Abstract
Trypanosoma cruzi infection causes intense myocarditis, leading to cardiomyopathy and severe cardiac dysfunction. Protective adaptive immunity depends on balanced signaling through a T cell receptor and coreceptors expressed on the T cell surface. Such coreceptors can trigger stimulatory or inhibitory signals after binding to their ligands in antigen-presenting cells (APC). T. cruzi modulates the expression of coreceptors in lymphocytes after infection. Deregulated inflammation may be due to unbalanced expression of these molecules. Programmed death cell receptor 1 (PD-1) is a negative T cell coreceptor that has been associated with T cell anergy or exhaustion and persistent intracellular infections. We aimed to study the role of PD-1 during T. cruzi-induced acute myocarditis in mice. Cytometry assays showed that PD-1 and its ligands are strongly upregulated in lymphocytes and APC in response to T. cruzi infection in vivo and in vitro. Lymphocytes infiltrating the myocardium exhibited high levels of expression of these molecules. An increased cardiac inflammatory response was found in mice treated with blocking antibodies against PD-1, PD-L1, and to a lesser extent, PD-L2, compared to that found in mice treated with rat IgG. Similar results in PD-1−/− mice were obtained. Moreover, the PD-1 blockade/deficiency led to reduced parasitemia and tissue parasitism but increased mortality. These results suggest the participation of a PD-1 signaling pathway in the control of acute myocarditis induced by T. cruzi and provide additional insight into the regulatory mechanisms in the pathogenesis of Chagas' disease.
INTRODUCTION
Chagas' disease is the most important cause of acquired cardiomyopathy in Latin America and is one of the outcomes resulting from the interaction between the human immune system and the hemoflagellate prokaryote Trypanosoma cruzi. In the natural infection, the flagellated forms in the feces of infected hematophagous insects of the Triatominae subfamily invade the host through skin lesions or intact mucosa. The parasite then proliferates intracellularly and disseminates systemically from the site of inoculation, causing an inflammatory reaction of variable intensity, along with splenomegaly, cardiac parasitism, and myocarditis, which is largely associated with morbidity. More frequently, a chronic asymptomatic infection is established, which eventually leads to dilated cardiomyopathy and heart failure as well as esophageal or intestinal dilatations due to the combined effects of parasite persistence, immune deregulation, autonomic denervation, and microvascular damages (21, 30).
The immunological mechanisms underlying this silent, relentless infection and heart pathology remain elusive despite several decades of research. It is known that T cell-mediated immune responses are essential to control the parasite replication during the acute phase of the infection (33). The cytokines gamma interferon (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α) strengthen the activation of innate and adaptive effector immune responses, resulting in more efficient killing of the parasite and a strong inflammatory response in several tissues where parasites replicate, including the myocardium. On the other hand, the cytokines IL-10 and transforming growth factor β (TGF-β) counterregulate the inflammatory process, indirectly favoring parasite persistence within infected host cells because they are potent inhibitors of nitric oxide (NO) production and other IFN-γ- and IL-12-mediated cell activation processes that are important for the killing of the parasite (12, 32). The extent of this regulation seems to be crucial for the final outcome of the illness, since patients with the indeterminate (asymptomatic) form of the disease have a more controlled immune response (38) than patients with advanced stages of infection.
The intensity of a protective immune response can be determined by the balanced expression of costimulatory and coinhibitory molecules during priming of T cells by antigen-presenting cells (APC). CD28 and inducible T cell costimulator (ICOS) are costimulatory receptors, while cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death cell receptor 1 (PD-1) exert a negative function to prevent excessive T cell activation (8). PD-1 is a member of the CD28 family, expressed mainly on activated T, B, and myeloid lineage cells (1). It signals through the ligands PD-L1 (9) and PD-L2 (18), which are expressed by an increasing number of cell types, including myeloid, lymphoid, and nonlymphoid cells. Engagement of PD-1 with any of its two ligands inhibits the activation of T cells and the production of cytokines, especially IL-2 and IFN-γ (8). Mice of the BALB/c strain which are deficient in PD-1 lost their immunological tolerance to cardiac autoantigens and can suffer spontaneous autoimmune dilated cardiomyopathy (25). In addition, the blockade of PD-1 engagement accelerates autoimmune disorders (3, 31) and graft-versus-host disease (7, 24). Induction of PD-L1 expression has also been demonstrated as a mechanism for immune evasion by intracellular pathogens (15). Recent evidence suggests that avoiding signaling through coinhibitory molecules could constitute promising immunotherapeutic strategies in antiviral and antitumor cellular immunity (14). For example, treatment with anti-CTLA-4 antibodies improves the cellular immune response against T. cruzi (23). It therefore seems reasonable to hypothesize that PD-1 may participate in the cell-mediated immune response and in the maintenance of cardiac tolerance during an infection with T. cruzi. Here we show that this infection induces increased expression of PD-1 by cells of the immune system and that this regulatory pathway is involved in the control of acute myocarditis, as its inhibition or gene deletion leads to increased cardiac inflammation.
MATERIALS AND METHODS
Mice, antibodies, and treatments.
