Abstract
We describe the pharmacokinetics (PKs) of caspofungin, an echinocandin antifungal, administered once daily as a 1-hour intravenous infusion in children and adolescents (ages, 3 months to 17 years), based on pooled data from four prospective pediatric studies. Caspofungin dosing was body-surface-area (BSA) based (50 mg/m2 daily after 70 mg/m2 on day 1). The area under the concentration-time curve from time zero to 24 h (AUC0–24), the concentration at the end of infusion (1 h after the start of infusion; C1), and the trough concentration (24 h after the start of infusion; C24) were obtained for 32 pediatric patients with invasive candidiasis, 10 with invasive aspergillosis, and 82 in the setting of empirical therapy with fever and neutropenia. Exposures were modestly higher (93 to 134% for C1, 45 to 78% for C24, ∼40% for AUC0–24) in pediatric patients than in adults receiving the standard 50-mg daily dose. The potential for covariates (age, gender, weight, race, renal status, serum albumin level, and disease state) to alter PKs was evaluated with a multiple-linear-regression model. Weight and disease state had statistically significant (P < 0.05) yet small effects on caspofungin PKs in pediatric patients. Concomitant use of dexamethasone (a cytochrome p450 inducer) was associated with a statistically significant reduction (44%) in C24 in a limited number of patients (n = 4). Odds ratios were estimated for the association between log-transformed PKs and treatment outcome or adverse events. No PK parameter or hybrid parameter (AUC/MIC, C1/MIC, and C24/MIC) was significantly correlated with treatment outcome or adverse events in the setting of similar response levels as adults, which suggests that the concentrations examined fall within the therapeutic window for caspofungin in pediatric patients. These results support a 50-mg/m2 daily dosing regimen (after a 70-mg/m2 loading dose) in children ages 3 months to 17 years.
INTRODUCTION
Caspofungin is an intravenous (i.v.) echinocandin antifungal agent that inhibits the synthesis of β-(1,3)-d-glucan, an essential component of the cell wall of many pathogenic fungi, including Candida and Aspergillus species. In vitro and in vivo studies have demonstrated that caspofungin has fungicidal activity against Candida spp. and potent activity against various species of Aspergillus (9). Caspofungin has recently been approved by the FDA and other health authorities for use in pediatric patients (3 months of age and older) for the treatment of esophageal candidiasis, invasive candidiasis, and invasive aspergillosis or as empirical therapy for presumed fungal infections in patients with persistent fever and neutropenia (13).
Candida and Aspergillus species are the fungi most frequently responsible for invasive infections in children. Mortality rates range from 19 to 31% in children with candidemia (17, 18, 32) and from 68 to 77% in those with invasive aspergillosis (10, 28). Even higher rates are observed in children with significant immune suppression, such as those receiving hematopoietic stem cell transplantation (HSCT) or bone marrow transplantation (BMT) (1, 5, 7, 22). Bloodstream infections with Candida are also occurring at increasing rates in children who do not suffer from childhood cancers but who have similar hospital exposures and risks, such as the presence of central venous catheters, broad-spectrum antibiotic use, receipt of total parenteral nutrition (TPN), and immunosuppressive therapy (31).
Results from phase I pediatric pharmacokinetic (PK) studies (16, 28) were used to determine the doses to be used in pediatric efficacy trials (11, 33) that were expected to provide exposures similar to those in adults. In this report, we present the results of a logistical population PK analysis to aid in confirming the dosing recommendations for caspofungin in pediatric patients, utilizing data from four prospective clinical studies (including previously unpublished PK data from the efficacy studies) conducted to support the approval of caspofungin for use in this population. The pharmacokinetics of caspofungin observed in pediatric patients were compared to those observed in adult patients, and the effects of underlying patient characteristics and concomitant medications were examined. In addition, the relationship between caspofungin PK parameters (as well as hybrid PK/microbiologic parameters) and both treatment outcome and the incidence of adverse events was explored.
MATERIALS AND METHODS
Study designs.
Data from 125 pediatric patients from four studies of caspofungin were pooled to assess the pharmacokinetics of caspofungin in the treatment of children and adolescents (ages, 3 months to 17 years) with new-onset fever and neutropenia (16, 29) or with documented Candida or Aspergillus infection (33) and in pediatric patients 2 to 17 years of age with persistent fever and neutropenia (11). Details of these studies appear in Table 1. Patients were monitored daily for adverse events during caspofungin therapy and for 14 days following the end of caspofungin therapy. Efficacy was defined separately for the different disease indications (Table 1).
Table 1.
