Abstract
P glycoproteins (Pgp), members of the ABC transporter superfamily, play a major role in chemoresistance. In nematodes, Pgp are responsible for resistance to anthelmintics, suggesting that they are Pgp substrates, as they are in mammalian cells. However, their binding to nematode Pgp and the functional consequences of this interaction have not been investigated. Our study showed that levamisole and most of the macrocyclic lactones (MLs) are Pgp substrates in nematodes. Ivermectin, although a very good substrate in mammalian cells, is poorly transported. In contrast to their inhibitory effect on mammalian Pgp, these drugs had a stimulatory effect on the transport activity of the reference Pgp substrate rhodamine 123 (R123) in the nematode. This may be due to a specific sequence of nematode Pgp, which shares only 44% identity with mammalian Pgp. Other factors, such as the affinity of anthelmintics for Pgp and their concentration in the Pgp microenvironment, could also differ in nematodes, as suggested by the specific relationship observed between the octanol-water partition coefficient (log P) of MLs and R123 efflux. Nevertheless, some similarities were also observed in the functional activities of the mammalian and nematode Pgp. As in mammalian cells, substrates known to bind the H site (Hoechst 33342 and colchicine) activated the R site, resulting in an increased R123 efflux. Our findings thus show that ML anthelmintics, which inhibit Pgp-mediated efflux in mammals, activate transport activity in nematodes and suggest that several substituents in the ML structure are involved in modulating the stimulatory effect.
INTRODUCTION
P glycoproteins (Pgp) are large membrane proteins belonging to the ABC (ATP binding cassette) superfamily (23). They function as ATP hydrolysis-dependent transporters whose substrates include a wide range of chemically unrelated compounds. Pgp play an important role in drug transport by pumping drugs out of cells and are thus often responsible for therapeutic failure in humans and animals. They have been studied mainly for their role in chemoresistance to antitumor agents. However, they have been described for their role in other biological models, including protozoan and nematode parasites of humans and animals (17).
Pgp-encoding genes have been identified in nematodes (55). Active Pgp was later detected in the gastrointestinal nematode Haemonchus contortus (31) by use of UIC2 monoclonal antibody (MAb), which specifically binds the active form of the protein (40). The role of Pgp in xenobiotic transport has also been demonstrated in this nematode by use of rhodamine 123 (R123) (29) as a specific Pgp substrate (44).
The role of Pgp in the transport of antiparasitic drugs has been studied less extensively but has been demonstrated in vivo in mice (56) and dogs (39, 52). Recently, a major group of anthelmintics, the macrocyclic lactones (MLs), were shown to inhibit Pgp function in mammalian cells, thus increasing the intracellular R123 concentration (19, 35).
However, no data are available on the effect of anthelmintics on nematode Pgp, whose putative protein sequence shows about 64% similarity with mouse mdr1a and 65% similarity with human mdr1 (65). The objectives of the present study were to determine (i) whether anthelmintics also bind nematode Pgp, (ii) whether binding depends on the different chemical structures of anthelmintics, and (iii) the effects of the binding of anthelmintics to nematode Pgp on the activity of the protein. We also examined the mechanisms underlying the binding of anthelmintics to nematode Pgp by comparing the effects of these drugs on Pgp transport activity with the effects of several other compounds known to interact in a different manner with Pgp in mammalian cells.
The life cycle of H. contortus includes several developmental stages. Free-living parasites were chosen in the present study. They are classically used for evaluation of helminth chemotherapy (11, 12, 37, 64), including analysis of structure-activity relationships of MLs in nematodes (42). Moreover, eggs can be obtained from feces without slaughtering the donor sheep.
MATERIALS AND METHODS
Parasites.
