Abstract
Introduction
The mandatory fortification of staple foods with folic acid to prevent neural tube defects (NTDs) began in the USA in 1998. Since then, more than 50 countries around the world have followed suit.
Methods
Selective literature review including national study results.
Results and discussion
Women of child-bearing age need sufficient body stores of folate before conception to prevent folate-sensitive NTDs, which make up 20% to 60% of all NTDs. Merely recommending folic acid supplementation before conception has been found to be an unsuitable strategy. Ingestion of folate-fortified food markedly increases folate intake, generally by about 50% of the recommended daily total intake. In Germany at present, debate surrounds the issue whether folate intake should be raised by mandatory folate supplementation, which will affect the entire population. Folate deficiency is associated with a higher risk of cancer and other diseases; on the other hand, there is concern that very high folic acid intake might promote the growth of pre-neoplastic lesions. There are no consistent study findings to support the latter hypothesis¸ and the evidence for it is derived from research in animals whose folate metabolism differs from that in humans. About 800 pregnancies with NTD are diagnosed each year in Germany; in most cases, the pregnancy is terminated after positive prenatal screening. The incidence of NTDs in Germany is estimated at 12.36 per 10 000 births (a mean figure derived from registry data in Mainz and Saxony-Anhalt) and is thus much higher than the mean incidence across Europe, 7.88 per 10 000 births (EUROCAT data for 2004–2008). Mandatory folic acid fortification should be adopted, as it is a highly effective and inexpensive way to prevent NTDs.
The incidence of neural tube defects (NTDs) ranges from 0.5/1000 to 14/1000 live births (e1, e2). The most common neural tube defects are spina bifida, where the spinal cord does not close completely, and anencephaly, where the cranial regions of the brain do not develop (e3). The neural tube closes about 28 days after conception. The most common maternal risk factors for giving birth to a baby with neural tube defects are folate deficiency, but also overweight and diabetes. Folate deficiency during pregnancy is a risk factor not only for NTDs but also for other birth defects (e4– e6).
Periconceptional folic acid supplementation lowers the incidence of neural tube defects significantly, by 20% to 60% (1, 2, e7– e8). A Cochrane review that included 4 studies with a total of 6425 women showed that periconceptional folic acid supplementation substantially reduces the incidence of neural tube defects (relative risk [RR] 0.28, 95% confidence interval 0.13 to 0.58) (2). A meta-analysis showed that folate supplementation can prevent recurrent neural tube defects in 85% to 100% of cases (e9, e10).
The present article focuses on folate supply relative to the risk of neural tube defects and additionally on the potential risks of folic acid fortification of foods for the general population. This review article is based on a selective literature search in PubMed (“folate fortification”, “NTD prevention”, “folate in Germany”, “folic acid in pregnancy”) and national reports.
Biological functions of folate
5-methyltetrahydrofolate (MTHF) is the physiologically active form of folate. When the methyl group from 5-MTHF is transferred to homocysteine, methionine is formed, from which the universal methyl group donor S-adenosyl-methionin (SAM) develops. 5,10-Methylen-THF is required for the synthesis of thymidine and 10-formyl-THF for the synthesis of purin.
Folate intake, recommendations
Natural folates are 30% to 80% less bioavailable than folic acid (e11, e12). The recommended daily intake (RDI) for folate is set at 400 µg of dietary folate equivalents (DFE). The upper tolerable limit for folic acid intake is set at 1 mg/d (e13). The usual fortification programs do not reach this upper limit nor do they exceed it, provided no additional supplements of >400 µg folic acid are taken (approximately 34% of Americans take additional preparations containing folic acid) (e14).
The RDI for folate is not population-based and therefore does not consider the special requirements of young women and elderly people. In postmenopausal women on a diet deficient in folates who then switched to a diet rich in folate it was found that a total amount of approximately 400 µg folate per day is required in order to normalize the blood parameters serum folate or homocysteine (e15, e16). The mean homocysteine concentration reaches a steady-state level of almost 11.0 µmol/L in people whose folate intake is 400 µg/d (e17) or in those with a serum folate concentration of 14 nmol/L (e18). Folic acid supplementation of approximately 200 µg/d (340 DFE, factor 1.7) lowered the plasma concentration of homocysteine significantly, according to a study from Ireland (the mean dietary intake of folate in Ireland is about 281 µg/d) (3). Increasing the dosage of folic acid to 400 µg /d did not have any additional homocysteine lowering effect, and 100 µg/d did not affect plasma homocysteine.
