predictors during schistosomiasis. Using this approach, we found that IL-2, MUP, and peroxiredoxin 6 together could predict 65% of the splenomegaly, while IL-2 correlated with 43% of the variation in hepatomegaly. Together, these results support our initial findings suggesting that these proteins are associated with pathology and may be useful as markers of disease.
Mouse serum analysis using targeted assays.
From the multiple regression analyses we found that MUP, IL-2, peroxiredoxin 6, hydroxyproline, and cytokeratin 18 were strong predictors of disease. However, because MUP is exclusively a mouse protein and because IL-2 and peroxiredoxin 6 detect the presence of infection but are not HSS specific, these proteins were not investigated as serologic markers. Liver cytokeratin 18 and hydroxyproline were associated with HSS and were included for further analysis. In addition, because our 20-week-infected mouse data showed significantly increased collagen isoforms, we also evaluated connective tissue growth factor (CTGF) in the serum as a marker of collagen synthesis (19).
Similar to the liver results, we found that the levels of mouse serum cytokeratin 18 were higher in animals with HSS than in those with MSS and uninfected mice (Table 2). Additionally, as anticipated from our DIGE results, CTGF and hydroxyproline levels were significantly increased in sera from mice with HSS compared to sera from mice with MSS and from uninfected mice (Table 2). In line with the findings for liver hydroxyproline (see Table S1b in the supplemental material), this is the first report investigating the levels of hydroxyproline in schistosomiasis sera. Taken together, we found that all three candidates were detected in mouse serum and correlated to HSS disease.
Table 2.
Summary of results for mouse serum analysis
| Mouse serum test (unit) | Result (mean ± SEM) for: |
P valuea | ||
|---|---|---|---|---|
| Uninfected mice | Mice with MSS | Mice with HSS | ||
| Cytokeratin 18 (band vol) | (6.8 ± 0.5) × 106 | (6.2 ± 0.3) × 106 | (10.8 ± 0.5) × 106 | ≤0.001 |
| CTGF (ng/ml) | 20.1 ± 0.4 | 37.2 ± 7.9 | 108.1 ± 23.7 | ≤0.01 |
| Hydroxyproline (μg/ml) | 5.3 ± 1.3 | 9.2 ± 2.6 | 19.4 ± 3.9 | ≤0.05 |
For mice with HSS compared to uninfected mice and mice with MSS.
Human serum analysis using targeted assays.
As a first step toward validation of the biomarkers in human serum, we tested the potential of cytokeratin 18, CTGF, and hydroxyproline as markers for HS. These candidates were tested in human sera from subjects with either HS or INT disease forms, as determined by ultrasound examination. The characteristics of the subjects analyzed in our study are shown in Table 3. Because coinfection with HIV-1 was common where the human samples were collected, we also considered a possible confounding effect of HIV-1 infection status.
Table 3.
Characteristics of human serum samples based on ultrasound image patterns and HIV-1 infection status
| Subject groupa | No. of subjects |
||||||
|---|---|---|---|---|---|---|---|
| Total | With image patternb: |
||||||
| IPA |
IPC |
IPD/E |
|||||
| HIV-1 negative | HIV-1 positive | HIV-1 negative | HIV-1 positive | HIV-1 negative | HIV-1 positive | ||
| Uninfected | 13 | ||||||
| INT | 23 | 11 | 12 | ||||
| HS | 14 | 6 | 4 | 4 | |||
INT, intestinal schistosomiasis; HS, hepatosplenic schistosomiasis.
IPA, normal liver texture; IPC, liver scan with “ring echoes” that appeared as pipe stems; IPD/E, liver scan with “ruff” around the main portal vein and in the liver parenchyma with patches of fibrosis.