We used a Haemonchus contortus (Hc) isolate resistant (R) to anthelmintics that was isolated from sheep in the French West Indies (Guadeloupe [G]), referred to here as HcR-G. This isolate is resistant to benzimidazoles and ivermectin. Its Pgp activity is higher than that of susceptible isolates (50). Three-month-old sheep were infected with 6,000 infective larvae (L3) 5 weeks before the experiments. The experiments complied with the current French laws on animal experimentation and received approval. The infection procedures and the technique for the isolation of a pure suspension of eggs without debris were described previously (28, 29). The eggs were then stored in deionized water at 4°C until use.
Anthelmintics and other compounds.
Two benzimidazole derivatives (thiabendazole [CAS 148-79-9] and albendazole [CAS 54965-21-8]), one imidazothiazole derivative (levamisole [CAS 16595-80-5]), and seven macrocyclic lactones (abamectin [CAS 71751-41-2], doramectin [CAS 117704-25-3], emamectin [CAS 155569-91-8], eprinomectin [CAS 123997-26-2], ivermectin [CAS 70288-86-7], moxidectin [CAS 113507-06-5], and selamectin [CAS 165108-07-6]) were used in the various transport assays.
Known inhibitors of Pgp activity in mammalian cells, namely, verapamil (CAS 152-11-4) and sodium orthovanadate (SOV) (CAS 13721-39-6), were used to modulate nematode Pgp. Several other compounds previously used to identify binding sites in vertebrate cells were also studied: daunorubicin (CAS 23541-50-6), colchicine (CAS 64-86-8), vinblastine (CAS 143-67-9), and Hoechst 33342 (CAS 23491-52-3). All compounds were used in increasing concentrations up to the highest concentration that could be reached without forming a precipitate upon addition to the eggs.
R123 (CAS 62669-70-9) was the reference substrate for Pgp activity.
All of these compounds were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France), except for eprinomectin (HalloChem Pharma, Chongqing, China), selamectin (European Pharmacopoeia, Strasbourg, France), and Hoechst 33342 (Invitrogen, Cergy-Pontoise, France).
All compounds were solubilized in dimethyl sulfoxide (DMSO) and then diluted in deionized water to a final concentration of 1% in contact with eggs.
The mouse monoclonal anti-human MDR1 antibody UIC2 (1 mg/ml; clone IgG2a) was purchased from Immunotech (Marseille, France).
Assays of P-glycoprotein-mediated xenobiotic transport.
The role of active transport mediated by ABC transporters in the efflux of xenobiotics was demonstrated by inhibition tests using sodium orthovanadate or the Pgp-specific MAb UIC2 as an inhibitor and R123 as the Pgp substrate. R123 fluorescence was estimated after exposure of eggs to R123 for 60 min followed by three washings at 1.5-min intervals.
Xenobiotic efflux was quantified by accumulation of R123 in eggs, which was estimated by measuring the specific fluorescence (λ for excitation = 495 nm and λ for emission = 525 nm) using a Quanta Master spectrofluorometer (PTI, NJ) equipped with a 75-W xenon lamp. Deionized water (1 ml) containing 15,000 eggs was centrifuged and the supernatant discarded. R123 solution (2 ml of 1.5 μM R123; treated eggs) or deionized water (control eggs) was added to the eggs, and the eggs were left to incubate for 5 min at 20°C in the dark. The various test compounds (20 μl) were then added. Eggs were further incubated for 10 min at 20°C and then centrifuged. The supernatant was discarded, and eggs were washed with 5 ml of ice-cold deionized water. They were centrifuged again, and after removing the supernatant, 1 ml of deionized water was added. Eggs were kept in the dark for 60 min before analyses. Data are given in arbitrary units corrected for the native green fluorescence of eggs and expressed as percentages of the green fluorescence in control eggs. Three replicates per condition were analyzed.