C57BL/6 mice aged 6 to 8 weeks, obtained from local animal facilities (FMRP-USP), were treated with anti-PD-1 (RPM1-14), anti-PD-L1 (MIH5), anti-PD-L2 (TY25), or normal rat IgG starting 48 h before infection and lasting for 2 weeks. During this period, intraperitoneal (i.p.) injections containing 250 μg of antibody were administered to mice every 72 h. Antibodies against PD-1 (RPM1-14), PD-L1 (MIH5), and PD-L2 (TY25) were produced in the labs of T. Honjo and M. Azuma. Four or five hearts were collected from mice at 14 and 20 days postinfection (p.i.) for histology, immunohistochemistry, PCR, and enzyme-linked immunosorbent assay (ELISA) studies. Noninfected age-matched mice were used as controls. For survival studies, two independent (anti-PD-1-treated and rat IgG-treated) groups of 8 animals were followed until 35 days postinfection. Mice were cared for according to the local guidelines on ethics in animal experiments.
Parasites and experimental infection.
Mice were infected (i.p.) with 1,000 bloodstream forms of T. cruzi (Y strain) obtained from intermediary strain-matched mice. Parasitemia levels were evaluated in 5 μl of blood drawn from the tail. Before infection of intermediary mice, parasites were grown and purified from the monkey kidney fibroblast line LLC-MK2 (ATCC).
Histological analysis.
Quantification of heart tissue inflammation was assessed by stereologically counting inflammatory cells in four representative nonconsecutive hematoxylin-eosin (H&E)-stained sections (thickness of 5 μm) per organ (n = 3) at days 14, 20, and 25 postinfection. A Zeiss Integrationsplatte II eyepiece reticule (Oberkochen, Germany) and an Olympus BHS microscope (magnification of ×400) were used, as previously described (29).
Immunofluorescence.
Hearts from 3 to 5 infected mice were removed, embedded in the tissue-freezing medium Tissue-Tek (Sakura Finetek, Torrance, CA), and stored in liquid N2. Serial 5- to 7-μm-thick sections were fixed in cold acetone and subjected to immunofluorescence staining using fluorescein isothiocyanate (FITC)-conjugated antibodies against PD-1 (RPM1-14) or phycoerythrin (PE)-conjugated antibodies PD-L1 (MIH5) or PD-L2 (TY25) (BD).
Measurement of cytokine/chemokine production.
The concentrations of cytokines and chemokines in heart homogenates or sera were measured by ELISA. The following ELISA sets were used: IFN-γ (BD Biosciences, San Jose, CA), TNF-α (R&D Systems, Minneapolis, MN), and CCL2, CCL3, and CCL5 (PeproTech, Rocky Hill, NJ), according to the manufacturers' instructions. The reaction was revealed with peroxidase-conjugated streptavidin (Sigma), followed by use of the substrate mixture containing hydrogen peroxide and tetramethyl benzidine (TMB; Kirkegaard & Perry Laboratories, MD) as a chromogen.
RNA extraction.
Total RNA was extracted from the homogenates of ventricular tissues of infected mice using the Trizol reagent (Invitrogen, Carlsbad, CA). Briefly, each organ was homogenized in Trizol, followed by addition of 0.2 ml of chloroform and centrifugation at 12,000 × g for 15 min. RNA was isolated from supernatants using the SV total RNA isolation system kit (Promega, Fitchburg, WI). The purified RNA was eluted in 50 μl of RNase-free water and quantified in a spectrophotometer, BioMate 3 (Thermo, Waltham, MA), and its integrity was evaluated in 1.5% agarose gel.
Synthesis of cDNA and real-time PCR.
cDNA was synthesized using 2 μg of RNA by a reverse transcriptase reaction, using ImProm-II reagents (Promega, Fitchburg, WI) in a PTC 100 thermal cycler (MJ Research, Watertown, MA). The conditions used for the reaction were as follows: 5 min at 70°C and 1 h at 42°C, followed by refrigeration at 4°C. The total volume of the reaction was 25 μl, which was diluted by 8-fold, reaching a total volume of 200 μl. Real-time PCRs were performed using the Platinum SYBR green qPCR SuperMix uracil-DNA glycosylase (UDG) with ROX reference dyes (Invitrogen, Carlsbad, CA) with 5 μl of diluted cDNA. cDNA samples obtained from mice belonging to different groups (uninfected and infected at various time points) were amplified in the 7000 Sequence Detection Systems device (Applied Biosystems, Foster City, CA) using forward and reverse primers (sequences are listed in Table 1) that we designed with Primer Express software (Applied Biosystems, Foster city, CA), according to nucleotide sequences available in the GenBank database. Expression of each mRNA was normalized to a constitutive mRNA (β-actin) by the threshold cycle (ΔΔCT) method as previously described (27).
Table 1.
Sequences of primers used in real-time PCR
| Primer | Sequence |
|
|---|---|---|
| Sense | Antisense | |
| β-actin | 5′-AGC TGC GTT TTA CAC CCT TT-3′ | 5′-AAG CCA TGC CAA TGT TGT CT-3′ |
| PD-1 mouse | 5′-TTC AGG TTT ACC ACA AGC TGG-3′ | 5′-TGA CAA TAG GAA ACC GGG AA-3′ |
| PD-L1 mouse | 5′-GCT GAA GT CAA TGC CCC ATA-3′ | 5′-TCC ACG GAA ATT CTC TGG TTG-3′ |
| PD-L2 mouse | 5′-TTG TCG GTG TGA TTG GCT TC-3′ | 5′-AAA AGG CAG CAC ACA GTT GC-3′ |
Isolation of inflammatory cells from cardiac tissues and cytometry.