Study design details
| Protocol (reference) | No. of patients in PK analysis | Description | Subjects | Treatment | PK sampling schemea | Primary efficacy evaluation |
|---|---|---|---|---|---|---|
| 033 (29) | 16 | Multicenter, open-label, sequential dose-escalation study | Children (ages, 2 to 11 yr) and adolescents (ages, 12 to 17 yr) with hematological or solid organ malignancies and fever and neutropenia at onset of empirical antibacterial therapy | Caspofungin monotherapy at 50-mg/m2 and 70-mg/m2 daily doses (maximum of 70 mg/day) administered as a 1-h i.v. infusion | A 7-point (predose, end-of-infusion [C1], 2, 4, 8, 12, and 24 h after start of infusion) plasma profile was collected on days 1, 4, and 9; trough concentrations (C24) were also collected on days 3, 7, 12, 14, 21, and 28 | Not applicable |
| 042 (16) | 9 | Multicenter, open-label study | Young children (ages, 3 to 24 mo) with hematological or solid organ malignancies and fever and neutropenia at onset of empirical antibacterial therapy | Caspofungin monotherapy at 50-mg/m2 daily dose (maximum, 70 mg/day) administered as a 1-h i.v. infusion | A 7-point (predose, end-of-infusion [C1], 2, 4, 8, 12, and 24 h after start of infusion) plasma profile was collected on days 1 and 4; trough concentrations (C24) were also collected on day 3 (and days 7, 14, and 28, when applicable) | Not applicable |
| 043 (33) | 43 | Multicenter, open-label, noncomparative study | Children or adolescents (ages, 3 mo to 17 yr) with documented Candida or Aspergillus infection | Caspofungin as a 1-h i.v. infusion at 50 mg/m2 daily following a 70-mg/m2 loading dose on day 1 (maximum, 70 mg/day) | Pretreatment, end-of-infusion (C1), and trough (C24) concentrations were collected on days 4, 7, and 14; in addition, a 5-point plasma profile was collected for a subset of patients on day 4 | Proportion of patients with a favorable response, which included assessment of clinical response and, where applicable, microbiologic, radiographic, or endoscopic response; primary efficacy time point was at end of caspofungin therapy |
| 044 (11) | 57 | Multicenter, double-blind, randomized, comparative study | Pediatric patients (ages, 2 to 17 yr) with suspected fungal infection in setting of persistent fever and neutropenia | Caspofungin as a 1-h i.v. infusion at 50 mg/m2 daily following a 70-mg/m2 loading dose on day 1 (maximum, 70 mg/day) or liposomal amphotericin B given at 3 mg/kg of body wt/day | Pretreatment, end-of-infusion (C1), and trough (C24) concentrations were collected on days 4, 7, and 14; in addition, a 5-point plasma profile was collected for a subset of patients on day 4 | A favorable response was defined as meeting all of the following endpoints: (i) successful treatment of baseline IFI,b if any, (ii) absence of breakthrough IFI up to 7 days posttherapy, (iii) survival to 7 days posttherapy, (iv) no discontinuation due to lack of efficacy or study drug toxicity, and (v) resolution of fever for 48 h during period of neutropenia |
PK sampling times are given in relation to the start of infusion.
IFI, invasive fungal infection.
Bioanalytical methods.
Plasma samples were analyzed for caspofungin and internal standard (an isostere) by using solid-phase extraction and high-performance liquid chromatography as previously described (4, 20). The calibration curve was over the concentration range of 125 to 10,000 ng/ml. The intraday assay precision was better than 5.7% coefficient of variation, and intraday accuracy was within 1.7% of nominal at all points of the standard curve. Detection was by fluorescence using an excitation wavelength of 220 nm and an emission wavelength of 304 nm. The lower limit of quantitation was 125 ng/ml.
Pharmacokinetic analyses.
The parameters used in the population PK analyses are the concentration at the end of infusion (1 h after the start of infusion; C1), the trough concentration (24 h after the start of infusion; C24), and the area under the concentration-time curve from time zero to 24 h (AUC0–24) time averaged over days 3 to 14 or over day 3 and later. Sampling time criteria were developed to define acceptable deviations from nominal time for C1 and C24 sampling. For C1, a sampling window from 20 min before to 30 min after the end of infusion was used, since concentrations obtained during that time period were expected to be within ∼30% of the true end-of-infusion concentration. Similarly, for C24, a sampling window from 3 h before to 3 h after the 24-hour postdose time point was used, since concentrations obtained during that time period were expected to be within ∼20% of the true C24. Concentration values for C1 and C24 obtained outside these windows were excluded and treated as missing. AUC0–24 was used in the analysis only if AUC data were available for 10 or more patients in that analysis. The day 1 PK parameters were excluded from the analysis, as no day 1 PK data were available from protocols 043 and 044 (11, 33).
Time-averaged values over days 3 to 14 were used in the covariate analysis and the comparison to adult data, since it was expected that patient characteristics obtained at the baseline would remain reasonably stable over a 2-week period and the PKs would be reasonably stable after day 3 of multiple daily dosing. In the treatment outcome and adverse event analyses, time-averaged parameters over day 3 and later were used in order to reflect the average exposure over the entire course of caspofungin therapy, since treatment outcome was predominantly assessed at the end of caspofungin therapy and adverse events were assessed throughout caspofungin therapy. Time-averaged PKs (day 3 and later) were calculated by taking the geometric mean of all values obtained during the specified interval, regardless of up- or downdosing. The time-averaged day 3 to 14 and day 3 and later parameters were equal in many cases due to the limited number of patients with data available beyond day 14.
In the treatment outcome analysis, three hybrid parameters were also used for patients with invasive candidiasis: the ratio of AUC to the MIC of caspofungin for the associated Candida isolate (AUC/MIC), the ratio of trough concentration to the caspofungin MIC (C24/MIC), and the ratio of peak concentration to the caspofungin MIC (C1/MIC). These parameters incorporated the MIC data for caspofungin determined from in vitro susceptibility testing (prominent inhibition in RPMI medium) of unique baseline Candida isolates obtained from each patient; guidelines of the Clinical and Laboratory Standards Institute (CLSI; M27-A3 for the yeasts and M38-A2 for the filamentous fungi) were followed. These parameters were determined only for patients with infections due to a single fungal (Candida) species and, thus, a single MIC measure. Patients with infections due to multiple Candida species were excluded from this analysis.
Statistical analysis.
All pediatric patients included in the analyses received caspofungin at 50 mg/m2 (with or without a 70-mg/m2 loading dose on day 1). All PK parameters were natural log transformed prior to analysis, and all tests were two-sided and assumed a significance level (α) of 0.05. No multiplicity adjustments were made, given the exploratory nonprospective nature of the analyses. Analyses were performed using SAS software (version 9.1.3).