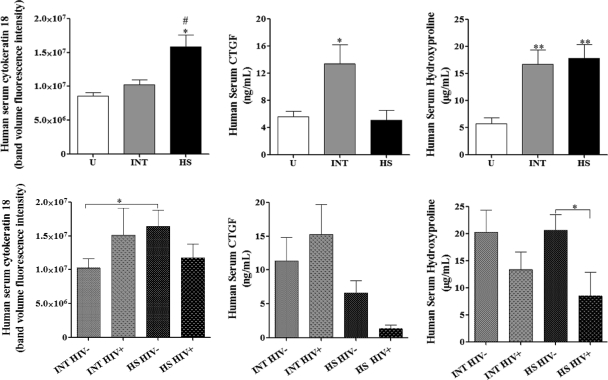
Consistent with the findings for mouse sera, serum cytokeratin 18 was significantly higher in HS sera than in INT sera and uninfected human sera (Fig. 3). This relationship remained statistically significant (P ≤ 0.05) even when only HIV-1-seronegative INT and HS groups were compared (Fig. 3); cytokeratin 18 levels in INT and HS HIV-1-seropositive sera were similar. These results suggest that for HIV-1-seronegative subjects, cytokeratin 18 levels are associated with hepatosplenic schistosomiasis.
Fig. 3.
Human serum analysis. Human serum cytokeratin 18, CTGF, and hydroxyproline comparisons for INT and HS and within the groups separated by HIV-1 coinfection status. Means ± SEM are shown. ANOVA, P ≤ 0.01 for all biomarkers. Posttest group comparisons were performed by Newman-Keuls multiple-comparison test: #, P ≤ 0.05 compared to uninfected human sera (U); ##, P ≤ 0.01 compared to INT sera; *, P ≤ 0.05 compared to uninfected human and HS sera; **, P ≤ 0.01 compared to uninfected human sera; *, P ≤ 0.05 for HIV-1 serostatus comparison.
Although the mouse serum results indicated that CTGF levels were higher in sera from animals with HSS than in those from animals with MSS, in humans INT sera showed significantly higher CTGF values in HS and uninfected sera (Fig. 3). When subjects categorized into HIV-1-seronegative and -seropositive groups were examined, subjects with HS had lower levels of CTGF than subjects with INT irrespective of HIV status (Fig. 3). These results suggest that in contrast to our findings in mice, CTGF levels are associated with moderate rather than severe disease in humans.
Although serum hydroxyproline levels were higher in the mice with HSS, the human serum analysis showed high levels in both chronic human disease forms (INT and HS) compared to those in uninfected individuals (Fig. 3). When all serum hydroxyproline concentrations from infected subjects were analyzed based on the HIV-1 serostatus, lower levels were found in HIV-1-seropositive subjects than in HIV-1-seronegative subjects, although this difference was not statistically significant. However, for those subjects with HS, this difference was significant (P ≤ 0.05) (Fig. 3).
When all the candidate markers were reviewed according to ultrasound image patterns (21) and the HIV-1 infection status, none of the protein levels were statistically significant among IPA, IPC, and IPD/E groups except for serum hydroxyproline. Serum hydroxyproline was significantly higher in the IPD/E patient group than in the sera from individuals with IPA or IPC. This increase correlates with the higher degree of fibrosis detected by ultrasound (see Fig. S2 in the supplemental material). Categorizing the serum hydroxyproline image patterns according to the HIV-1-seronegative and -seropositive groups showed no statistical significance; however, the hydroxyproline levels were lower in HIV-1-seropositive subjects than the HIV-1-seronegative subjects (see Fig. S2 in the supplemental material).
Multiple regression analysis using human serum data.
The candidate liver markers analyzed using human sera were normalized logarithmically (loge) and entered into stepwise multivariate regression analysis to identify predictors of ultrasound-detected fibrosis in human schistosomiasis. When all the ultrasound image patterns were combined together, cytokeratin 18 (r2 = 0.142; standardized β = +0.377; P = 0.031) was the only predictor of ultrasound-detected fibrosis in humans. These results indicate that cytokeratin 18 has significant potential as a serum biomarker for HS.
DISCUSSION
The aim of this study was to use the mouse model of hepatosplenic schistosomiasis to identify candidate liver markers for hepatosplenic schistosomiasis and to investigate their relevance to human disease. Initially, the candidate markers that were selected were IL-2, cytokeratin 18, peroxiredoxin 6, MUP, an apparently modified form of S. mansoni PEPCK, CTGF and hydroxyproline. After regression analyses of the liver 2D-DIGE results, cytokeratin 18, hydroxyproline, and CTGF were chosen for further analysis using targeted assays with mouse and human sera. These targeted assays highlighted the potential of cytokeratin 18 to be used as a marker for HS.