Hearts collected from 5 mice at day 20 p.i. were minced, pooled, and incubated for 1 h at 37°C with RPMI 1640, supplemented with NaHCO3, penicillin-streptomycin-gentamicin, and 0.05 g/ml of liberase blendzyme CI (Roche, Basel, Switzerland). The organs were processed in a Medimachine (BD Biosciences) in phosphate-buffered saline (PBS) containing 0.01% bovine serum albumin (BSA). After tissue digestion and washes, cell viability was assessed by trypan blue exclusion, counted in a hemocytometer, and stained with a 1:1,000 dilution of each fluorescent-labeled antibody. Phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated antibodies against CD3, CD4, CD8, PD-1, PD-L1, PD-L2, and the respective isotype controls were employed (BD Biosciences). For cytometry of cells obtained from the spleen, the organs were minced with scissors, and the suspension was filtered through 50 μm of nylon mesh. Fluorocytometric analysis was performed with a FACScan apparatus and CellQuest software (both obtained from BD Biosciences) as well as FlowJo software (Tree Star, Ashland, OR).
Lymphocyte proliferation assays.
Analysis of lymphocyte proliferation was performed by carboxyfluorescein diacetate succinimidyl ester (CFSE) staining. In brief, spleen-derived leukocytes (1 × 107 cells/ml) were stained with 5 μmol/liter CFSE (5 min, 37°C, and protected from light). Staining was stopped by addition of complete culture medium, and the cells were centrifuged (5 min, 300 × g). The cell suspension was adjusted to 5 × 106 cells/ml and plated in a 96-well culture plate (Nunc) at 200 μl cells/well, and then the cells treated with 1 μg of each antibody/well or left alone in medium for 72 h. Some wells were precoated with anti-CD3 (2.5 μg/ml). Data analysis was performed using a flow cytometer on a FACSCanto II apparatus (BD) using FACSDiva (BD) and FlowJo (Tree Star) softwares by setting a gate on the live cells to side-scatter versus forward-scatter dot plots and determining the expression of the CFSE.
Statistic analysis.
Data are expressed as means ± standard errors of the means (SEM). Analysis of variance (ANOVA) followed by Student's t test was used to determine the statistical significance of the observed differences between the treated and control groups. The Kaplan-Meier method was used to compare the survival times of the study groups. Differences were considered statistically significant at P values of <0.05. All analyses were performed using the PRISM 3.0 program (GraphPad Software, San Diego, CA).
RESULTS
Modulation of PD-1 expression in immune cells by T. cruzi infection.
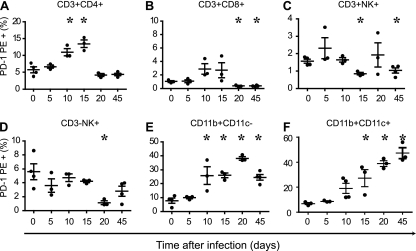
To evaluate if T. cruzi infection upregulates the expression of PD-1 in immune cells in vivo, flow cytometry was performed on spleen cells at several time points after infection, and the percentage of cells expressing PD-1 on the surface was quantified. The results showed that the infection leads to a gradual increase in the expression of PD-1 in spleen cells (Fig. 1). This was particularly seen within TCD4 cells and APC (CD11b+ CD11c+, CD11b+), where a curve was observed along the time course of the infection (Fig. 1A, E, and F). In the case of CD4 T cells, the expression of PD-1 fell to normal levels after day 20. Expression of PD-1 on CD8, NK, or NKT cells showed no correlation with the time of infection. These results demonstrated for the first time that T. cruzi is able to modulate the expression levels of the negative coreceptor PD-1, a molecule known to trigger a regulatory pathway that inhibits T cell activation.
Fig. 1.
Time course experiment showing expression of PD-1 in spleen cells from mice infected with T. cruzi. Spleen cells were obtained from mice at different time points after infection with T. cruzi, and the expression of PD-1 was determined in each cell subset by cytometry. (A to F) Gated on lymphocytes (A to D) and on monocytes (E, F). Data are representative values obtained from three independent experiments. Asterisks denote P values of <0.005 by ANOVA compared to uninfected values (0).
Increased levels of mRNA transcripts for PD-1 and its ligands in heart tissue from T. cruzi-infected mice.
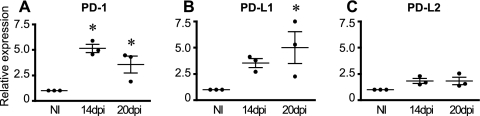
To assess the levels of mRNA for PD-1 and its ligands, cardiac tissues were collected at days 14 and 20 p.i. and compared to normal hearts. The results showed significantly increased levels of mRNA transcripts for PD-1 on both of the examined time points (by 5- and ∼3-fold, respectively) (Fig. 2A). Transcripts for PD-L1 were significantly increased on day 20 p.i. (Fig. 2B), while the PD-L2 transcripts remained unchanged (Fig. 2C). We further confirmed the presence of PD-1 and its ligands by immunofluorescence of the heart and spleen by comparing infected and normal tissues. The results revealed marked expression of PD-L1 (see Fig. S1E in the supplemental material) and low expression of PD-L2 (see Fig. S1F) in cardiac tissue obtained from infected mice at day 20 p.i., while no perceptible expression of PD-1 was detected by this methodology. Of note, no expression of the three molecules in normal hearts (see Fig. S1A to C in the supplemental material) was observed. Interestingly, reduced expression levels of PD-1 and its ligands in the spleen after infection were observed. From being expressed typically in the B-T cell region to agglomerating mostly within T cell zones (see Fig. S1G and J in the supplemental material), these data show that T. cruzi infection modulates the expression of PD-1 and its ligands in the heart and spleen.