The average daily natural folate intake in the different European countries ranges from 230 µg to 280 µg (e19– e22). According to the German National Nutrition Survey II (Nationale Verzehrsstudie, NVS II), the median intake of folate equivalents in Germany for men is 283 µg/d and for women, 252 µg/d (4). In the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study, a minimum of 80% of the recommended daily folate intake was only achieved by means of supplementation in 50% of German children and adolescents (e23). Only children younger than 1 year had sufficient intake of folate (probably explained by fortified foods). In the European Prospective Investigation into Cancer and Nutrition (EPIC) Study, 50% of subjects had a DFE intake of <103 µg/d (e24). According to controlled dietary studies, folate intake (dietary folate and folic acid) correlates to metabolic markers in the blood (homocysteine) (e12). In people older than 60 years in Germany we measured lowered folate concentrations in about 25% of cases (5, e25). Compared with US subjects in the National Health and Nutrition Examination (NHANES) Study, who had a mean serum concentration of folate of 39 nmol/L, the mean folate concentration in elderly German subjects was extremely low, at 13.9 nmol/L (5). Data showed that 22% of pregnant women in Germany smoked during their pregnancy and only 17% took folic acid preparations up to the birth (6). Smoking mothers had significantly lower serum folate concentrations than non-smoking mothers (18.4 vs. 30.6 nmol/L), and the same was true for umbilical cord blood (median 57.1 vs 61.0 nmol/L) (6).
Folic acid supplementation before and during pregnancy
The US Centers for Disease Control and Prevention in 1992 called for an additional intake of 400 to 800 µg/d of folic acid for all women of childbearing age. In countries with folic acid fortification of staple foods, a notable fall in the incidence of neural tube defects was observed as early as one year after fortification had been introduced (e2, e26). 800 µg/d of folic acid are no more effective in preventing neural tube defects than 400 µg/d (1, e27). For women with a history of neural tube defect–affected pregnancy, a daily intake of 4 to 5 mg folic acid is recommended. However, the lowest effective dose for preventing neural tube defects is not known thus far.
Different international strategies to increase folate intake in young women have altogether shown very little success. Educational measures and prevention programs in the Netherlands led to sufficient preconceptional intake of folic acid in only 30% to 42% of young women after 10 years (e28, e29). In Germany periconceptional total folate intake (including the intake of folic acid–containing multivitamin drinks) has been reported to be sufficient in only 8.6% of women (7). The Bundesrat (the upper chamber of Germany’s parliament) recommended in 2006 to improve folate intake (information campaigns targeting women and the staff of healthcare institutions) (http://www.bundesrat.de). Thus far, these measures have not resulted in a fall in neural tube defects in Germany. In non-pregnant German women, mean dietary intake of food folate is 225 to 252 µg/d (8). According to a study in three European countries, mean daily folate intake in pregnant women is between 311 and 327 µg (9). The corresponding figures for folate intake in Germany are between 254 and 271 µg/d (9). Only 6% of study participants reached the recommended folate intake for pregnant women of 600 µg/d for Germany, Austria, and Switzerland, and only 26% had a total dietary folate intake of 400 µg/d. In Germany, only 36% of pregnant women were taking folic acid supplementation in their 20th week of gestation (9).
The EUROCAT registry (European Registration of Congenital Abnormalities and Twins) reports a significantly higher prevalence of neural tube defects for most European countries than for countries that have fortification programs (www.eurocat-network.eu). Reports of congenital defects in Germany for 2008 showed the highest incidence of neural tube defects since 1996 (16.84/10 000 vs 15.76/10 000 births in Saxony-Anhalt). EUROCAT’s mean rate is 7.88/10 000 births (between 2004 and 2008), in Germany, the rate is 12.36/10 000 births (mean value for the registries Mainz and Saxony-Anhalt). This translates as some 800 neural tube defects in Germany every year. Some 50% of these could be prevented with folic acid.
Folic acid supplementation should start at least one month before conception, since the neural tube closes in the first month after conception. Most pregnancies are unplanned and the mean timespan to the first antenatal visit is 9 weeks; this is too late to prevent neural tube defects. Further impairing factors include a lack of awareness—especially in socially disadvantaged women—, a lack of willingness to take folic acid supplementation, and cost. In 1998, the US and Canada therefore introduced mandatory folic acid fortification of grain products. The costs of folic acid fortification are low (in the US they amount to 1.5 to 3 dollars per ton of wheat flour) (www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a4.htm). In Chile, the annual costs of fortification are covered by the saved expenses of as few as two prevented cases of neural tube defects (e30).
In the meantime, more than 50 countries have started mandatory folic acid fortification of staple foods to prevent neural tube defects.
