Abstract
Methicillin-resistant Staphylococcus aureus with a MIC of linezolid of 4 μg/ml, isolated from a patient who had undergone unsuccessful linezolid therapy, yielded linezolid-resistant mutants in blood agar at 48 h of incubation. The resistant clones showed a MIC of linezolid ranging from 8 to 64 μg/ml and accumulated the T2500A mutation(s) of the rRNA genes. Emergence of these resistant clones appears to be facilitated by a cryptic mutation or mutations associated with chloramphenicol resistance.
INTRODUCTION
Infection by methicillin-resistant Staphylococcus aureus (MRSA) is a serious problem in hospitals and communities because this bacterium shows resistance to major chemotherapeutic agents. To combat MRSA infections, powerful antibiotics, such as vancomycin and linezolid, have been developed. However, vancomycin-resistant MRSA has emerged worldwide. The recently developed antimicrobial agent linezolid is probably one of a few choices for the treatment of vancomycin-resistant MRSA. Most S. aureus isolates, including MRSA, are found to be linezolid susceptible, with a breakpoint of 4 μg/ml (3, 4). However, the emergence of linezolid-resistant MRSA with MICs of >4 μg/ml has been reported recently (1, 5, 6, 8, 11, 12). The resistant cells were found to have the G2576T (5, 11, 12) or T2500A (8) mutation in the gene(s) encoding the 23S rRNA. Moreover, some of the mutants accumulated the mutation in multiple copies of the rRNA genes, rendering the cells increasingly resistant to linezolid (2, 6, 9). Other types of linezolid resistance reported are enzymatic methylation of the 23S rRNA by chloramphenicol methyltransferase (5) and a mutation in the ribosomal protein genes encoding L3 and L4 (7, 13). We report here that linezolid-resistant cells, isolated from a patient for whom linezolid therapy was unsuccessful, emerged from the susceptible cell population.
(A part of this work was presented as poster A-037 at the 109th General Meeting, American Society for Microbiology, Philadelphia, PA, 2009.)
A patient with pyogenic spondylosis suffered from MRSA bacteremia and was treated orally with 600 mg of linezolid twice a day for 68 consecutive days. However, S. aureus cells were frequently isolated from the blood, resulting in the failure of linezolid chemotherapy. The MRSA isolates from the pre- and post-linezolid blood (referred to as HG503pre and HG503post, respectively) had linezolid MICs of 2 and 4 μg/ml, respectively, determined by the Clinical and Laboratory Standards Institute (CLSI) method (Table 1) (4). Therefore, both strains were classified as linezolid-susceptible MRSA. The relatedness of these strains was confirmed by comparing the pulsed-field gel electrophoretograms of their chromosomal DNA treated with SmaI (not shown).
Table 1.
Properties of the linezolid-resistant clones isolated from HG503post
| Strain | Copy no. of rRNA genea with T2500A mutation | MICb (μg/ml) |
Doubling timec (h) | |
|---|---|---|---|---|
| LZD | CHL | |||
| HG503pre | None | 2 | 8 | 0.96 |
| HG503post | None | 4 | 32 | 1.26 |
| HG503post-4R | 2 | 8 | 64 | 1.33 |
| HG503post-8R | 3 | 16 | 128 | 1.53 |
| HG503post-7D | 4 | 64 | 128 | 1.96 |
Both HG503pre and HG503post had five copies of the rRNA gene.
MICs of antibiotics were determined by the CLSI method. Abbreviations: LZD, linezolid; CHL, chloramphenicol. MICs of other antibiotics were presented in the supplemental material.
Doubling times were calculated from the growth curves in MH broth as shown in the supplemental material.