Fig. 2.
Expression of PD-1 in the heart and spleen upon infection with T. cruzi. Heart tissues from naïve mice (NI) or infected mice (14 or 20 days postinfection [dpi]) were subjected to RNA extraction and real-time PCR for detection of PD-1 (A), PD-L1 (B), or PD-L2 (C) transcripts. The relative expression levels (to uninfected mice) for each transcript, corrected for β-actin, are shown. Data are representative values obtained from three independent experiments. Asterisks denote P values of <0.005 by one-way ANOVA compared to those of NI.
Heart-infiltrating CD4+ and CD8+ T cells express PD-1.
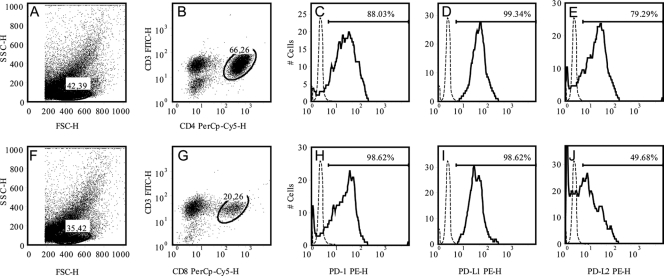
The expression of PD-1 was determined by flow cytometry in CD4 and CD8 T cells extracted from heart tissues on day 20 p.i. (Fig. 3). The results showed that 88.03% and 99.34% of CD4 T cells express PD-1 and PD-L1 (Fig. 3C and D). The frequencies of CD8 T cells expressing PD-1 and PD-L1 was 98.62% and 98.62%, respectively, while the frequencies of being PD-L2+ among CD8 and CD4 T cells was 79.29% and 49.68%, respectively. These results clearly show that PD-1 and its ligands are highly expressed in lymphocytes found in the hearts of T. cruzi-infected mice.
Fig. 3.
Expression of PD-1 in T cells infiltrating the myocardium during T. cruzi infection. Heart tissues from infected mice (20 dpi) were processed to isolate inflammatory cells, and the levels of expression of PD-1 and the ligands PD-L1 and PD-L2 in the gate of T CD4 (B to E) or T CD8 (G to J) cells were assessed by cytometry. (A and F) Representative dots of the ungated cells; (B and G) representative dots of each T cell subset studied. The bar in each histogram represents the expression level of each molecule according to the IgG control (which is shown by a dashed line). Data are representative values obtained from three independent experiments. SSC-H, side scatter; FSC-H, forward scatter.
PD-1 blockade results in increased acute myocarditis and reduced survival in mice infected with T. cruzi.
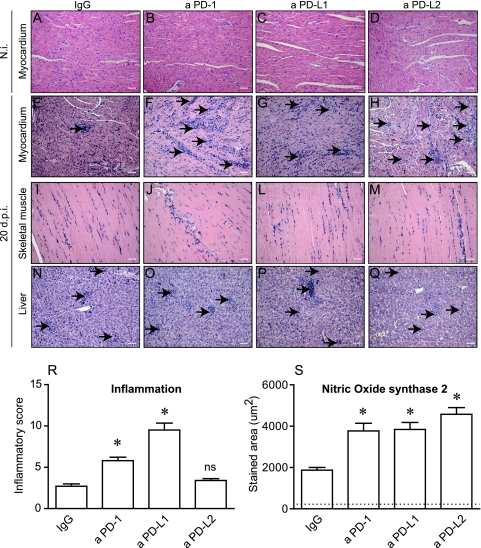
Aiming to test if the PD-1-dependent regulatory pathway is indeed involved in the maintenance of cardiac tissue tolerance during T. cruzi infection in vivo, we treated T. cruzi-infected mice with blocking antibodies against each one of the PD-1-related molecules (PD-1, PD-L1, and PD-L2) and studied the heart histopathology as well as the local production of cytokines and chemokines. The results showed that the blockade of PD-1 and PD-L1 (but not of PD-L2) led to increased myocarditis (Fig. 4 E and F). This was not observed in the skeletal muscle or in the hepatic tissue (Fig. 4I to Q). In addition, all treatments induced increased expression of NO synthase 2 (NOS2) in the cardiac tissue observed at the same time point (Fig. 4S). The treatment with anti-PD-1, anti-PD-L1 or anti-PD-L2 MAb did not induce any cell migration to the heart tissue of normal C57BL/6 mice (Fig. 4B to D). These results suggest a role for the PD-1 pathway in regulating the inflammatory response at the myocardium during T. cruzi infection. In fact, the analysis of inflammatory cells infiltrating the heart tissue of PD-1−/− and wild-type (WT) mice showed significantly increased numbers of macrophages and T cells in PD-1−/− mice (see Fig. S2G and C, respectively, in the supplemental material), and this was in association with increased expression of pStat5 (see Fig. S2M).
Fig. 4.