Folic acid and cancer risk
An increase in folic acid intake for the entire population is the subject of discussion, with several objections raised (10), such as:
An increased cancer risk (e31)
Masking cobalamin deficiency (folic acid may mask the signs of anemia in cobalamin deficiency, while neurodegeneration progresses (an aspect that is rather more theoretical than of practical relevance), or
Interactions with medications (antifolates; although this has not been proved so far, fortification with folic acid may necessitate a higher dosage of antifolate) (10).
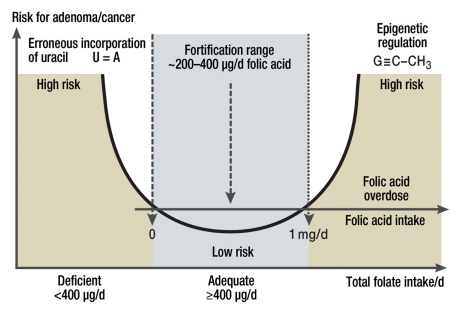
In animal studies, high dosages of folic acid prevented the development of new tumors, but supported the progression of pre-existing tumors (11). The latter role is consistent with the therapeutic use of antifolates. By contrast, no negative consequences have been reported for increased folate intake from natural sources. Furthermore, clinical studies have thus far not shown unequivocal results regarding a consistent relation between folic acid supplementation and a raised cancer risk, which militates against a causal role, especially in the concentration range below the upper tolerable limit of 1 mg/d of folic acid (Figure).
Figure.
The dual role of folic acid regarding the risk for adenoma and cancer. Folate deficiency is regarded as a risk factor for different tumors (incorporation of uracil rather than thymidine into the DNA). Excess supplementation with folic acid is also being discussed in relation to an increased risk for cancer (genetic dysregulation). No upper limit exists for natural folates
Folate deficiency as well as an excess of folic acid may impair DNA stability, gene expression, and cell proliferation. Folate deficiency can have genetic and epigenetic consequences and thus support tumor development (e32). Low folate concentrations in vitro or in vivo lead to erroneous incorporation of uracil into the DNA (instead of thymidine) (e33– e35), which is reversible by means of folate supplementation (12, e36). Compared with controls, the serum concentrations of folate were lower in patients with colorectal tumors, and DNA hypomethylation of the colonic mucosa was found (13). However, not all studies showed a negative association between folate supplementation and erroneous incorporation of uracil into the DNA (e37). A report from the World Cancer Research Fund and the American Institute for Cancer Research confirmed limited protection against pancreatic cancer for foods rich in folate (14). Compared with demographic (age) and environmental factors (such as smoking or alcohol), folic acid apparently does not play a proved part as a carcinogenic factor.
Colon cancer
After folic acid fortification was introduced in the US and Canada in 1998, a rise in colorectal cancer was observed for the following 3 years, which amounted to 4 to 6 cases per 100 000 persons (15). This does, however, not prove a causal role for folic acid, especially since the diagnostic possibilities for colorectal cancers were notably improved over this time period. Furthermore, the study did not investigate mortality due to colorectal tumors. It is therefore not clear whether the presumed role of folic acid regarding accelerated tumor growth is associated with increased mortality.
Case-control studies have shown that high serum concentrations of folate are associated with a 50% lower risk for colorectal tumors than low serum folate concentrations (median 31.0 vs 12.2 nmol/L) (16). In patients whose folate concentrations were low at the start of the study, folic acid reduced the risk for colorectal adenomas (e38). A prospective study showed that total mortality for colorectal tumors did not depend on serum folate concentrations measured before the tumor diagnosis (e39). On the other hand, low blood folate concentrations were associated with a higher risk for advanced adenomas and hyperplastic polyps (e40). According to a Swedish study, the relation between plasma folate and colorectal cancer risk is bell-shaped. Compared with the highest quintile (≥15 nmol/L), a folate concentration in the lowest quintile (<5 nmol/L) was protective against colorectal tumors if the observation period was longer than 4 years (e32).
According to a meta-analysis (5 cross-sectional studies and 7 case-control studies) the risk for colorectal tumors was reduced by some 25% if dietary folate intake was high (17). If 1 mg/d folic acid was taken for 6 to 8 years, however, a tendency towards an increased risk for advanced or multiple lesions was found (relative risk 1.67, 95% confidence interval 1.00 to 2.80; p = 0.05); the recurrence rate of colorectal adenomas was not increased (e41). A recently published meta-analysis (3 studies) of folic acid supplementation (0.5 to 5 mg/d for 3 years) did not find a significant influence on the risk of colorectal cancer in patients with a history of adenomas (e42). Similarly, three further studies (folic acid supplementation over 5 to 7 years of 2.5 mg/d in 2 studies and 20 mg/d in one study) in populations without an increased risk for colorectal cancer before the start of the study did not show a significant effect of folic acid on the relative risk of colon cancer (e42).