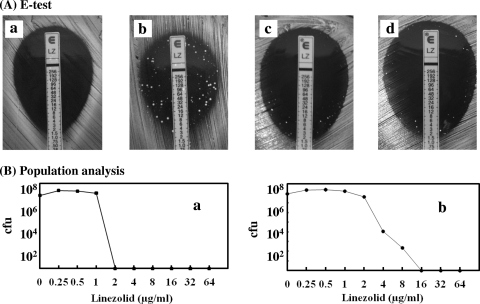
Since the MIC of linezolid in HG503post was close to the border of the breakpoint and that of chloramphenicol was 4-fold higher than that of HG503pre, we wondered whether or not this strain was genuinely linezolid susceptible. Thus, the linezolid susceptibility of HG503post was examined again using the Etest (AB Biodisk, Solna, Sweden). The MIC of linezolid in Mueller-Hinton (MH) agar was 0.19 μg/ml at 24 h of incubation, while those in MH agar supplemented with 5% sheep blood (MH-blood agar) appeared to be 1.5 and 4 μg/ml at 24 and 48 h, respectively. An important observation made was that many microcolonies appeared in the clear inhibitory zone in the MH-blood agar at 48 h (Fig. 1Ab). This result suggested that the linezolid-resistant mutants spontaneously emerged from the susceptible cell population, HG503post, while this phenomenon was not seen in HG503pre (Fig. 1Aa). To test whether or not the resistant cells appeared only in the presence of linezolid, HG503post cells were streaked on antibiotic-free MH-blood agar and 20 randomly selected microcolonies were subjected to the Etest. The result showed that all the subclones exhibited microcolonies in the clear inhibitory zone without exception, suggesting that they were segregated from a single susceptible cell (Fig. 1Ac and 1Ad).
Fig. 1.
Emergence of linezolid-resistant subclones by Etest and their population analysis. (A) Etest. Cells were grown in drug-free MH broth overnight, and a 100-μl aliquot was streaked on MH-blood agar with a linezolid-impregnated Etest strip. Plates were incubated at 35°C for 48 h. HG503pre (a), HG503post (b), and randomly selected HG503post cells from the drug-free agar medium (c and d) were subjected to Etest. Only two subclones are shown. (B) Population analysis. Cells were grown overnight in drug-free MH broth, and 100-μl aliquots were streaked on BHI agar impregnated with various concentrations of linezolid. The numbers of colonies on the plates were counted after incubation at 35°C for 48 h. (a) HG503pre. (b) HG503post.
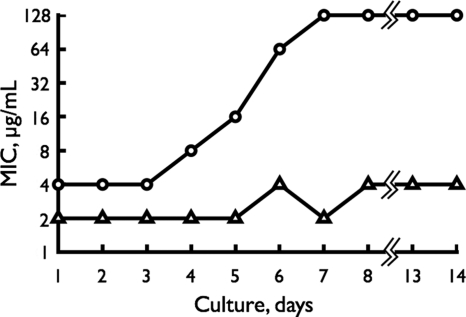
To test the homogeneity of the HG503 cells, we analyzed the population distribution of HG503pre and HG503post in terms of linezolid susceptibility. The cells grown in antibiotic-free MH broth were plated on brain heart infusion (BHI) agar impregnated with various concentrations of linezolid. The MIC for the HG503post population ranged from 1 to 16 μg/ml of linezolid (Fig. 1Bb), while that for HG503pre was restricted to within 1 to 2 μg/ml (Fig. 1Ba). These data suggest that the emergence of a resistant subpopulation in HG503post is due to a cryptic mutation or mutations. The colonies that appeared at the linezolid concentrations of 4 and 8 μg/ml in BHI agar were designated HG503post-4R and HG503post-8R, respectively. Clinically, it is important to know how early such resistant mutants emerge during linezolid treatment. An in vitro experiment was designed to simulate such a situation by exposing HG503post to medium containing linezolid concentrations one-fourth the MIC for 14 consecutive days (Fig. 2). The MIC of linezolid progressively increased to 8, 16, 64, and 128 μg/ml by the fourth, fifth, sixth, and seventh days, respectively, as determined by the broth dilution method. The strain on the seventh day was designated HG503post-7D. This experiment revealed that once the HG503post-like cell appeared, cells with high linezolid resistance emerged within a short time in the presence of a sub-MIC concentration of linezolid. A similar experiment with HG503pre revealed that the MIC of linezolid increased only 2-fold.
Fig. 2.