Comparison of pharmacokinetics in pediatric patients and adult subjects.
Each of the time-averaged parameters (AUC0–24, C1, and C24) in pediatric patients were compared with those in adult patients with fungal infections (invasive candidiasis, esophageal candidiasis, or invasive aspergillosis) (2, 12, 15, 24, 26, 27) and adult patients treated empirically for suspected fungal infections in the setting of persistent fever and neutropenia (30). The log-transformed values of the PK parameters for each pediatric age group (young children [ages, 3 to 24 months], older children [ages, 2 to 11 years], and adolescents [ages, 12 to 17 years]) were compared to the log-transformed values of the parameters for the pooled adult control group on the basis of the two-sample t test. Geometric mean ratios were provided for each comparison.
Analysis of covariates as a predictor of patient pharmacokinetics.
A simple linear regression was first run on each covariate (e.g., gender, age, and creatinine clearance at the baseline) to identify a set of significant covariates. Stepwise (backward) regression was then used to construct the final multiple-regression model. Appropriate regression diagnostics were assessed. The final determination of significance of covariates for PKs was based on the final multiple-regression model.
The covariate analysis included a drug interaction screening analysis to assess the potential for concomitant medications to alter caspofungin PK parameters. It was decided a priori that a concomitant medication would be analyzed only if 10 or more patients with PK data available received the medication for 90 to 100% of the time while on caspofungin therapy up to day 14. In addition, comparisons of the data in patients receiving concomitant tacrolimus, sirolimus, dexamethasone, or a pooled category of cytochrome p450 (CYP) inducers were also evaluated, even though PK data were available for less than 10 patients in those groups. The explanatory variable for the analysis of each concomitant medication for patients with 0% coverage was compared with that for patients with 90 to 100% coverage; all other patients (i.e., those using the concomitant medication intermittently) were excluded in order to obtain the most meaningful comparison. Geometric mean ratios (95% confidence intervals [CIs]) comparing 90 to 100% coverage versus no coverage were obtained for each PK parameter.
Analysis of patient PKs as a predictor of treatment outcome, microbiologic outcome, and adverse event.
Treatment outcome was defined as a favorable or unfavorable response in the evaluable patient (per protocol) population. Treatment outcome was based on the same efficacy criteria used in the original study for the respective indication, as previously described (11, 33). For pediatric patients with invasive candidiasis, the microbiologic response at the end of caspofungin therapy was also analyzed. To examine the effects of PK parameters on treatment outcome, a logistic regression model was constructed for each time-averaged PK parameter. An odds ratio (95% CI) measuring the association between the log-transformed PK parameter and treatment outcome was estimated from each model. In addition, a subgroup analysis was conducted using logistic regression for pediatric patients who were empirically treated for suspected fungal infections in the setting of persistent fever and neutropenia (from protocol 044) on the basis of predefined risk category (high versus low risk), as previously described (11).
A two-stage model-building procedure was used to analyze the effect of caspofungin PK parameters on clinically important adverse events or laboratory abnormalities and account for the differences in the effects by disease indication. The dependent variable was binary, indicating the presence or absence of a particular adverse experience or lab abnormality. Clinical adverse events that occurred during the course of caspofungin therapy or the 14-day posttherapy follow-up period were included in the analysis if the investigator rated the adverse events as possibly, probably, or definitely drug related. Laboratory abnormalities that occurred during caspofungin therapy were included if they met certain predefined criteria for a clinically significant laboratory abnormality. An odds ratio (95% CI) was estimated from each logistic regression model.
RESULTS
Comparison of pharmacokinetics in pediatric patients and adult subjects.
The log-transformed values of the caspofungin PK parameters (C1 and C24) for each pediatric age group (young children [ages, 3 to 24 months], older children [ages, 2 to 11 years], and adolescents [ages, 12 to 17 years]) were compared to the same log-transformed values of the parameters for adult patients (Table 2). In all comparisons, the time-averaged day 3 to 14 C1 for caspofungin was moderately increased (93% to 134%) in all pediatric age groups relative to that in all adult groups. In addition, the time-averaged day 3 to 14 C24 for caspofungin was moderately increased (45% to 78%) in older children and in adolescents but not in young children relative to that in all adult groups. In pediatric patients, the clearance of caspofungin (normalized by body surface area [BSA]) was about 6 ml/min/m2 (Table 3), was generally consistent across all age groups, and was similar to clearance values reported for adults receiving caspofungin at 50 mg daily (16).
Table 2.