Breast cancer
In a 5-year follow-up study, the risk for breast cancer in women who ingested >1272 DFE per day (>748 µg total folate) was 22% lower than for <345 DFE (<203 µg total folate) (18). Another follow-up study over 9 years in >11 000 postmenopausal women also showed a protective effect of folate against breast cancer for a total daily intake of >456 µg compared with 160 µg/d (hazard ratio [HR] 0.56) (19). A third prospective study with >25 000 postmenopausal women showed that those women whose folate intake was >337 µg/d did not have a higher risk of breast cancer than women taking ≤233 µg/d, nor did women with supplemental folic acid intake <400 µg/d compared with women not taking folic acid supplementation (e43). In the same study, folic acid supplementation at ≥400 µg/d (equating to a total folate intake of >853 µg/d) was associated with a higher risk for breast cancer (HR 1.32). The currently available data do not show a consistent association between breast cancer risk and folate intake in the concentration range of folic acid supplementation.
Pancreatic cancer, ovarian cancer, and prostate cancer
In a 13-year follow-up study including >27 000 male smokers, those in the upper quintile (total folate intake >373 µg/d) had a lower risk for pancreas carcinoma compared with those in the lowest quintile (folate intake <280 µg/d) (20). A recent study including more than 100 000 participants (21) showed that a daily folate intake of ≥253 µg/d was protective, compared with ≤179 µg/d, in women, whereas in men, folic acid supplementation did not influence the risk of pancreatic cancer (12). Plasma folate and homocysteine were not associated with the risk of pancreatic cancer (22, e44). A Swedish prospective study over 8.6 years in >81 000 men and women found that a folate intake of >350 µg/d was associated with a lower risk of pancreatic cancer than and intake of ≤200 µg/d (23).
A higher folate intake was not associated with an increased risk for ovarian cancer or prostate cancer (e45). A prospective study over 22 years did not show a statistically significant relation between the risk for ovarian cancer and folate intake (24). In a 9-year follow-up study including 65 836 men (American Cancer Society Cancer Prevention Study II Nutrition Cohort), in which prostate cancer was diagnosed in 5158 men, no correlation was found between the risk for prostate cancer and dietary and total folate intake (e46).
Adenomas, solid tumors, role of folic acid
According to animal experiments, the dose as well as the timing of folic acid supplementation can accelerate the growth of adenomas and their transformation into carcinomas in animals with preneoplastic lesions (e47– e48). In the mouse model, folate deficiency induced intestinal tumors (e49). A recent meta-analysis did, however, not find a statistically significant influence of high folic acid dosages (0.5 to 20 mg/d over 3 to 7 years) on the recurrence or incidence of advanced adenomas in persons with a history of adenomas (e42).
Solid tumors have a relatively long latency period. The cumulative occurrence of tumors in the first 3 years after the start of folic acid fortification (15) is therefore more likely to be associated with other factors (for example, the introduction of colonoscopy screening) than with folic acid. A potentially harmful effect of non-metabolized free folic acid in the blood has thus far not been confirmed in clinical studies (e50– e53).
Folic acid is proposed to have a dual role with regard to the risk of cancer. In moderate intake it would be protective with regard to the development of neoplastic foci, whereas excess intake (far more than 1 mg/d) over a longer period of time can accelerate tumor growth (e54). Thus far no proof exists regarding a causal role for a raised cancer incidence nor cancer related mortality.
National strategies for folic acid fortification, concluding remarks
Before the implementation of folic acid fortification the benefit for the population should be evaluated by assessing all the items listed in the Box (25). The high incidence of neural tube defects in Germany could be lowered by 30% to 50% by means of mandatory folic acid fortification; furthermore, substantial cost savings could be made. Accelerated tumor progression is unlikely if the order of magnitude of fortification is that of current programs, because neither consistent results nor causal relations exist.
Box. Strategies for preventing neural tube defects by means of a strategy of folic acid fortification and data from Germany.
-
Representative data should be collected about the frequency of neural tube defects in the population; only two registries exist in Germany:
Mainz (active registry)
Saxony-Anhalt (passive registry)
The cost-benefit ratio for each country in question should be determined (e55, e6).
Data on the mean folate intake of the population should be collected; as well as data on serum folate concentrations in representative population samples (5, 6, e23, e24, e57).