The kinetics of R123 efflux was analyzed using flow cytometry at time point zero (T0) and at 1.5-min intervals for 9 min. Deionized water (1 ml) containing 120,000 eggs was centrifuged and the supernatant discarded. Aliquots (3 ml) of a 1.5 μM R123 solution were added to the egg pellets, which were then incubated for 15 min at 4°C in the dark. Deionized water (control eggs) or anthelmintics (15 μl) were then added to a final concentration of 7.5 μM. Egg suspensions were homogenized and further incubated for 45 min at 4°C in the dark. The supernatant was discarded, and eggs were washed with 3.5 ml of deionized water before analyses.
P-glycoprotein sequences.
The protein sequences of three mammalian Pgp (human, mouse, and sheep) known to be involved in drug transport and three equivalent Pgp in nematodes (H. contortus, Caenorhabditis elegans, and Onchocerca volvulus) were obtained from the Universal Protein Resource (http://www.uniprot.org/uniprot/) and compared using the ClustalW2 multisequence alignment program (http://www.ebi.ac.uk/Tools/clustalw2/).
Statistics.
Data were expressed as means ± standard deviations, and statistical analyses were carried out using Prism software (v5.02; GraphPad Software, San Diego, CA).
For accumulation tests, the log agonist versus response variable-slope model was used by calculating the following equation: y = bottom + (top − bottom)/1 + 10∧[log (EC50 − x) × hill slope], where “bottom” is the baseline and “top” is the maximum effect (Emax), EC50 is the dose giving half the maximum effect, x is the anthelmintic concentration (log), and hill slope is the slope factor. Bottom values were adjusted to 100, corresponding to the fluorescence in the control eggs. Best-fit values were obtained for “top,” EC50, and hill slope.
For efflux tests, the dissociation kinetic model was applied using the equation y = (y0 − NS)∧(−kx) + NS, where y0 is R123 binding at time zero, NS is the nonspecific binding at infinite times, and k is the rate constant. The half-life is equal to ln 2/k. Best-fit values for NS, k, and half-life were obtained.
For both statistical analyses, we calculated 95% confidence intervals for each parameter. Curves were plotted, and the goodness of fit (R and degrees of freedom) was estimated for each curve. Curves obtained for treated eggs were compared to those observed for control eggs.
RESULTS
Role of active efflux in R123 transport.
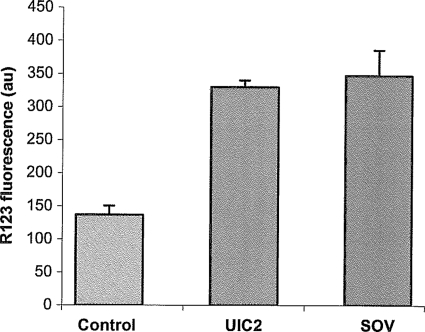
The role of active transport in the observed changes in R123 fluorescence was confirmed by inhibition tests using the Pgp-specific MAb UIC2 and SOV as inhibitors, which increased the R123 fluorescence in eggs about 2.4 and 2.5 times, respectively, compared to that in the control eggs (Fig. 1).
Fig. 1.
R123 is transported by Pgp in Haemonchus contortus eggs. Increased green fluorescence (arbitrary units [au]) indicates inhibition of R123 efflux in the presence of the Pgp-specific MAb UIC2 or the ATPase inhibitor SOV.
Accumulation tests.