We found a significantly increased abundance of cytokeratin 18 in mice with HSS compared to other study groups (Fig. 1). The linear regression analysis showed significant correlations with pathology at 20 weeks of infection. When all the cytokeratin 18 spot volumes were assessed together, the linear regression remained significant independent of the time of infection (Fig. 2). An increased abundance of cytokeratin 18 has been reported in alcoholic liver fibrosis (25) and chronic hepatitis C virus (HCV) infection (26). Acidic forms of cytokeratin 18, including apparent phosphorylation products, have been detected in alcoholic hepatitis and hepatic steatosis (25); whether protein spots 10 and 11 in our study represent similarly modified isoforms is unclear (see Fig. S1 in the supplemental material). Cytokeratins 8 and 18 provide hepatocyte structural stability and act as modulators of toxic stress and apoptosis. In addition, imbalanced expression of liver cytokeratins 8 and 18 has been related to increased oxidative stress and reactive oxygen species during liver disease (34). In schistosomiasis, reactive oxygen species play an important role in granuloma formation and disease progression (15). Thus, the increased abundances of spots 9 to 11 (cytokeratin 18) and spot 12 (cytokeratin 8) may be related to stress modulation of the scarred liver during schistosomiasis. Furthermore, studies have confirmed the role of cytokeratin 18 in liver regeneration (5); a high abundance of cytokeratin 18 in schistosomiasis may be the result of a healing process for the damaged liver. Previous studies suggest that cytokeratin 18 is hepatoprotective (22) and that mutations in the protein predispose the liver to fibrosis (27), indicating its importance in liver stability.
The mouse serum cytokeratin 18 Western blot confirmed the liver 2D-DIGE results and showed a strong relationship between mice with HSS and serum cytokeratin 18 levels (Table 1). Supporting the mouse serum data, the human serum Western blot showed significantly increased cytokeratin 18 levels in subjects with HS compared to those with INT and uninfected subjects (Fig. 3). Studies of nonalcoholic liver disease (22) and chronic HCV infection-associated fibrosis (26) have recorded high levels of serum cytokeratin 18, similar to the case in our study. The results for HIV-1 coinfection showed no significant difference between seronegative and seropositive subjects, although the limited number of HIV-1-positive individuals and the convenience sampling methodology makes it difficult to derive any firm conclusions about the effect of HIV-1 (Fig. 3). Our study showed increased levels of cytokeratin 18 in sera from mice and humans with severe disease. Verifying these results with a larger number of human serum samples may generate a clearer picture of the relevance of this protein to the pathogenesis and detection of schistosomiasis-associated fibrosis. A marker that detects fibrosis during schistosomiasis, even if it also detects fibrosis resulting from other causes, could be coupled with serodiagnostic assays for schistosomiasis as a screening tool for people at risk of HS in areas of endemicity.
Hydroxyproline is an amino acid found in collagen and is formed by hydroxylation of proline in preformed collagen. Hydroxyproline makes up 10% of the weight of the collagen and may therefore be used as an as a measure of tissue collagen content. During fibrosis, accumulation of collagen due to an imbalance in collagen synthesis and degradation (28) may lead to increased hydroxyproline. We found an increased abundance of collagen isoforms during 20 weeks of murine schistosomiasis (19) and therefore assessed liver hydroxyproline. We found an increased abundance of liver hydroxyproline in mice with HSS compared to 8-week- and 12-week-infected mice and mice with MSS (Fig. 1). Other studies have demonstrated high liver hydroxyproline levels during S. mansoni infection (8, 12, 14, 20) and chemically induced hepatic fibrosis (16). In our study the linear regression analysis showed the strongest correlation with splenomegaly (Fig. 2) at 12 weeks of infection (r2 = 0.8666; P < 0.0001). Liver hydroxyproline proved to be the strongest predictor of splenomegaly and hepatomegaly (Table 1). Changes in mouse liver hydroxyproline levels were reflected in mouse serum, as mice with HSS showed higher serum hydroxyproline levels than those with MSS and uninfected mice (Table 2), consistent with the high levels of liver hydroxyproline in mice with HSS (see Table S1b in the supplemental material).