Comparison of time-averaged pharmacokinetic parameters (days 3 to 14) between all pediatric patients who received 50 mg/m2/day and adult patients who received 50 mg/day
| Parametera | No. of pediatric patients/no. of adult patients | Geometric mean (95% CI) |
GMRb (95% CI) | P value | |
|---|---|---|---|---|---|
| Pediatric patients | Adult patients | ||||
| AUC0–24 | |||||
| Overall | 67/38 | 144.37 (133.70, 155.89) | 103.38 (93.36, 114.47) | 1.40 (1.23, 1.59) | <0.001 |
| Young children | 10/38 | 142.60 (116.68, 174.29) | 103.38 (93.26, 114.58) | 1.38 (1.10, 1.73) | 0.006 |
| Older children | 35/38 | 145.99 (131.14, 162.52) | 103.38 (93.26, 114.58) | 1.41 (1.22, 1.64) | <0.001 |
| Adolescents | 22/38 | 142.64 (124.59, 163.30) | 103.38 (93.26, 114.58) | 1.38 (1.16, 1.64) | <0.001 |
| C1 | |||||
| Overall | 94/393 | 16.63 (15.38, 17.98) | 7.85 (7.56, 8.16) | 2.12 (1.94, 2.31) | <0.001 |
| Young children | 10/393 | 18.39 (14.48, 23.36) | 7.85 (7.56, 8.15) | 2.34 (1.84, 2.99) | <0.001 |
| Older children | 55/393 | 17.17 (15.51, 19.02) | 7.85 (7.56, 8.15) | 2.19 (1.96, 2.44) | <0.001 |
| Adolescents | 29/393 | 15.12 (13.13, 17.40) | 7.85 (7.56, 8.15) | 1.93 (1.66, 2.23) | <0.001 |
| C24 | |||||
| Overall | 97/420 | 2.50 (2.27, 2.76) | 1.66 (1.59, 1.74) | 1.51 (1.35, 1.68) | <0.001 |
| Young children | 10/420 | 1.90 (1.41, 2.56) | 1.66 (1.59, 1.74) | 1.14 (0.84, 1.55) | 0.388 |
| Older children | 57/420 | 2.41 (2.12, 2.73) | 1.66 (1.59, 1.74) | 1.45 (1.27, 1.65) | <0.001 |
| Adolescents | 30/420 | 2.96 (2.49, 3.52) | 1.66 (1.59, 1.74) | 1.78 (1.49, 2.13) | <0.001 |
Overall, values averaged across all age groups (age as a categorical variable); young children, ages 3 to 24 months; older children, ages 2 to 11 years; adolescents, ages 12 to 17 years.
GMR, geometric mean ratio (pediatric patients/adult patients).
Table 3.
Summary of time-averaged (day 3 to 14) clearance of caspofungin normalized by BSA in pediatric patients who received 50 mg/m2/day
| Pediatric age group | No. of patients | Clearance (ml/min/m2)a |
|---|---|---|
| Young children | 10 | 5.84 (1.36) |
| Older children | 37 | 5.67 (2.09) |
| Adolescents | 25 | 5.74 (2.03) |
Values are geometric means (standard deviations).
Covariates as predictors of caspofungin pharmacokinetics.
Among all covariates analyzed (age, gender, disease state, race, weight, creatinine clearance, and serum albumin level), only disease state and weight were found to be statistically significant determinants of caspofungin PKs (see Table S1 in the supplemental material). In particular, disease state was a significant covariate for caspofungin AUC0–24 and C24 but not for C1. On average, modest decreases (25% for AUC0–24 and 35.9% for C24) were observed in pediatric patients with new-onset fever and neutropenia relative to that observed in pediatric patients empirically treated for suspected fungal infections in the setting of persistent fever and neutropenia. The decrease in trough concentrations and AUC0–24 in pediatric patients with new-onset fever and neutropenia was consistent across age groups (older children [ages, 2 to 11 years] and adolescents [ages, 12 to 17 years]). Weight was found to be a significant determinant of C1 but not AUC0–24 or C24. The current analysis predicts that the ratio of the average C1 in a pediatric patient weighing 10 kg relative to that in a pediatric patient weighing 20 kg would be 1.07 (95% CI, 1.03, 1.12). Similarly, the ratio of the average C1 in a pediatric patient weighing 30 kg relative to that in a pediatric patient weighing 20 kg would be 0.93 (95% CI, 0.89, 0.97). Overall, a 10-kg reduction in weight was, on average, associated with a 7% increase in C1. In addition, PK variability appeared to be greater in lighter pediatric patients than heavier pediatric patients.
Caspofungin PK parameters were also evaluated in the presence or absence of 12 different concomitant medications on the basis of prespecified criteria (i.e., 10 or more patients received the concomitant medication for 90% of the time or greater during caspofungin therapy up to day 14). Concomitant use of acyclovir was associated with a 17% decrease in the geometric mean C1 for caspofungin, and concomitant use of vancomycin was associated with a 28% decrease in the geometric mean C24 for caspofungin (see Table S2 in the supplemental material). No concomitant medication was associated with a significant change in caspofungin AUC0–24 or with changes in both C1 and C24. In addition, the potential for drug interactions with tacrolimus, sirolimus, dexamethasone, and a pooled set of CYP inducers was also examined, as these interactions have been noted in adults. The concurrent use of dexamethasone was associated with a statistically significant decrease in the caspofungin geometric mean C24 (44% decrease, P = 0.033), but there was no reduction in caspofungin AUC0–24 or C1 during concomitant dexamethasone use (see Table 5). Prior therapy with dexamethasone was not associated with a reduction in any of the caspofungin PK parameters. None of the other additional medications were associated with a statistically significant change in caspofungin AUC0–24, C1, or C24.
Table 5.