Data on the folate status of young women (target group) should be collected by means of clinical markers (folate concentrations in blood and serum); homocysteine in plasma). The more common folate deficiency is in the target group, the higher the effect that may be expected from folic acid supplementation.
Decisions are needed about the mode of administration or the food that should be fortified, which the target group will consume (bread, milk).
The level of folic acid fortification should be decided for each country individually, on the basis of items 2–4 and the average eating behavior of the population, in order to achieve a total dosage of 400 µg/d of folate.
Deficiencies in other nutrients associated with folate should be estimated at the population level (cobalamin, choline) (e23).
Folic acid fortification should be accompanied by regulations that limit uncontrolled use of folic acid supplements, so as to avoid that folic acid intake in certain populations exceeds the upper tolerable limit.
The effect of fortification on the target group (young women) and other relevant population groups (elderly people, children) should be monitored over the long term.
10. Fortification can be introduced step by step, combined with the labeling of fortified products.
Key Messages.
Neural tube defects (NTDs) are common congenital abnormalities whose incidence can be lowered significantly by means of supplementation with a single micronutrient—folic acid—given before conception. Periconceptional folic acid supplementation lowers the incidence of NTDs significantly, by 20% to 60%.
Folate intake in Germany is low and folate deficiency is common; the median intake of folate equivalents in Germany is 283 µg/d for men and 252 µg/d for women. The recommended dosages are 400 µg/d and, for pregnant women, 600 µg/d.
Mere recommendations for folate supplementation in young women have not lowered the prevalence of NTDs in Germany, nor in other countries.
A national program to decrease NTDs in Germany by folic acid supplementation or targeted fortification of staple foods with low amounts of folic acid should urgently be started.
A causal relation between increased folic acid intake—in the range of the amounts used in most fortification programs—and the development or progression of tumors has not been proved and seems very unlikely.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of Interest Statement
Professor Herrmann has received honoraria for acting as an advisor from Axis-Shield-Diagnostics and Synervit GmbH. Furthermore he was reimbursed for delegate fees for conferences organized by Abbot Diagnostics and Axis-Shield. Professor Herrmann received research funding from Synervit GmbH. Professor Obeid received research funding from Merck and Synervit GmbH.
References
- 1.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 2.Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001056. CD001056. [DOI] [PubMed] [Google Scholar]
- 3.Ward M, McNulty H, McPartlin J, Strain JJ, Weir DG, Scott JM. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. QJM. 1997;90:519–524. doi: 10.1093/qjmed/90.8.519. [DOI] [PubMed] [Google Scholar]
- 4.Nationale Verzehrsstudie II. http://www.bmelv.de/SharedDocs/Downloads/Ernaehrung/NVS_ErgebnisberichtTeil2.pdf?__blob=publicationFile. Max Rubner-Institut, Bundesforschungsinstitut für Ernährung und Lebensmittel. 2008. pp. 121–122.
- 5.Obeid R, Schorr H, Eckert R, Herrmann W. Vitamin B12 status in the elderly as judged by available biochemical markers. Clin Chem. 2004;50:238–241. doi: 10.1373/clinchem.2003.021717. [DOI] [PubMed] [Google Scholar]
- 6.Obeid R, Munz W, Jager M, Schmidt W, Herrmann W. Biochemical indexes of the B vitamins in cord serum are predicted by maternal B vitamin status. Am J Clin Nutr. 2005;82:133–139. doi: 10.1093/ajcn.82.1.133. [DOI] [PubMed] [Google Scholar]
- 7.Genzel-Boroviczény O, Hachmeister A, von Kries R. Unverändertes Risiko für Neuralrohrdefekte. Mangelhafte Umsetzung der Empfehlungen zur Folsäureprophylaxe in der Frühschwangerschaft. Kinderärztliche Praxis. 1997;1:6–9. [Google Scholar]
- 8.Lamers Y, Prinz-Langenohl R, Bramswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006;84:156–161. doi: 10.1093/ajcn/84.1.156. [DOI] [PubMed] [Google Scholar]
- 9.Franke C, Verwied-Jorky S, Campoy C, et al. Dietary intake of natural sources of docosahexaenoic acid and folate in pregnant women of three European cohorts. Ann Nutr Metab. 2008;53:167–174. doi: 10.1159/000172978. [DOI] [PubMed] [Google Scholar]