The serum hydroxyproline levels in subjects with INT and HS were also greater than those in uninfected human sera (Fig. 3), similar to the case for patients with hepatitis C-associated fibrosis (4). Our human serum data differed from the mouse serum data in that serum hydroxyproline levels failed to differentiate between the moderate and severe disease. In human chronic schistosomiasis, the increased serum hydroxyproline levels may indicate successive or continuous reinfections and therefore a constant initiation and imbalance of collagen synthesis and degradation. However, when serum hydroxyproline levels were analyzed according to the ultrasound image pattern categories (see Fig. S2 in the supplemental material), subjects with IPD/E had significantly higher levels than subjects with IPA and IPC, suggesting that serum hydroxyproline levels may reflect the degree of fibrosis in human schistosomiasis.
CTGF production is triggered by transforming growth factor β during hepatic fibrosis and is highly expressed in fibrotic tissues, inducing collagen synthesis (31). As a result, CTGF can be used as a measure of collagen synthesis. Our study showed that CTGF levels were significantly elevated in mice with HSS compared to uninfected mice and mice with MSS, reflecting extensive fibrosis in the severe form of the disease (Table 2) and supporting the increased abundance of collagen isoforms in chronic schistosomiasis (2, 19). Another study showed that high serum CTGF levels are a useful biomarker for assessment of liver fibrosis (18), which is in line with our findings and supports CTGF as a candidate marker for human schistosomiasis. However, our human serum study showed that human serum CTGF levels were higher in subjects with INT than in subjects with HS (P ≤ 0.01) (Fig. 3). Further supporting this connection between CTGF and schistosomiasis pathology, a recent study conducted with schistosome-infected Chinese, Sudanese, and Brazilian subjects reported two single-nucleotide polymorphisms located close to the CTGF gene as valuable markers for disease progression in schistosomiasis hepatic fibrosis (11). Our results indicate that CTGF was a potential marker for INT, although further investigation is required to study the mechanisms of action of this factor during human schistosomiasis.
The high abundance of parasite protein S. mansoni PEPCK at 12 weeks of infection and with HSS suggests increased gluconeogenesis for the energy needs of the parasite, indicating active infection at these time points postinfection. A recent study reported that during host-schistosome interactions, glyceroneogenesis, a PEPCK-dependent pathway, is active and uses glutamine as a precursor (33). Indeed, this indicates that during murine-schistosome interactions, this pathway is active and acts as an alternative source of energy for the parasite in the liver portal area. Another study demonstrated S. mansoni PEPCK to be a novel egg antigen with a T-cell epitope (3). This information, in combination with the results in our study, suggests that S. mansoni PEPCK can possibly be an infection-specific marker for human schistosomiasis and merits further elucidation and investigation.
We found an almost 7-fold-decreased abundance of the urinary pheromone binding protein MUP as a marked feature of mice with HSS (Fig. 1). The decreased abundance of MUP in murine hepatocellular carcinoma (13) is similar to the findings in our study, possibly indicative of comparable pathological mechanisms in hepatocellular carcinoma and chronic schistosomiasis. Interestingly, the pattern of MUP isoforms differs between male and female mice (7), and this protein is similar to human epididymis-specific lipocalin 9 (30); whether lipocalin 9 correlates with degree of pathology in human schistosomiasis merits investigation. Taken together, the results in our study indicate that proteins such as S. mansoni PEPCK and MUP are interesting potential candidates, but technical issues limit their current usefulness. In particular, MUP is an interesting candidate protein, but its decrease in response to disease and variation in patterns in male and female mice (7) make it a complex candidate for serum study, while the parasite S. mansoni PEPCK has no commercially available method of detection. However, if these issues can be addressed, we believe that these proteins will merit further investigation.