Potential for caspofungin pharmacokinetic parameters to predict occurrence of selected clinical adverse events and/or laboratory abnormalities
| Caspofungin PK parametera | Type of clinical adverse event and/or laboratory abnormality | No. of patients |
Odds ratio (95% CI)b,c | P valueb | |
|---|---|---|---|---|---|
| With event | Total | ||||
| AUC0–24 (μg · h/ml) | ALT > 2.5 times ULNd | 5 | 66 | 0.08 (0.00, 9.30) | 0.294 |
| ALT > 5 times ULN | 5 | 66 | 0.26 (0.01, 6.06) | 0.399 | |
| ALT > 2.5 times BSLe | 9 | 66 | 1.03 (0.04, 27.20) | 0.987 | |
| AST > 2.5 times ULN | 6 | 66 | 2.33 (0.07, 80.62) | 0.640 | |
| AST > 2.5 times BSL | 11 | 66 | 0.56 (0.04, 8.85) | 0.678 | |
| Potassium < 2.5 mEq | 3 | 68 | 1.60 (0.03, 94.45) | 0.820 | |
| Feverf | 6 | 69 | 5.94 (0.07, 487.81) | 0.428 | |
| Headachef | 2 | 69 | 1.67 (0.00, >999) | 0.891 | |
| C1 (μg/ml) | ALT > 2.5 times ULN | 9 | 97 | 0.50 (0.07, 3.76) | 0.501 |
| ALT > 5 times ULN | 7 | 97 | 1.86 (0.17, 20.23) | 0.609 | |
| ALT > 2.5 times BSL | 20 | 97 | 1.63 (0.35, 7.67) | 0.536 | |
| AST > 2.5 times ULN | 10 | 97 | 0.71 (0.10, 5.26) | 0.736 | |
| AST > 2.5 times BSL | 17 | 97 | 0.88 (0.18, 4.29) | 0.878 | |
| Potassium < 2.5 mEq | 4 | 98 | 0.29 (0.02, 4.32) | 0.370 | |
| Feverf | 14 | 99 | 4.44 (0.62, 31.68) | 0.137 | |
| Headachef | 5 | 99 | 0.36 (0.02, 5.32) | 0.460 | |
| C24 (μg/ml) | ALT > 2.5 times ULN | 9 | 99 | 1.68 (0.37, 7.64) | 0.499 |
| ALT > 5 times ULN | 6 | 99 | 0.78 (0.16, 3.88) | 0.760 | |
| ALT > 2.5 times BSLg | 20 | 99 | |||
| Persistent fever and neutropenia | 10 | 39 | 36.31 (2.29, 574.83) | 0.011 | |
| Invasive aspergillosis | 4 | 8 | 1.21 (0.10, 14.79) | 0.880 | |
| Invasive candidiasis | 2 | 28 | 534.74 (0.21, >999) | 0.117 | |
| New fever | 4 | 24 | 0.40 (0.06, 2.51) | 0.331 | |
| AST > 2.5 times ULN | 10 | 99 | 2.33 (0.49, 11.00) | 0.286 | |
| AST > 2.5 times BSL | 18 | 99 | 1.58 (0.51, 4.91) | 0.431 | |
| Potassium < 2.5 mEq | 5 | 101 | 2.74 (0.36, 21.06) | 0.332 | |
| Feverf | 17 | 102 | 3.10 (0.78, 12.31) | 0.107 | |
| Headachef | 5 | 102 | 3.45 (0.28, 42.27) | 0.333 | |
Time averaged (day 3 and later).
The odds ratios (95% CIs) and the P values are based on the model that included disease indication and the log-transformed pharmacokinetic parameter without their interaction term.
Fold change in odds (probability of event occurring/probability of event not occurring) per unit increase (on the log scale) in pharmacokinetic parameters.
ULN, upper limit of normal.
BSL, baseline.
Rated possibly, probably, or definitely drug related by investigator.
Statistically significant (P = 0.027). The relationship between PKs and occurrence of adverse events/laboratory abnormality was further investigated for each disease indication. The results suggested that C24 was positively associated with the occurrence of an ALT level greater than 2.5 times the baseline level with an odds ratio of 36.31 (95% CI, 2.29, 574.83) and a P value of 0.011 for pediatric patients empirically treated for suspected fungal infections but not for pediatric patients with other disease indications.
Caspofungin pharmacokinetics as a predictor of treatment outcome.
None of the three caspofungin PK parameters examined (AUC0–24, C1, and C24) was found to be a significant factor for predicting efficacy outcome for caspofungin within the range of available PK parameter values (Table 4). In all cases, the 95% CIs for the odds ratio estimates were wide and extended both below and above 1. Three hybrid parameters (AUC0–24/MIC, C1/MIC, and C24/MIC) were also examined for patients with invasive candidiasis; none was found to be a significant factor for predicting overall response or microbiologic response following caspofungin therapy (Table 4). In addition, in a subgroup analysis based on the predefined risk category (low risk and high risk) for pediatric patients receiving empirical therapy, none of the caspofungin PK parameters (AUC0–24, C1, and C24) was found to be a significant factor for predicting overall response.
Table 4.
Potential for caspofungin pharmacokinetic parameters to predict favorable treatment outcome in pediatric patients
| Patient group and caspofungin PK parametera | No. of patients |
Odds ratiob (95% CI) | P value | |
|---|---|---|---|---|
| With favorable outcome | Total | |||
| Empirical therapy | ||||
| AUC0–24 (μg · h/ml) | 5 | 16 | 0.18 (0.00, 38.36) | 0.529 |
| C1 (μg/ml) | 14 | 38 | 0.66 (0.09, 4.76) | 0.677 |
| C24 (μg/ml) | 15 | 40 | 1.24 (0.28, 5.43) | 0.774 |
| Invasive aspergillosis | ||||
| AUC0–24 (μg · h/ml) | 1 | 2 | NDc | ND |
| C1 (μg/ml) | 5 | 8 | 0.08 (0.00, 9.02) | 0.300 |
| C24 (μg/ml) | 5 | 8 | 0.01 (0.00, 12.19) | 0.216 |
| Invasive candidiasis | ||||
| AUC0–24 (μg · h/ml) | 23 | 26 | 5.39 (0.07, 400.25) | 0.444 |
| AUC0–24/MIC (h) | 21 | 24 | 0.47 (0.10, 2.26) | 0.349 |
| C1 (μg/ml) | 23 | 28 | 9.49 (0.27, 333.88) | 0.216 |
| C1/MIC | 21 | 25 | 0.99 (0.31, 3.14) | 0.983 |
| C24 (μg/ml) | 24 | 29 | 1.67 (0.19, 14.36) | 0.642 |
| C24/MIC | 21 | 25 | 0.94 (0.34, 2.55) | 0.898 |
| Invasive candidiasis with a favorable microbiologic response | ||||
| AUC0–24 (μg · h/ml) | 24 | 26 | 4.18 (0.03, 644.74) | 0.578 |
| AUC0–24/MIC (h) | 22 | 24 | 0.48 (0.07, 3.10) | 0.441 |
| C1 (μg/ml) | 25 | 27 | 0.38 (0.01, 22.76) | 0.646 |
| C1/MIC | 23 | 25 | 0.29 (0.02, 3.44) | 0.323 |
| C24 (μg/ml) | 26 | 28 | 8.96 (0.11, 749.97) | 0.332 |
| C24/MIC | 23 | 25 | 0.72 (0.17, 3.05) | 0.660 |
Time averaged (day 3 and greater).