Effect of the PD-1 blockade in the regulation of T. cruzi-induced pathology. (A to Q) Heart, skeletal muscle, or liver from mice belonging to each of the following groups was processed for conventional H&E staining: mice treated with rat IgG, a PD-1, a PD-L1, or a PD-L2. (R) Additionally, the inflammatory index in serial slides obtained from normal (N.i.) or infected (20 dpi) cardiac tissues was assessed, and the result of the quantification is shown. (S) The extension of staining for NOS2 in cardiac tissues was also quantified. The dashed line represents the mean value obtained from uninfected, untreated controls. Asterisks denote P values of <0.005 by ANOVA. Data are representative values obtained from three independent experiments. ns, not significant.
To further explore the effects of the PD1/PDL1/PDL2 blockade treatments in the induction of increased myocarditis, we assayed the levels of the proinflammatory chemokines CCL5, CCL3, and CCL2 and the cytokines IFN-γ and TNF-α in heart homogenates obtained from mice belonging to each experimental group (see Fig. S3 in the supplemental material). Increased levels of all analyzed markers were found in hearts obtained from anti-PD-1-treated mice at day 14 p.i. CCL3 and CCL2 were reduced at day 20 p.i. in mice receiving anti-PD-L1 or anti-PD-L2. In addition, the expression of mRNA for CCR5, which is a receptor for these chemoattractants involved in the Th-1-biased immune response, was higher in the group that received anti-PD-L1 treatment (not shown).
PD-1-dependent regulation is involved in the mechanism that mediates host protection during the acute phase of T. cruzi infection.
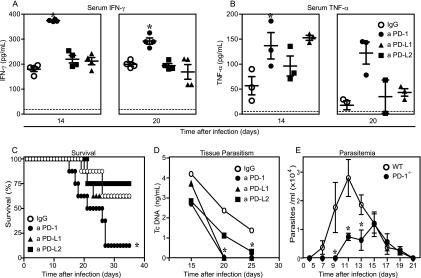
The increased myocardial inflammation observed in T. cruzi-infected mice after the blockade of PD-1 signaling molecules suggests that it could be also involved in the resistance to T. cruzi infection. In agreement with the increased levels of proinflammatory factors observed in cardiac tissues after the blockade of PD-1, the results showed that mice treated with anti-PD-1 present higher serum levels of IFN-γ and TNF-α than the control mice (Fig. 5 A and B) at day 14 p.i. Furthermore, this increased production of IFN-γ (but not of TNF-α) was maintained at day 20 p.i. These results point to a role for PD-1 in the control of the systemic inflammatory response during this infection. To determine the role of PD-1 in the control of parasite proliferation and resistance to the acute phase of the infection, we studied the parasitism and mortality of mice belonging to each experimental group. The results demonstrated that anti-PD-1-treated mice have a significant reduction in tissue parasitism (Fig. 5D), and the same results were obtained with PD-1−/− mice, which also exhibited reduced parasitemia (Fig. 5E) compared to that of the control group. However, the blockade of PD-1 also lead to a significantly decreased survival rate compared to that of the control group, as they started to die by day 15 p.i. By day 35 p.i., more than 90% of them had succumbed, while in the control group, the survival rate at this date was more than 55% (Fig. 5C). Additionally, PD-1−/− mice showed significantly reduced parasitemia compared to that of the WT mice (Fig. 5E). These results demonstrate that, in spite of mice becoming more resistant to infection, PD-1 blockade/deletion makes the host less tolerant to the inflammatory response.
Fig. 5.
PD-1 blockade/gene deletion leads to increased resistance to T. cruzi in vivo and increases the mortality rate. The effect of treatment with blocking antibodies against PD-1 or its ligands on the production of the cytokines IFN-γ (A) and TNF-α (B) in the sera of infected mice belonging to each group was assessed by ELISA at 14 and 20 dpi. (C) Survival in mice receiving each treatment was also assessed. The parasitism in cardiac tissue was assessed by real-time PCR (D), and parasitemia in C57BL/6 or PD-1−/− mice was studied (E). Data are representative values obtained from three independent experiments. Asterisks denote P values of <0.005 by ANOVA. Dashed lines represent the mean values obtained from uninfected, untreated mice.
PD-1 blockade induced the increased proliferative response by lymphocytes.
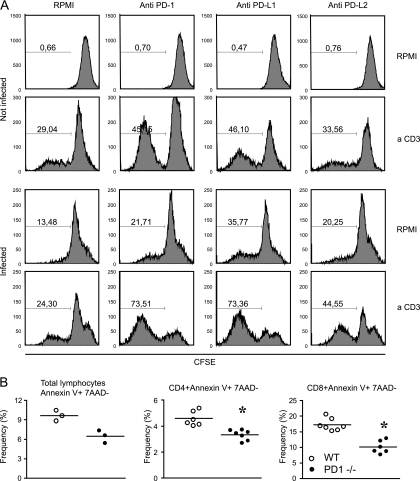
To gain further insight into the mechanisms underlying the increased inflammation after PD-1 blockade/gene deletion, CFSE-stained naïve or primed spleen cells were analyzed for proliferation after being incubated with anti-PD-1, anti-PD-L1, or anti-PD-L2. Our results showed that the blockade of PD-1 and PD-L1 led to the increased proliferation of spleen cells from uninfected or infected mice (Fig. 6 A). These data provide definitive evidence that the blockade of PD-1 and its ligand PD-L1 induces an increased proliferative response by lymphocytes.