In vitro simulation of the appearance of linezolid-resistant subclones in the presence of the sub-MIC level of linezolid. The HG503post cells were grown in MH broth overnight and adjusted to an A587 of 0.3 (108 CFU/ml), and a 100-μl aliquot was inoculated into a fresh MH broth containing one-fourth the MIC of linezolid and incubated overnight. The MIC of linezolid in the culture was determined by the broth dilution method. Then, the cells grown in the culture were again exposed to one-fourth the MIC of linezolid as described above, and this procedure was repeated for 14 consecutive days. Symbols, ▵, HG503pre; ○, HG503post.
The MICs of linezolid in HG503post, HG503post-4R, HG503post-8R, and HG503post-7D appeared to be 4, 8, 16, and 64 μg/ml, respectively (Table 1) as determined by the CLSI method. MICs of other antibiotics were comparable among these strains, except for that of chloramphenicol (see the supplemental material). Cross-resistance between chloramphenicol and linezolid was reported earlier (10). Acquisition of resistance to chloramphenicol by the HG503post isolate is further evidence for a mutation that could facilitate the emergence of linezolid resistance.
Since the major factor associated with linezolid resistance was reported to be a chromosomal mutation in the gene(s) encoding the domain V region of the 23S rRNA, we analyzed this region by PCR (refer to the supplemental material). The following results were obtained (Table 1). (i) Both HG503pre and HG503post cells had a domain V sequence identical with that of the reference strain. (ii) The HG503post-4R, HG503post-8R, and HG503post-7D cells had the T2500A substitution in two, three, and four copies, respectively, of the rRNA genes. These results revealed that the MIC of linezolid was closely associated with the number of mutations in the 23S rRNA genes.
In summary, linezolid treatment failure was associated with a heterogeneously resistant isolate. Although this isolate appeared to be susceptible by standard susceptibility test methods, a linezolid-resistant subpopulation was detected by Etest after a 48-h incubation or with passage in a sub-MIC level of linezolid. Characterization of resistant clones showed that they had mutations in multiple copies of the 23S rRNA gene. Although differences in growth rates were observed for more resistant clones than for the parent posttreatment isolate (Table 1 and supplemental material), these differences were relatively small. Moreover, testing of individual colonies of the posttreatment isolate makes it unlikely that a mixed culture in the original posttreatment isolate accounted for the presence of the resistant subpopulation. A cryptic mutation (or mutations), outside domain V of rRNA and associated with a higher MIC of chloramphenicol, may account for this heterogeneous phenotype and facilitate the selection and emergence of higher-level resistant mutants with rRNA mutations.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Arias C. A., et al. 2008. Clinical and microbiological aspects of linezolid resistance mediated by the cfr gene encoding a 23S rRNA methyltransferase. J. Clin. Microbiol. 46:892–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Besier S., Ludwig A., Zander J., Brade V., Wichelhaus T. A. 2008. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob. Agents Chemother. 52:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozdogan B., Appelbaum P. C. 2004. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 23:113–119 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Hentschke M., et al. 2008. Emergence of linezolid resistance in a methicillin resistant Staphylococcus aureus strain. Infection 36:85–87 [DOI] [PubMed] [Google Scholar]
- 6. Ikeda-Dantsuji Y., et al. 2011. Linezolid-resistant Staphylococcus aureus isolated from 2006 through 2008 at six hospitals in Japan. J. Infect. Chemother. 17:45–51 [DOI] [PubMed] [Google Scholar]
- 7. Locke J. B., Hilgers M., Shaw K. J. 2009. Novel ribosomal mutations in Staphylococcus aureus identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob. Agents Chemother. 53:5265–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meka V. G., et al. 2004. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J. Infect. Dis. 190:311–317 [DOI] [PubMed] [Google Scholar]
- 9. Pillai S. K., et al. 2002. Linezolid resistance in Staphylococcus aureus: characterization and stability of resistant phenotype. J. Infect. Dis. 186:1603–1607 [DOI] [PubMed] [Google Scholar]
- 10. Toh S.-M., et al. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 64:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsiodras S., et al. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 3358:207–208 [DOI] [PubMed] [Google Scholar]
- 12. Wilson P., et al. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186–188 [DOI] [PubMed] [Google Scholar]
- 13. Wolter N., et al. 2005. Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob. Agents Chemother. 49:3554–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.