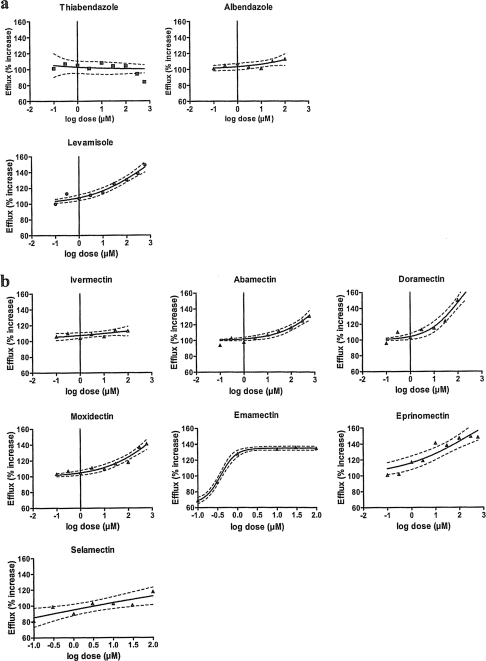
Most of the anthelmintics tested caused an increased efflux, i.e., a decreased accumulation of R123, the extent of which depended on the compound and concentration used. The smallest effects were observed with the benzimidazole derivatives (r = 0.71, n = 7, and P < 0.05 for albendazole; nonsignificant effects for thiabendazole), whereas highly significant effects were recorded for levamisole (r = 0.97; n = 9; P < 0.001) (Fig. 2a). For the ML group, the effects differed depending on the chemical structure of the drug (Fig. 2b). We observed only a very slight, nonsignificant effect for ivermectin, whereas significant effects (P < 0.01) were observed for all other MLs, with particularly high values obtained for doramectin and emamectin. The EC50 values also varied greatly between the MLs, with selamectin, emamectin, and doramectin showing the lowest EC50 values (Table 1). Two of the MLs (emamectin and selamectin) showed inhibitory effects at the lowest anthelmintic concentrations.
Fig. 2.
Changes in efflux activity of Pgp in Haemonchus contortus eggs in the presence of various anthelmintics. Regression lines were obtained from the log agonist versus response variable-slope model by use of Prism software. (a) Two benzimidazole derivatives (thiabendazole and albendazole) and an imidazothiazole (levamisole). A significant effect was observed for levamisole (P < 0.001). (b) Seven MLs with different structures. Regression analysis revealed significant effects for six of these compounds (P < 0.001) but not for ivermectin.
Table 1.
Stimulatory effects of anthelmintics and other compounds on R123 transport by Pgp
| Compound | Mean log EC50a | SE | Hill slope | SE |
|---|---|---|---|---|
| Selamectin | −2.016 | 0.721 | 0.118 | 0.034 |
| Emamectin | −0.425 | 0.016 | 2.228 | 0.166 |
| Hoechst 33342 | 0.862 | 0.065 | 0.941 | 0.12 |
| Vinblastine | 1.168 | 0.106 | 0.588 | 0.089 |
| Daunorubicin | 1.56 | 0.065 | 0.099 | 0.099 |
| Albendazole | 1.787 | 0.538 | 0.383 | 0.199 |
| Doramectin | 2.086 | 0.084 | 0.648 | 0.087 |
| Eprinomectin | 2.416 | 0.253 | 0.295 | 0.059 |
| Levamisole | 2.951 | 0.143 | 0.36 | 0.042 |
| Moxidectin | 3.217 | 0.163 | 0.402 | 0.042 |
| Colchicine | 3.431 | 0.507 | 0.240 | 0.062 |
| Abamectin | 3.465 | 0.196 | 0.486 | 0.062 |
The EC50 (μM) is the concentration of compound giving a response halfway between the baseline (bottom) and maximal (top) responses.
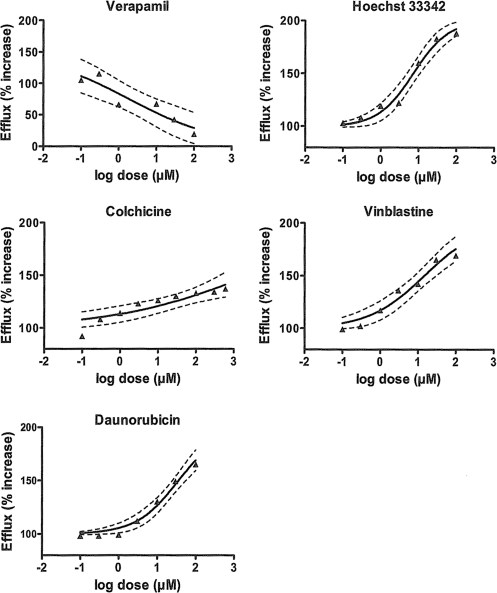
All other Pgp substrates tested, particularly Hoechst 33342, daunorubicin, and vinblastine, showed significant stimulating effects on R123 transport (Fig. 3). The corresponding EC50 values for the three substrates were generally much lower than those for anthelmintics (with the exception of selamectin and emamectin) (Table 1). As expected, the known Pgp inhibitor verapamil increased R123 accumulation (Fig. 3). Table 2 summarizes the differences in the biological activities of the compounds between mammalian cells and nematodes.