- 10.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 11.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–1128. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 12.Basten GP, Duthie SJ, Pirie L, Vaughan N, Hill MH, Powers HJ. Sensitivity of markers of DNA stability and DNA repair activity to folate supplementation in healthy volunteers. Br J Cancer. 2006;94:1942–1947. doi: 10.1038/sj.bjc.6603197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pufulete M, Al-Ghnaniem R, Leather AJ, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124:1240–1248. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 14.Food, nutrition physical activity and the prevention of cancer. a global perspective. 2007:106–107. [Google Scholar]
- 15.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–1329. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 16.Kato I, Dnistrian AM, Schwartz M, et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer. 1999;79:1917–1921. doi: 10.1038/sj.bjc.6690305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113:825–828. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 18.Maruti SS, Ulrich CM, White E. Folate and one-carbon metabolism nutrients from supplements and diet in relation to breast cancer risk. Am J Clin Nutr. 2009;89:624–633. doi: 10.3945/ajcn.2008.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ericson U, Sonestedt E, Gullberg B, Olsson H, Wirfalt E. High folate intake is associated with lower breast cancer incidence in postmenopausal women in the Malmo Diet and Cancer cohort. Am J Clin Nutr. 2007;86:434–443. doi: 10.1093/ajcn/86.2.434. [DOI] [PubMed] [Google Scholar]
- 20.Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol. 2001;153:680–687. doi: 10.1093/aje/153.7.680. [DOI] [PubMed] [Google Scholar]
- 21.Oaks BM, Dodd KW, Meinhold CL, Jiao L, Church TR, Stolzenberg-Solomon RZ. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2010;91:449–455. doi: 10.3945/ajcn.2009.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keszei AP, Verhage BA, Heinen MM, Goldbohm RA, van den Brandt PA. Dietary folate and folate vitamers and the risk of pancreatic cancer in the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:1785–1791. doi: 10.1158/1055-9965.EPI-08-1220. [DOI] [PubMed] [Google Scholar]
- 23.Larsson SC, Giovannucci E, Wolk A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology. 2007;132:113–118. doi: 10.1053/j.gastro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Tworoger SS, Hecht JL, Giovannucci E, Hankinson SE. Intake of folate and related nutrients in relation to risk of epithelial ovarian cancer. Am J Epidemiol. 2006;163:1101–1111. doi: 10.1093/aje/kwj128. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence MA, Chai W, Kara R, Rosenberg IH, Scott J, Tedstone A. Examination of selected national policies towards mandatory folic acid fortification. Nutr Rev. 2009;67(Suppl 1):73–78. doi: 10.1111/j.1753-4887.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- e1.Heseker HB, Mason JB, Selhub J, Rosenberg IH, Jacques PF. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br J Nutr. 2008:1–8. doi: 10.1017/S0007114508149200. [DOI] [PubMed] [Google Scholar]
- e2.Busby A, Abramsky L, Dolk H, et al. Preventing neural tube defects in Europe: a missed opportunity. Reprod Toxicol. 2005;20:393–402. doi: 10.1016/j.reprotox.2005.03.009. [DOI] [PubMed] [Google Scholar]
- e3.Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ. 2009;338 doi: 10.1136/bmj.b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Beaudin AE, Stover PJ. Insights into metabolic mechanisms underlying folate-responsive neural tube defects: a minireview. Birth Defects Res A Clin Mol Teratol. 2009;85:274–284. doi: 10.1002/bdra.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–708. [PubMed] [Google Scholar]
- e7.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- e8.Smithells RW, Sheppard S, Schorah CJ, et al. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet. 1980;1:339–340. doi: 10.1016/s0140-6736(80)90886-7. [DOI] [PubMed] [Google Scholar]
- e9.Grosse SD, Collins JS. Folic acid supplementation and neural tube defect recurrence prevention. Birth Defects Res A Clin Mol Teratol. 2007;79:737–742. doi: 10.1002/bdra.20394. [DOI] [PubMed] [Google Scholar]
- e10.Smithells RW. Can vitamins prevent neural tube defects? Can Med Assoc J. 1984;131:273-274–276. [PMC free article] [PubMed] [Google Scholar]
- e11.Winkels RM, Brouwer IA, Siebelink E, Katan MB, Verhoef P. Bioavailability of food folates is 80% of that of folic acid. Am J Clin Nutr. 2007;85:465–473. doi: 10.1093/ajcn/85.2.465. [DOI] [PubMed] [Google Scholar]