In conclusion, our studies using the mouse model demonstrated a strong correlation between the serum and liver data, and using these results, we identified several candidate markers in human sera. Importantly, this study provides support for the use of proteomics to identify potential biomarkers for more targeted studies, as it has pinpointed specific molecular changes, such as changes in cytokeratin 18 or MUP expression, that correlate closely with disease development. These candidates would not have been identified using previous targeted approaches, which focus on the major components of altered pathways or processes such as fibrosis. Finally, while this study assessed candidates in only a small and well-defined cohort, future studies with larger and more diverse patient populations will help to validate the usefulness of these candidates as predictors of schistosomiasis pathology.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the University Research Fund and Wellington Medical Research Foundation. The mouse and human serum analysis at the Centers for Disease Control and Prevention, Atlanta, GA, was possible due to the following travel grants: New Zealand Postgraduate Study Abroad Awards 2009, J. L. and Kathleen Stewart Research Experience Awards 2009, and Victoria University of Wellington Faculty Strategic Research Grant 2009. Schistosome life cycle stages for this work were supplied through NIH-NIAID contract N01-A1-55270. Collection of human sera and ultrasound analyses were supported in part by NIH/NIAID grant R01 053695.
We thank Danyl McLauchlan for bioinformatics advice and Pete Augostini for performing the infections and assisting with the mouse liver and serum sample collection. We also thank Daniel G. Colley and Molly Hyde for critical reading of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Aitchison J. 1986. The statistical analysis of compositional data. Chapman and Hall, London, United Kingdom [Google Scholar]
- 2. Andrade Z. A., Peixoto E., Guerret S., Grimaud J. 1992. Hepatic connective tissue changes in hepatosplenic schistosomiasis. Hum. Pathol. 23:566–573 [DOI] [PubMed] [Google Scholar]
- 3. Asahi H., Osman A., Cook R. M., LoVerde P. T., Stadecker M. J. 2000. Schistosoma mansoni phosphoenolpyruvate carboxykinase, a novel egg antigen: immunological properties of the recombinant protein and identification of a T-cell epitope. Infect. Immun. 68:3385–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Attallah A. M., et al. 2007. Evaluation of serum procollagen aminoterminal propeptide III, laminin, and hydroxyproline as predictors of severe fibrosis in patients with chronic Hepatitis C. J. Immunoassay Immunochem. 28:199–211 [DOI] [PubMed] [Google Scholar]
- 5. Bastos R., et al. 1992. Increase of cytokeratin D during liver regeneration: association with the nuclear matrix. Hepatology 16:1434–1446 [DOI] [PubMed] [Google Scholar]
- 6. Beddek A. J., et al. 2008. Profiling the metabolic proteome of bovine mammary tissue. Proteomics 8:1502–1515 [DOI] [PubMed] [Google Scholar]
- 7. Beynon R. J., Hurst J. L. 2004. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides 25:1553–1563 [DOI] [PubMed] [Google Scholar]
- 8. Cheever A. W., Barral-Netto M. 1985. Fibroblast stimulating activity of extracts of hepatic granulomata of Schistosoma mansoni-infected rodents with marked or slight hepatic fibrosis. Trans. R. Soc. Trop. Med. Hyg. 79:319–321 [DOI] [PubMed] [Google Scholar]
- 9. Chiavaroli R., Grima P. 2008. Detection of early liver fibrosis in patients with intestinal schistosomiasis: sonographic and histologic findings in Schistosoma mansoni infection. Infection 36:585–589 [DOI] [PubMed] [Google Scholar]
- 10. Crompton D. W. T. 1999. How much human helminthiasis is there in the world? J. Parasitol. 85:397–403 [PubMed] [Google Scholar]
- 11. Dessein A., et al. 2009. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes. J. Exp. Med. 206:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn M. A., Rojkind M., Warren K. S. 1977. Liver collagen synthesis in murine schistosomiasis. J. Clin. Invest. 59:666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elchuri S., Naeemuddin M., Sharpe O., Robinson W. H., Huang T. T. 2007. Identification of biomarkers associated with the development of hepatocellular carcinoma in CuZn superoxide dismutase deficient mice. Proteomics 7:2121–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Meneza S., Olds G. R., Kresina T. F., Mahmoud A. A. F. 1989. Dynamics of hepatic connective tissue matrix constituents during murine Schistosoma mansoni infection. Hepatology 9:50–56 [DOI] [PubMed] [Google Scholar]