Fold change in odds (probability of a favorable outcome/probability of an unfavorable outcome) per unit increase (on the log scale) in parameter.
ND, not done due to small number of patients.
Caspofungin pharmacokinetics as a predictor of clinical adverse events or laboratory abnormalities.
Of the 24 combinations of caspofungin PK parameter values and adverse events investigated, only the interaction between log-transformed C24 and an alanine aminotransferase (ALT) concentration >2.5 times the baseline concentration was statistically significant (P = 0.027). No other adverse events or laboratory abnormalities were found to be significantly predicted by AUC0–24, C1, or C24 (Table 5). The potential for log-transformed C24 to predict the occurrence of an ALT concentration >2.5 times the baseline concentration was further investigated for each disease indication. C24 was found to be positively associated with the occurrence of an ALT concentration >2.5 times the baseline concentration (P = 0.011) for pediatric patients empirically treated for suspected fungal infections but not for pediatric patients with other disease indications (Table 5).
DISCUSSION
This report describes the population PK analysis of caspofungin performed with data obtained from pediatric patients (ages, 3 months to 17 years) from four prospective clinical trials. Pediatric patients with confirmed documented infections (invasive candidiasis or invasive aspergillosis) and those receiving empirical therapy in the setting of fever and neutropenia (absolute neutrophil count < 500 cells/mm3) received caspofungin as a 1-hour intravenous infusion on the basis of BSA-based dosing with 50 or 70 mg/m2/day (with or without a 70-mg/m2 loading dose).
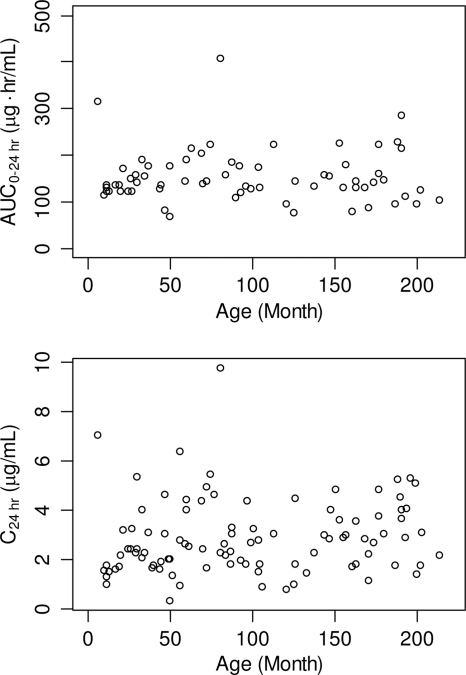
In comparison to adult patients receiving caspofungin at 50 mg daily, C1 was significantly increased (93% to 134%) in pediatric patients of all age groups, and C24 was significantly increased (45% to 78%) in older children (ages, 2 to 11 years) and adolescents (ages, 12 to 17 years) but not in young children (ages, 3 to 24 months). Significant but modest increases (38% to 41%) in AUC0–24 were also found in pediatric patients of all age groups compared to those in adults. The averaged C1 values in pediatric patients receiving 50 mg/m2/day were roughly comparable to those obtained in adults receiving caspofungin at 70 mg/day (2, 13, 23) and less than those seen in adults receiving caspofungin at 100 mg/day (14). Previous studies have shown decreased elimination (β)-phase half-lives (t1/2s) in children and adolescents compared to those in adults (29); a further decrease in β-phase t1/2 was observed in younger children compared to that in adolescents (16). The decrease in β-phase t1/2 may be related to increased plasma clearance, decreased distributional volume, or both (29). Thus, it is not possible to identify a dose that would provide comparable values for all three PK parameters (C1, C24, and AUC0–24). The 70-mg daily dose of caspofungin has generally been well tolerated in over 100 adults (12, 19, 30). In fact, doses as high as 150 mg daily have generally been well tolerated in adult patients and shown to have a safety profile similar to that seen with the 50-mg daily maintenance dose (3). In addition, the lower range of individual C24 values is generally similar in adults and pediatric patients. This suggests that reducing the pediatric dosage below that studied in the pediatric efficacy trial could leave some patients with trough values lower than the trough range observed in the adult efficacy trials. Given the life-threatening nature of invasive fungal disease in pediatric patients and the favorable safety results found in the caspofungin pediatric studies, maintaining the current dosing regimen appropriately errs on the side of maintaining adequate drug exposure for efficacy. Overall, consistent trough concentrations and overall exposure (AUC) values were observed across pediatric patients receiving caspofungin at 50 mg/m2 daily (Fig. 1), supporting the BSA-based dosing regimen.
Fig. 1.
Variation in caspofungin pharmacokinetics with age in pediatric patients (ages, 3 months to 17 years) receiving caspofungin at 50 mg/m2 once daily.