Fig. 3.
Effects of classically used Pgp substrates on R123 efflux by Pgp in Haemonchus contortus eggs. Verapamil is both a substrate and an inhibitor. Other compounds are known to bind to the R site (daunorubicin), the H site (Hoechst 33342 and colchicine), or both (vinblastine) in other eukaryotic models. The regression lines, obtained from the log agonist versus response variable-slope model by use of Prism software, were significant for all compounds (P < 0.001).
Table 2.
Effects of MLs and Pgp modulators on transport activity of Pgp in nematodes and mammalian cells
| Compound | Effluxa |
Reference(s) | |
|---|---|---|---|
| Nematodesb | Mammalian cellsc | ||
| Selamectin | + | − | 19 |
| Emamectin | + | ? | |
| Hoechst | + | + | 61 |
| Vinblastine | + | − | 61 |
| Daunorubicin | + | − | 61 |
| Albendazole | (+) | 0 | 41 |
| Doramectin | + | ? | |
| Eprinomectin | + | ? | |
| Levamisole | + | 0 | 14, 25 |
| Moxidectin | + | (−) | 46 |
| Colchicine | + | + | 61 |
| Abamectin | + | − | 13 |
| Ivermectin | 0 | − | 13, 45, 57 |
| Thiabendazole | 0 | − | 21 |
| Verapamil | − | − | 16, 26, 54 |
+, increased efflux; −, decreased efflux; (+) or (−), low-level increased or decreased efflux.
Results from the present study.
Results from the literature.
Kinetics of R123 efflux.
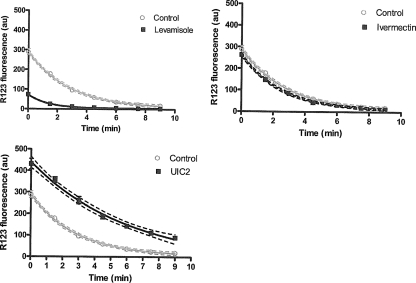
R123 efflux was rapid, with 50% of the compound being eliminated within less than 3 min in the control eggs (Fig. 4). R123 efflux was significantly inhibited by the UIC2 MAb, confirming the important role of Pgp in R123 elimination. Treatment with levamisole caused a highly significant (P < 0.00001) increase in R123 efflux, and ivermectin had a very slight, but nevertheless significant, effect (P < 0.0001). The rate constant k (0.36 for control eggs, 0.16 for UIC2-treated eggs, and 0.74 for levamisole-treated eggs) varied accordingly, giving k values ranging from twice to half the value obtained for eggs treated with R123 alone.
Fig. 4.
Kinetics of R123 efflux by Pgp in Haemonchus contortus eggs in the presence or absence of levamisole, an anthelmintic that activates R123 efflux by Pgp (significant; P < 0.001); ivermectin, an example of an anthelmintic that very slightly activates R123 efflux by Pgp (significant; P < 0.001); or MAb UIC2, which inhibits R123 efflux by Pgp with a very high specificity (significant; P < 0.001).
Differences according to ML structure.