Fig. 6.
The PD-1 and PD-L1 blockade efficiently restores proliferation (A), and the absence of PD-1 induces reduced levels of apoptosis in T cells obtained from T. cruzi-infected mice (B). (A) Spleen cells were obtained from naïve or infected mice (14 dpi), CFSE stained, and cultured in the presence of the described stimuli/treatment. After 72 h of culture, the levels of expression of CFSE were assessed in each case. The marker in each histogram represents the percentage of cells considered to be proliferating. (B) Spleen cells were collected from C57BL/6 (WT) mice or PD-1−/− mice (21 dpi), and the frequency of cells stained for annexin V that were negative for 7AAD staining was determined by cytometry within the indicated gates. Asterisks denote P values of <0.005 by Student's t test compared to those of WT mice. The lines represent the mean values obtained from uninfected WT mice. Data are representative values obtained from three independent experiments.
PD-1 deficiency induced diminished apoptosis of T cells during T. cruzi infection.
Aiming to study a possible mechanism behind the increased inflammatory response and lymphocyte proliferation observed in mice after PD-1 blockade, we finally studied the frequency of apoptosis of lymphocytes in WT or PD-1−/− mice. The results showed that T. cruzi-infected PD-1−/− mice exhibited significantly reduced numbers of apoptotic CD4+ and CD8+ T cells (Fig. 6B).
In summary, our data demonstrate that PD-1 blockade/deficiency lead to a reduction in the regulation of the immune response, which is related to increased lymphocyte proliferation and reduced apoptosis.
DISCUSSION
This study demonstrates for the first time that T. cruzi modulates the expression of PD-1, which has been widely involved in T cell exhaustion and persistent infections (5), features that have been associated with T. cruzi infection. The expression of PD-1 in association with parasite persistence has been reported for filariasis (4). In addition, increased expression of the ligands PD-L1 and PD-L2 has been reported to be induced by Taenia crassiceps in macrophages, which is associated with the inhibition of T cell proliferation by this parasite (36).
T. cruzi infection is characterized by acute parasitemia that is usually cleared out raising an intense cellular immune response against the parasite that usually causes extensive tissue damage, leading to fibrosis and dysfunction of the myocardium and other organs. However, tissue parasitism can persist, being responsible for continued tissue destruction. In this study, we show that T. cruzi infection upregulates the expression of PD-1 by CD8 and CD4 T cells migrating to the myocardium during the acute phase of this systemic infection. The high expression levels of PD-1 and its ligands reported in effectors T cells can be responsible for such pathogen persistence. In fact, the blockade of PD-1 and PD-L1 or deletion of the PD-1 gene ameliorated the control of parasite burden both systemically and in cardiac tissue.
Similar to our current results, we previously reported that T. cruzi induces upregulated expression of another coinhibitor molecule, CTLA-4, in lymphocytes in vivo and in vitro, and the blockade of this inhibitory signaling pathway lead to increased inflammation and decreased tissue parasitism (23).
T. cruzi appears to have evolved to manage the expression of costimulatory molecules as a strategy to persistently survive within the mammalian host. A recent study showed that the parasite is also able to exert immune evasion by downregulating CD28 ligands and major histocompatibility complex (MHC) molecules in dendritic cells (28). In addition, it has been proposed that differential expression levels of these costimulator molecules induced by the parasite can be associated with the intensity of the inflammatory response, leading to different clinical forms of the disease in humans (28, 34). Whether differential expression of PD-1 and CTLA-4 would be a marker of clinical forms of the disease in humans is currently under investigation in our laboratory.
The modulation of expression of PD-1, PD-L1, and to a lesser extent, PD-L2, by lymphocytes in response to T. cruzi infection in vivo became clear by the time course cytometry study. A wave of increasing expression of PD-1 in T cells (both CD4 and CD8) obtained from the spleen was detected on days 10 and 15 p.i. By day 20 p.i., however, the expression of PD-1 fell. This may correspond to a migration effect from the spleen to the heart and to other tissues. It can be confirmed by an indirect fluorescent-antibody assay (IFA) of the spleen, as shown in Fig. S1 in the supplemental material, in which the expression of these molecules is lower in infected mice than in uninfected mice. These data clearly show that T. cruzi infection lead to modulation in the normal patterns of expression of PD-1 and its ligands by lymphocytes and monocytes. In all cases, PD-L1 exhibited the most upregulated expression, which is known to participate in immune evasion in other microorganisms (6). Previous reports showed that PD-1 and its ligands are induced in immune cells late after activation, and PD-1 is now considered a marker of late activation and cell exhaustion during chronic infections and tumor immune evasion (6). The T cells present at the myocardium migrate in response to the parasite's presence and are known to exhibit a phenotype of activated cells, producing massive amounts of cytokines, predominantly those of the Th-1 immune response (13, 35). In addition, we demonstrated that nearly 100% of these cells express high levels of PD-1. Such high expression of PD-1 in T cells has already been described during viral infections (16). The apparent discrepancy in the detection of PD-1 by IFA and flow cytometry is due to differential sensibility among the two methods. In addition, the effect of concentration is also evident, as flow cytometry takes into account cells found in a whole heart, while IFA focuses only on a small area of the heart. Our findings suggest the participation of a novel regulatory mechanism in the control of the inflammatory response in cardiac tissues during this parasitic infection.