Weight and disease state were the only covariates found to be statistically significant determinants of caspofungin PK values. In particular, weight was found to be a significant determinant of C1 but not AUC0–24 or C24 in these pediatric patients receiving BSA-based doses. The current analysis predicts that in pediatric patients, on average, a 10-kg reduction in weight is associated with a 7% increase in C1. The modest alteration in C1 with weight is not anticipated to be clinically meaningful. Thus, no dose adjustment for weight is recommended beyond that provided by the BSA-based doses. Hope et al. performed a population PK analysis for micafungin and suggested that weight was a statistically significant determinant of the PKs of micafungin in pediatric patients 2 to 17 years of age (8). The appropriateness of a BSA-based regimen over a weight-based regimen of caspofungin in pediatric patients has been previously reported (29). The lack of a large effect with weight and age supports the dosing regimen based on BSA in pediatric patients (Fig. 1). Disease state was a significant covariate for AUC0–24 and C24 but not C1. Compared to pediatric patients with suspected fungal infection with persistent fever and neutropenia, pediatric patients with new-onset fever and neutropenia had modest but statistically significant reductions in AUC0–24 (25%, P = 0.004) and C24 (35.9%, P < 0.001). Although the possible mechanism is unclear, these alterations are unlikely to be clinically meaningful since all groups of pediatric patients had AUC0–24 and C24 values equivalent to or greater than those for the corresponding group of adult patients, as discussed above. Of note, no other covariates (including gender, race, age, renal status, and serum albumin level) were statistically significant determinants of caspofungin PK values.
The results of the drug interaction screen indicate that alterations in caspofungin concentrations as a result of potential drug interactions are not common. The current analyses identified three medications (acyclovir, vancomycin, and dexamethasone) that were associated with reductions in either C1 or C24 on the basis of a significant P value (uncorrected for multiplicity). Neither acyclovir nor vancomycin was identified in previous adult population PK studies to significantly increase or decrease caspofungin plasma concentrations or exposures. Furthermore, the magnitude of the associations was modest to moderate in each case, and the association was not consistent across all PK parameters. On the basis of the favorable efficacy data obtained for pediatric patients, decreases of this magnitude would not be expected to be clinically meaningful. The analyses also identified concurrent use of dexamethasone (a CYP inducer) to significantly decrease the caspofungin geometric mean C24 (44% decrease, P = 0.033), although no significant changes in caspofungin PK parameters were associated with prior use of dexamethasone or with prior or concurrent use of the pooled CYP inducer group. In adults, prior use and concomitant use of CYP inducers were associated with significant reductions in AUC0–24 and C24 of caspofungin (9, 13), and this drug-drug interaction mechanism was confirmed in phase I studies with rifampin (25). Trends seen with concomitant use of dexamethasone are consistent with a similar moderate reduction in caspofungin concentration associated with concomitant inducer use in adults and are supportive of the dosing recommendation of 70 mg/m2 daily (not to exceed an actual daily dose of 70 mg) of caspofungin in pediatric patients when it is coadministered with inducers of drug clearance, such as dexamethasone. Since multiplicity was not adjusted in this analysis, there is a 5% false-positive rate. In addition, it is important not to overinterpret the results, considering several confounding factors, such as the small number of patients for each concomitant medication, overlapping administration of different medications, and the potential effects of additional factors, such as underlying conditions of the patients. Overall, the results should be used only as a guide to the potential effects of concomitant medications on caspofungin PK values, which, if indicated, might then be more carefully evaluated in a controlled phase I study.
In the treatment outcome analysis, none of the caspofungin PK parameters (AUC0–24, C1, and C24) or hybrid parameters (AUC/MIC, C1/MIC, and C24/MIC) was found to be a significant factor for predicting efficacy outcome in any of the disease indications studied. Although the total number of pediatric patients included in this analysis is small, the lack of correlation between plasma concentrations and treatment outcome and the high proportion of favorable responses in the pediatric populations are consistent with the results of previous population PK/pharmacodynamic studies in adults, where similar caspofungin concentrations were shown to fall near the top of the concentration-response curve for adults and where treatment response has at most only a modest concentration dependency (24). Approximately half of the patients in the current analysis received caspofungin for suspected fungal infection in an empirical therapy study (11). Removal of the fever resolution component from the 5-point composite endpoint used in that study may provide a more meaningful indicator of antifungal efficacy (21); this modified endpoint leads to higher favorable response rates in our patients (11).
For the most part, the incidence of adverse events was not correlated with higher caspofungin plasma concentrations over the range of PK values examined. While no significant associations were identified for most adverse events, the point estimate of the odds ratio for C24 as a determinant of the occurrence of ALT levels >2.5 times the baseline level was considerably greater than 1, with a P value of 0.011, which suggests that C24 may be associated with ALT levels >2.5 times the baseline level for pediatric patients with suspected fungal infections. However, no similar association of PK parameters with any other liver function test biomarkers was identified, suggesting that the association between C24 and an elevated ALT level is not consistent with other related comparisons. Multiplicity was not adjusted in this analysis, and thus, there is a 5% false-positive rate. Results from previous population PK studies in adults suggested a lack of correlation between the occurrence of liver function test biomarkers (24).
These analyses support the use of a 50-mg/m2 daily dose of caspofungin (following a 70-mg/m2 loading dose on day 1) in pediatric patients (ages, 3 months to 17 years) for the treatment of invasive candidiasis or invasive aspergillosis or as empirical therapy in patients with fever and neutropenia. Overall, the results indicate that the caspofungin concentrations achieved with this dosing regimen are generally within the therapeutic window for caspofungin, since there was a high proportion of favorable responses with caspofungin treatment, and no significant and consistent correlations between caspofungin concentrations and clinical adverse events or laboratory abnormalities were found.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kim Strohmaier (Merck Research Laboratories) for her excellent assistance in the preparation and revision of the manuscript.