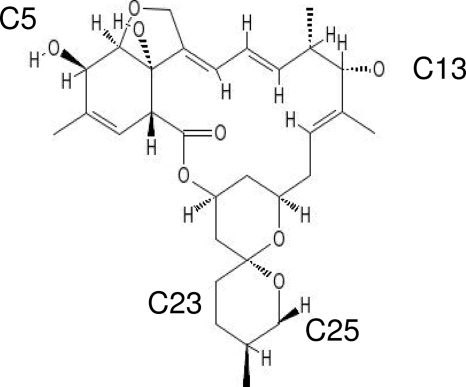
MLs are composed of a 16-membered macrocycle fused to spiroketal and benzofuran groups (62) and differ by their peripheral substituents, particularly at C-5, C-13, C-25, C-22, and C-23 (Fig. 5; Table 3). The inducing activities of these compounds, reflected by the EC50s, differed significantly between the MLs. Ivermectin had no significant effect, followed by abamectin, moxidectin, eprinomectin, doramectin, emamectin, and selamectin (greatest effect). The presence of a double bond at C-22–C-23 correlated with higher inducing activity (abamectin > ivermectin), as did the presence of the cyclohexyl group at C-25 (selamectin > all other MLs; doramectin > abamectin).
Fig. 5.
Structures of the MLs studied with respect to their C-5, C-13, C-23, and C-25 substituents and the double bond at C-22–C-23, which are known to play significant roles in anthelmintic properties. The substituents at C-25 and the double bond at C-22–C-23 also seemed to play a role in rhodamine 123 transport by P-glycoprotein. (Adapted from PubChem [http://pubchem.ncbi.nlm.nih.gov/].)
Table 3.
Substituents in MLsa
| ML | Substituent at C-5 | Sugar at C-13 | Bond at C-22–C-23 | Substituent at C-25 |
|---|---|---|---|---|
| Ivermectin | Hydroxyl | α-l-Arabino-hexapyranosyl | Single | Isopropyl/sec-butyl |
| α-l-Arabino-hexapyranosyl | ||||
| Abamectin | Hydroxyl | α-l-Arabino-hexapyranosyl | Double | Isopropyl/sec-butyl |
| α-l-Arabino-hexapyranosyl | ||||
| Moxidectin | Hydroxyl | No sugar | Single + oxime or methoxyimino? | Dimethyl-butenyl |
| Eprinomectin | Hydroxyl | α-l-Lyxo-hexapyranosyl | Double | Isopropyl/sec-butyl |
| α-l-Arabino-hexapyranosyl | ||||
| Doramectin | Hydroxyl | α-l-Arabino-hexapyranosyl | Double | Cyclohexyl |
| α-l-Arabino-hexapyranosyl | ||||
| Emamectin | Hydroxyl | α-l-Lyxo-hexapyranosyl | Double | Isopropyl/sec-butyl |
| α-l-Arabino-hexapyranosyl | ||||
| Selamectin | Ketoxime | α-l-Arabino-hexapyranosyl | Single | Cyclohexyl |
The main differences are shown in bold.
The nature of the sugar moiety at C-13 also affects the inducing activity (eprinomectin/emamectin > ivermectin/abamectin). Selamectin is the only ML tested with a monosaccharide at C-13; it also differs from the other MLs by containing a ketoxime instead of a hydroxyl radical at C-5 and having a single bond at C-22–C-23. Moxidectin, a milbemycin compound, does not have a sugar substituent at C-13, and it has an intermediate inducing activity. However, its substituents at C-23 and C-25 are also different from the substituents in this part of the structure of avermectins.
Pgp sequences in mammals and nematodes.