We believe that the balanced expression of positive and negative coreceptors is what determines the final outcome of infection in terms of the intensity and clearance of the parasite. The pathogenic role of collateral destruction of cardiac tissue during this inflammatory reaction is also undeniable. It is mediated by cellular and soluble components of the immune response, which is poorly regulated by classic immune regulatory mechanisms. For instance, regulatory cells do not play a strong role in the modulation of this inflammatory response (17, 20). Our data demonstrate that PD-1 is crucial to reduce the intensity of myocarditis.
It has been suggested that the effector lymphocytes recruited in response to the presence of the parasite in the myocardium may display altered tolerance mechanisms, leading to self-damage in an autoreactive fashion (10, 11, 19, 37) because activated T cells are not properly cleared out from circulation or because an altered peripheral tolerance is induced by the pathogen. Thus, it is possible that the inflammatory damage of cardiomyocytes cause an imbalanced expression of PD-L1, known to maintain the tolerance to cardiac troponin I (26), which is a protein exclusively expressed by cardiomyocytes. These hypotheses are currently under further investigation.
The role for PD-1 in maintaining immunological tolerance during acute myocarditis induced by T. cruzi was showed in this study by the treatment of mice with blocking antibodies against PD-1, PD-L1, or PD-L2. These treatments induced increased inflammation that was more remarkable in the cardiac tissues but not in other tissues where the presence of the parasites has been described during this infection. We also demonstrated that the blockade of PD-1 and PD-L1 was more effective in worsening myocarditis than the blockade of PD-L2. These data are in agreement with those from previous studies on the role of PD-1 in the immune response against intracellular pathogens and suggest that PD-L1 has a predominant regulatory role over PD-L2 during this parasitic infection. Although spontaneous cardiomyopathy has been described in BALB/c mice as a consequence of PD-1 deficiency, this autoimmune disorder has not been described in C57BL/c mice. We did not observe any myocarditis in uninfected C57BL/c PD-1−/− mice. In addition, we did not detect any cell migration to the heart tissue of normal C57BL/6 mice after treatment with anti-PD-1 MAb, indicating that PD-1 is one of the factors responsible for the deregulation of the immune response in the myocardium after T. cruzi infection.
The mechanism by which PD-1 regulates the immune response to T. cruzi appears to involve regulation of T cell proliferation and apoptosis. The induction of PD-1 expression in lymphocytes is known to be induced through the signaling by common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21. We showed that deletion of PD-1 leads to increased Stat-5 phosphorylation, which is crucial to T cell proliferation. In addition, the blockade of PD-1 and PD-L1 restored the proliferative capacity of lymphocytes obtained from infected mice. This result is very important since it explains one of the mechanisms that causes the inhibition of T cell proliferation that is well known to be induced after T. cruzi infection, which induces a large production of NO, which is responsible for induction of apoptosis (22).
It has been shown that the blockade/deletion of PD-1 leads to an improved immune response to intracellular pathogens (2), which correlates with our findings of reduced parasite load, an increased number of inflammatory infiltrates in the myocardium, and increased levels of the proinflammatory cytokines TNF-α and IFN-γ and chemokines CCL3, CCL5, and CCL2. These data support the regulatory role for PD-1 signaling, mainly through the PD-L1 ligand in the infected myocardium during the acute phase of T. cruzi infection.
In spite of favoring parasite persistence, PD-1 signaling is important for the survival of the infected host, as mice receiving the anti-PD-1 treatment die earlier than the control group mice or the mice receiving other treatments. We hypothesized that the increased mortality rate of these mice could be associated with the uncontrolled, intense myocardial inflammation observed as a consequence of the PD-1 blockade. This reduced tolerance to myocardial inflammation results from increased lymphocyte proliferation, proinflammatory cytokine production, and reduced apoptosis of T cells. The participation of regulatory T cells in the mechanisms involving PD-1 regulation during this infection was not studied and is an interesting issue for future reports.
In conclusion, our data demonstrated that PD-1 and PD-L1 participate in T. cruzi-induced myocarditis and that they regulate the inflammatory immune response.
Finally, recent studies have started to suggest that immune therapy involving soluble PD-1 could be beneficial under inflammatory conditions, as is the case with Chagas' heart disease. Soluble PD-1 does not appear to be produced under normal conditions. However, an alternative splicing variant of the PD-1 gene (PD-1Deltaex3), which leads to production of soluble PD-1, has recently been described (39). We did not perform any assays to test the presence of PD-1 or its ligands in mouse plasma. We hypothesize that a delicate manipulation of this signaling pathway on T cells of T. cruzi-infected hosts could become a potential strategy to design future therapeutic approaches for Chagas' heart disease.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful for the participation of and discussions with all former and present members of the working group on immunoparasitology at FMRP-USP, especially Beatriz R. Ferreira, Karen Cavassani, Ana P. Moreira, and Cristina Cardoso.