- e12.Hannon-Fletcher MP, Armstrong NC, Scott JM, et al. Determining bioavailability of food folates in a controlled intervention study. Am J Clin Nutr. 2004;80:911–918. doi: 10.1093/ajcn/80.4.911. [DOI] [PubMed] [Google Scholar]
- e13.Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press; 2000. pp. 150–195. [PubMed] [Google Scholar]
- e14.Yang Q, Cogswell ME, Hamner HC, et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am J Clin Nutr. 2010;91:64–72. doi: 10.3945/ajcn.2009.28401. [DOI] [PubMed] [Google Scholar]
- e15.Kauwell GP, Lippert BL, Wilsky CE, et al. Folate status of elderly women following moderate folate depletion responds only to a higher folate intake. J Nutr. 2000;130:1584–1590. doi: 10.1093/jn/130.6.1584. [DOI] [PubMed] [Google Scholar]
- e16.Jacob RA, Gretz DM, Taylor PC, et al. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr. 1998;128:1204–1212. doi: 10.1093/jn/128.7.1204. [DOI] [PubMed] [Google Scholar]
- e17.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population [see comments] JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- e18.Lewis CA, Pancharuniti N, Sauberlich HE. Plasma folate adequacy as determined by homocysteine level. Ann NY Acad Sci. 1992;669:360–362. doi: 10.1111/j.1749-6632.1992.tb17123.x. [DOI] [PubMed] [Google Scholar]
- e19.Stahl A, Vohmann C, Richter A, Heseker H, Mensink GB. Changes in food and nutrient intake of 6- to 17-year-old Germans between the 1980s and 2006. Public Health Nutr. 2009;12:1912–1923. doi: 10.1017/S1368980009004844. [DOI] [PubMed] [Google Scholar]
- e20.Flynn A, Hirvonen T, Mensink GB, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. 2009 doi: 10.3402/fnr.v53i0.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Gonzalez-Gross M, Prinz-Langenohl R, Pietrzik K. Folate status in Germany 1997-2000. Int J Vitam Nutr Res. 2002;72:351–359. doi: 10.1024/0300-9831.72.6.351. [DOI] [PubMed] [Google Scholar]
- e22.Kersting M, Alexy U. Vitamin and mineral supplements for the use of children on the German market: products, nutrients, dosages. Ann Nutr Metab. 2000;44:125–128. doi: 10.1159/000012834. [DOI] [PubMed] [Google Scholar]
- e23.Sichert-Hellert W, Wenz G, Kersting M. Vitamin intakes from supplements and fortified food in German children and adolescents: results from the DONALD study. J Nutr. 2006;136:1329–1333. doi: 10.1093/jn/136.5.1329. [DOI] [PubMed] [Google Scholar]
- e24.Drogan D, Klipstein-Grobusch K, Dierkes J, Weikert C, Boeing H. Dietary intake of folate equivalents and risk of myocardial infarction in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam study. Public Health Nutr. 2006;9:465–471. doi: 10.1079/phn2005863. [DOI] [PubMed] [Google Scholar]
- e25.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Lopez-Camelo JS, Orioli IM, da Graca DM, et al. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. Am J Med Genet A. 2005;135:120–125. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- e27.Laurence KM, James N, Miller MH, Tennant GB, Campbell H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. BMJ (Clin Res Ed) 1981;282:1509–1511. doi: 10.1136/bmj.282.6275.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Weggemans RM, Schaafsma G, Kromhout D. Toward an optimal use of folic acid: an advisory report of the Health Council of the Netherlands. Eur J Clin Nutr. 2009;63:1034–1036. doi: 10.1038/ejcn.2009.2. [DOI] [PubMed] [Google Scholar]
- e29.de Walle HE, de Jong-van den Berg LT. Ten years after the Dutch public health campaign on folic acid: the continuing challenge. Eur J Clin Pharmacol. 2008;64:539–543. doi: 10.1007/s00228-007-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e30.Hertrampf E, Cortes F. National food-fortification program with folic acid in Chile. Food Nutr Bull. 2008;29:231–237. doi: 10.1177/15648265080292S128. [DOI] [PubMed] [Google Scholar]
- e31.Hirsch S, Sanchez H, Albala C, et al. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol. 2009;21:436–439. doi: 10.1097/MEG.0b013e328306ccdb. [DOI] [PubMed] [Google Scholar]
- e32.Van Guelpen B, Hultdin J, Johansson I, et al. Low folate levels may protect against colorectal cancer. Gut. 2006;55:1461–1466. doi: 10.1136/gut.2005.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e33.Crott JW, Mashiyama ST, Ames BN, Fenech M. The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol Biomarkers Prev. 2001;10:1089–1096. [PubMed] [Google Scholar]
- e34.Duthie SJ, Narayanan S, Blum S, Pirie L, Brand GM. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37:245–251. doi: 10.1207/S15327914NC372_18. [DOI] [PubMed] [Google Scholar]