- 15. Elsammak M. Y., Al-Sharkaweey R. M., Ragab M. S., Amin G. A., Kandil M. H. 2008. IL-4 and reactive oxygen species are elevated in Egyptian patients affected with schistosomal liver disease. Parasite Immunol. 30:603–609 [DOI] [PubMed] [Google Scholar]
- 16. George J., Chandrakasan G. 2000. Biochemical abnormalities during the progression of hepatic fibrosis induced by dimethylnitrosamine. Clin. Biochem. 33:563–570 [DOI] [PubMed] [Google Scholar]
- 17. Henderson G. S., et al. 1993. Two distinct pathological syndromes in male CBA/J. inbred mice with chronic Schistosoma mansoni infections. Am. J. Pathol. 142:703–714 [PMC free article] [PubMed] [Google Scholar]
- 18. Kovalenko E., et al. 2009. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J. Viral Hepatitis 16:612–620 [DOI] [PubMed] [Google Scholar]
- 19. Manivannan B., Rawson P., Jordan T. W., Secor W. E., La Flamme A. C. 2010. Differential patterns of liver proteins in experimental murine hepatosplenic schistosomiasis. Infect. Immun. 78:618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montesano M. A., Colley D. G., Willard M. T., Freeman Jr. G. L., Secor W. E. 2002. Idiotypes expressed early in experimental Schistosoma mansoni infections predict clinical outcomes of chronic disease. J. Exp. Med. 195:1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mwinzi P. N. M., et al. 2004. Evaluation of hepatic fibrosis in persons co-infected with Schistosoma mansoni and human immunodefiency virus 1. Am. J. Trop. Med. Hyg. 71:783–786 [PubMed] [Google Scholar]
- 22. Oshima R. G. 2002. Apoptosis and keratin intermediate filaments. Cell Death Differ. 9:486–492 [DOI] [PubMed] [Google Scholar]
- 23. Richter J., Hatz C., Campagne G., Bergquist N. R., Jenkins J. M. 2000. Ultrasound in schistosomiasis: a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. World Health Organization, Geneva, Switzerland [Google Scholar]
- 24. Rumbley C. A., et al. 1998. The schistosome granuloma: characterization of lymphocyte migration, activation, and cytokine production. J. Immunol. 161:4129–4137 [PubMed] [Google Scholar]
- 25. Salmhofer H., Rainer I., Zatloukal K., Denk H. 1994. Posttranslational events involved in griseofulvin-induced keratin cytoskeleton alterations. Hepatology 20:731–740 [PubMed] [Google Scholar]
- 26. Strnad P., et al. 2006. Keratin variants associate with progression of fibrosis during chronic hepatitis C infection. Hepatology 43:1354–1363 [DOI] [PubMed] [Google Scholar]
- 27. Strnad P., et al. 2008. Keratin mutation predisposes to mouse liver fibrosis and unmasks differential effects of the carbon tetrachloride and thioacetamide models. Gastroenterology 134:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi S., Dunn M. A., Seifter S. 1980. Liver collagenase in murine schistosomiasis. Gastroenterology 78:1425–1431 [PubMed] [Google Scholar]
- 29. Uji Y., et al. 1994. Measurement of free and total hydroxyproline by automated flow injection of serum or urine samples from maintenance hemodialysis patients with renal osteodystrophy. J. Clin. Lab. Anal. 8:267–272 [DOI] [PubMed] [Google Scholar]
- 30. Virtanen T., Kinnunen T. 2008. Mammalian allergens, p. 201–208 In Lockey R. F., Ledford D. K. (ed.), Allergens and allergen immunotherapy, 4th ed. Informa Health Care, London, United Kingdom [Google Scholar]
- 31. Weng H.-L., et al. 2007. Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes. Hepatology 46:1257–1270 [DOI] [PubMed] [Google Scholar]
- 32. WHO, 2010, posting date Schistosomiasis. WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs115/en/index.html [Google Scholar]
- 33. Yang J., Kalhan S. C., Hanson R. W. 2009. What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 284:27025–27029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zatloukal K., et al. 2004. The keratin cytoskeleton in liver diseases. J. Pathol. 204:367–376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.