Funding for this study was provided by Merck & Co., Inc.
We are current or former employees of Merck Research Laboratories and may own stock and/or stock options in the company.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Abbasi S., Shenep J. L., Hughes W. T., Flynn P. M. 1999. Aspergillosis in children with cancer: a 34-year experience. Clin. Infect. Dis. 29:1210–1219 [DOI] [PubMed] [Google Scholar]
- 2. Arathoon E. G., et al. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiasis. Antimicrob. Agents Chemother. 46:451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Betts R., et al. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin. Infect. Dis. 48:1676–1684 [DOI] [PubMed] [Google Scholar]
- 4. Bi S., Schwartz M. S., Desai R. B., Miller A. R., Matuszewski B. K. 2005. A semi-automated procedure for the determination of caspofungin in human plasma using solid-phase extraction and HPLC with fluorescence detection using secondary ionic interactions to obtain a highly purified extract. J. Liq. Chromatogr. Related Technol. 28:2895–2908 [Google Scholar]
- 5. Chanock S. J., Walsh T. J. 1996. Evolving concepts of prevention and treatment of invasive fungal infections in pediatric bone marrow transplant recipients. Bone Marrow Transplant. 18(Suppl. 3):S15–S20 [PubMed] [Google Scholar]
- 6. Reference deleted.
- 7. Engelhard D. 1998. Bacterial and fungal infections in children undergoing bone marrow transplantation. Bone Marrow Transplant. 21(Suppl. 2):S78–S80 [PubMed] [Google Scholar]
- 8. Hope W. W., et al. 2007. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob. Agents Chemother. 51:3714–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kartsonis N. A., Nielsen J., Douglas C. M. 2003. Caspofungin: the first in a new class of antifungal agents. Drug Resist. Updat. 6:197–218 [DOI] [PubMed] [Google Scholar]
- 10. Lin S. J., Schranz J., Teutsch S. M. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358–366 [DOI] [PubMed] [Google Scholar]
- 11. Maertens J. A., et al. 2010. A randomized, double-blind, multicenter study of caspofungin versus liposomal amphotericin B for empiric antifungal therapy in pediatric patients with persistent fever and neutropenia. Pediatr. Infect. Dis. J. 29:415–420 [DOI] [PubMed] [Google Scholar]
- 12. Maertens J., et al. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563–1571 [DOI] [PubMed] [Google Scholar]
- 13. Merck & Co., Inc. 2008. Cancidas (caspofungin acetate) for injection, prescribing information, July. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 14. Migoya E., et al. 2011. Safety and pharmacokinetics of higher doses of caspofungin in healthy adult subjects. J. Clin. Pharmacol. 51:202–211 [DOI] [PubMed] [Google Scholar]
- 15. Mora-Duarte J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 16. Neely M., et al. 2009. The pharmacokinetics and safety of caspofungin in older infants and toddlers. Antimicrob. Agents Chemother. 53:1450–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappas P. G., et al. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634–643 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Nunez A. 2001. Incidence and mortality of proven invasive Candida infections in pediatric intensive care patients. Infect. Control Hosp. Epidemiol. 22:477–478 [DOI] [PubMed] [Google Scholar]
- 19. Sable C. A., Nguyen B.-Y. T., Chodakewitz J. A., DiNubile M. J. 2002. Safety and tolerability of caspofungin acetate in the treatment of fungal infections. Transpl. Infect. Dis. 4:25–30 [DOI] [PubMed] [Google Scholar]
- 20. Schwartz M., Kline W., Matuszewski B. 1997. Determination of a cyclic hexapeptide (L-743 872), a novel pneumocandin antifungal agent in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Anal. Chem. Acta 352:299–307 [Google Scholar]
- 21. Segal B. H., et al. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin. Infect. Dis. 47:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shetty D., Giri N., Gonzalez C. E., Pizzo P. A., Walsh T. J. 1997. Invasive aspergillosis in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 16:216–221 [DOI] [PubMed] [Google Scholar]
- 23. Stone J. A., et al. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone J., et al. 2003. Population pharmacokinetics of caspofungin in candidiasis patients, abstr. A-1571. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 25. Stone J., et al. 2004. Potential for interactions between caspofungin and nelfinavir or rifampin. Antimicrob. Agents Chemother. 48:4306–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villaneuva A., et al. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 33:1529–1535 [DOI] [PubMed] [Google Scholar]
- 27. Villaneuva A., et al. 2002. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am. J. Med. 113:294–299 [DOI] [PubMed] [Google Scholar]
- 28. Walmsley S., et al. 1993. Invasive Aspergillus infections in a pediatric hospital: a ten-year review. Pediatr. Infect. Dis. J. 12:673–682 [DOI] [PubMed] [Google Scholar]
- 29. Walsh T. J., et al. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 49:4536–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh T. J., et al. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391–1402 [DOI] [PubMed] [Google Scholar]
- 31. Zaoutis T. 2010. Candidemia in children. Curr. Med. Res. Opin. 26:1761–1768 [DOI] [PubMed] [Google Scholar]
- 32. Zaoutis T. E., et al. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41:1232–1239 [DOI] [PubMed] [Google Scholar]
- 33. Zaoutis T. E., et al. 2009. A prospective, multicenter study of caspofungin for the treatment of documented Candida or Aspergillus infections in pediatric patients. Pediatrics 123:877–884 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.