J.C.S.A. is supported by NIH grants (AI075038 and AI 078969). We were supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 2007/53940-0; scholarships to F.S.M. [04/05285-4] and F.R.S.G. [05/60762-5]), the Millennium Institute for Vaccine Development and Technology (grant 420067/2005-1), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; scholarship to J.S.S.). We have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Agata Y., et al. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8:765–772 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez I. B., et al. 2010. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J. Infect. Dis. 202:524–532 [DOI] [PubMed] [Google Scholar]
- 3. Ansari M. J., et al. 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babu S., Blauvelt C. P., Kumaraswami V., Nutman T. B. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176:3248–3256 [DOI] [PubMed] [Google Scholar]
- 5. Barber D. L., et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 6. Blank C., Mackensen A. 2007. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 56:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blazar B. R., et al. 2003. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 171:1272–1277 [DOI] [PubMed] [Google Scholar]
- 8. Carreno B. M., Collins M. 2002. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 20:29–53 [DOI] [PubMed] [Google Scholar]
- 9. Dong H., Zhu G., Tamada K., Chen L. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369 [DOI] [PubMed] [Google Scholar]
- 10. Engman D. M., Dragon E. A., Donelson J. E. 1990. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J. Immunol. 144:3987–3991 [PubMed] [Google Scholar]
- 11. Engman D. M., et al. 1989. A novel flagellar Ca2+-binding protein in trypanosomes. J. Biol. Chem. 264:18627–18631 [PubMed] [Google Scholar]
- 12. Golgher D., Gazzinelli R. T. 2004. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity 37:399–409 [DOI] [PubMed] [Google Scholar]
- 13. Gomes J. A., et al. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect. Immun. 71:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwai Y., et al. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 99:12293–12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwai Y., Terawaki S., Ikegawa M., Okazaki T., Honjo T. 2003. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 198:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasprowicz V., et al. 2008. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J. Virol. 82:3154–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kotner J., Tarleton R. 2007. Endogenous CD4(+) CD25(+) regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect. Immun. 75:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latchman Y., et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268 [DOI] [PubMed] [Google Scholar]
- 19. Levin M. J., et al. 1989. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' heart disease. Am. J. Trop. Med. Hyg. 41:530–538 [DOI] [PubMed] [Google Scholar]
- 20. Mariano F. S., et al. 2008. The involvement of CD4(+) CD25(+) T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 10:825–833 [DOI] [PubMed] [Google Scholar]
- 21. Marin-Neto J. A., Cunha-Neto E., Maciel B. C., Simoes M. V. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123 [DOI] [PubMed] [Google Scholar]
- 22. Martins G. A., Cardoso M. A., Aliberti J. C., Silva J. S. 1998. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immunol. Lett. 63:113–120 [DOI] [PubMed] [Google Scholar]
- 23. Martins G. A., Tadokoro C. E., Silva R. B., Silva J. S., Rizzo L. V. 2004. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J. Immunol. 172:4893–4901 [DOI] [PubMed] [Google Scholar]
- 24. Nishimura H., Honjo T. 2001. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 22:265–268 [DOI] [PubMed] [Google Scholar]
- 25. Nishimura H., et al. 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322 [DOI] [PubMed] [Google Scholar]
- 26. Okazaki T., et al. 2003. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9:1477–1483 [DOI] [PubMed] [Google Scholar]
- 27. Overbergh L., et al. 2003. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Tech. 14:33–43 [PMC free article] [PubMed] [Google Scholar]
- 28. Poncini C. V., Alba Soto C. D., Batalla E., Solana M. E., Gonzalez Cappa S. M. 2008. Trypanosoma cruzi induces regulatory dendritic cells in vitro. Infect. Immun. 76:2633–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roffe E., et al. 2007. Endothelin-1 receptors play a minor role in the protection against acute Trypanosoma cruzi infection in mice. Braz. J. Med. Biol. Res. 40:391–399 [DOI] [PubMed] [Google Scholar]
- 30. Rossi M. A. 1991. Patterns of myocardial fibrosis in idiopathic cardiomyopathies and chronic Chagasic cardiopathy. Can. J. Cardiol. 7:287–294 [PubMed] [Google Scholar]
- 31. Salama A. D., et al. 2003. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Savino W., et al. 2007. Cytokines and cell adhesion receptors in the regulation of immunity to Trypanosoma cruzi. Cytokine Growth Factor Rev. 18:107–124 [DOI] [PubMed] [Google Scholar]
- 33. Soares M. B., Pontes-De-Carvalho L., Ribeiro-Dos-Santos R. 2001. The pathogenesis of Chagas' disease: when autoimmune and parasite-specific immune responses meet. An. Acad. Bras. Cienc. 73:547–559 [DOI] [PubMed] [Google Scholar]
- 34. Souza P. E., et al. 2007. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infect. Immun. 75:1886–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teixeira M. M., Gazzinelli R. T., Silva J. S. 2002. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 18:262–265 [DOI] [PubMed] [Google Scholar]
- 36. Terrazas L. I., Montero D., Terrazas C. A., Reyes J. L., Rodriguez-Sosa M. 2005. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 35:1349–1358 [DOI] [PubMed] [Google Scholar]
- 37. Van Voorhis W. C., Eisen H. 1989. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J. Exp. Med. 169:641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vitelli-Avelar D. M., et al. 2005. Chagasic patients with indeterminate clinical form of the disease have high frequencies of circulating CD3+ CD16− CD56+ natural killer T cells and CD4+ CD25 high regulatory T lymphocytes. Scand. J. Immunol. 62:297–308 [DOI] [PubMed] [Google Scholar]
- 39. Wan B., et al. 2006. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 177:8844–8850 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.