- e35.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil mis-incorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998;12:1491–1497. [PubMed] [Google Scholar]
- e36.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome break-age: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e37.Hazra A, Selhub J, Chao WH, Ueland PM, Hunter DJ, Baron JA. Uracil misincorporation into DNA and folic acid supplementation. Am J Clin Nutr. 2010;91:160–165. doi: 10.3945/ajcn.2009.28527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e38.Wu K, Platz EA, Willett WC, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. 2009;90:1623–1631. doi: 10.3945/ajcn.2009.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e39.Wolpin BM, Wei EK, Ng K, et al. Prediagnostic plasma folate and the risk of death in patients with colorectal cancer. J Clin Oncol. 2008;26:3222–3228. doi: 10.1200/JCO.2008.16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e40.Ulvik A, Evensen ET, Lien EA, et al. Smoking, folate and methyl-enetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum. Am J Med Genet. 2001;101:246–254. doi: 10.1002/ajmg.1370. [DOI] [PubMed] [Google Scholar]
- e41.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- e42.Cooper K, Squires H, Carroll C, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010;14:1–206. doi: 10.3310/hta14320. [DOI] [PubMed] [Google Scholar]
- e43.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006;83:895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- e44.Schernhammer E, Wolpin B, Rifai N, et al. Plasma folate, vitamin B6, vitamin B12, and homocysteine and pancreatic cancer risk in four large cohorts. Cancer Res. 2007;67:5553–5560. doi: 10.1158/0008-5472.CAN-06-4463. [DOI] [PubMed] [Google Scholar]
- e45.Kotsopoulos J, Hecht JL, Marotti JD, Kelemen LE, Tworoger SS. Relationship between dietary and supplemental intake of folate, methionine, vitamin B6 and folate receptor alpha expression in ovarian tumors. Int J Cancer. 2010;126:2191–2198. doi: 10.1002/ijc.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e46.Stevens VL, Rodriguez C, Pavluck AL, McCullough ML, Thun MJ, Calle EE. Folate nutrition and prostate cancer incidence in a large cohort of US men. Am J Epidemiol. 2006;163:989–996. doi: 10.1093/aje/kwj126. [DOI] [PubMed] [Google Scholar]
- e47.Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–5440. [PubMed] [Google Scholar]
- e48.Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009;30:1536–1543. doi: 10.1093/carcin/bgp152. [DOI] [PubMed] [Google Scholar]
- e49.Knock E, Deng L, Wu Q, Leclerc D, Wang XL, Rozen R. Low dietary folate initiates intestinal tumors in mice, with altered expression of G2-M checkpoint regulators polo-like kinase 1 and cell division cycle 25c. Cancer Res. 2006;66:10349–10356. doi: 10.1158/0008-5472.CAN-06-2477. [DOI] [PubMed] [Google Scholar]
- e50.Kalmbach RD, Choumenkovitch SF, Troen AM, D’Agostino R, Jacques PF, Selhub J. Circulating folic acid in plasma: relation to folic acid fortification. Am J Clin Nutr. 2008;88:763–768. doi: 10.1093/ajcn/88.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e51.Sweeney MR, Staines A, Daly L, et al. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e52.Sweeney MR, McPartlin J, Weir DG, et al. Evidence of unmetabolised folic acid in cord blood of newborn and serum of 4-day-old infants. Br J Nutr. 2005;94:727–730. doi: 10.1079/bjn20051572. [DOI] [PubMed] [Google Scholar]
- e53.Obeid R, Kirsch SH, Kasoha M, Eckert R, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism. 2010 doi: 10.1016/j.metabol.2010.06.020. in press. [DOI] [PubMed] [Google Scholar]
- e54.Hubner RA, Houlston RS. Folate and colorectal cancer prevention. Br J Cancer. 2009;100:233–239. doi: 10.1038/sj.bjc.6604823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e55.Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence-prevention program. Am J Prev Med. 2008;35:572–577. doi: 10.1016/j.amepre.2008.07.008. [DOI] [PubMed] [Google Scholar]
- e56.Grosse SD, Waitzman NJ, Romano PS, Mulinare J. Reevaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am J Public Health. 2005;95:1917–1922. doi: 10.2105/AJPH.2004.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e57.Herrmann W, Quast S, Ullrich M, Schultze H, Bodis M, Geisel J. Hyperhomocysteinemia in high-aged subjects: relation of B-vitamins, folic acid, renal function and the methylenetetrahydrofolate reductase mutation. Atherosclerosis. 1999;144:91–101. doi: 10.1016/s0021-9150(99)00036-2. [DOI] [PubMed] [Google Scholar]