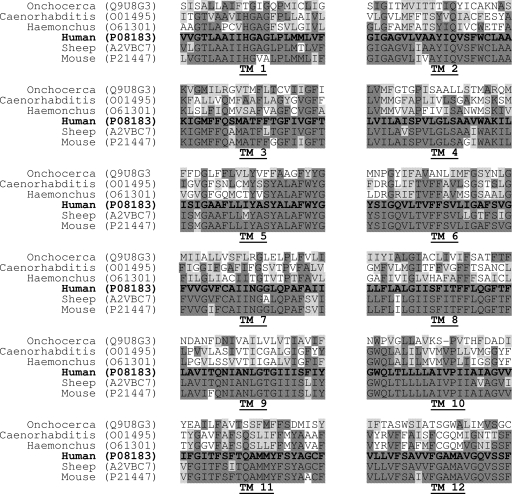
Compared to human Pgp, nematode Pgp show low similarities (53 to 64%), while the three mammalian Pgp show 90 to 91% similarities. The transmembrane domains (TM), especially those classically implicated in drug binding in mammalian Pgp (TM5, TM6, TM11, and TM12), differ highly between mammals and nematodes (Fig. 6). Fewer than 50% of the residues are similar in these domains, and their sequences indicate two distinct subgroups. However, sequences corresponding to the nucleotide binding domain (NBD) and linker regions (Walker A and Walker B motifs) appeared to be highly conserved (sequences not shown).
Fig. 6.
Protein sequence alignment of the 12 TMs of ABCB1 P-glycoproteins from three mammals (human, mouse, and sheep) and three nematodes (Haemonchus contortus, Caenorhabditis elegans, and Onchocerca volvulus). Two subgroups (mammals and nematodes) can be distinguished. The GenBank accession numbers for the sequences used are as follows: human, P08183; mouse, P21447; sheep, A2VBC7; Caenorhabditis elegans, O01495; Onchocerca volvulus, Q9U8G3; and Haemonchus contortus, O61301.
Biochemical properties of drugs and stimulatory effects.
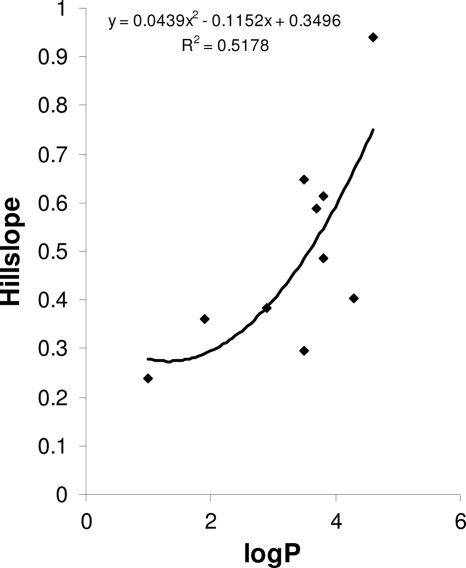
We studied the characteristic biophysical properties of the drugs tested. The octanol-water coefficient (log P) appeared to be associated significantly with the hill slope calculated from the relationship between the stimulatory effect of the drug on Pgp activity and the drug concentration (Fig. 7). Selamectin could not be included in the curve due to its curve having very different coordinates.
Fig. 7.
The hill slope coefficient, calculated from the regression curves for the percent increase in rhodamine 123 efflux in Haemonchus contortus eggs in the presence of different doses of P-glycoprotein substrates, was shown to depend on the log P values of these substrates (R = 0.720; n = 11; P < 0.01).
DISCUSSION
We found that Pgp transport the fluorescent dye R123 in nematodes as in mammalian cells (36). Nematode Pgp possess similar functional features, such as inhibition by the ATPase inhibitor sodium orthovanadate or the monoclonal antibody UIC2, which is highly specific for the active conformation of Pgp (40), as previously observed (29). In addition, we examined compounds never studied in nematodes by cotreating the parasites with R123 and antiparasitic drugs belonging to the main groups of anthelmintics or other compounds known to bind Pgp in other biological models. None of these compounds affected the excitation or emission spectra of R123 fluorescence (data not shown). Thus, all observed changes in fluorescence resulted from increased or decreased transport of R123.
Our findings demonstrate that most anthelmintics that are active against nematodes interact with nematode Pgp and modify R123 transport. A broad range of substrates have been described for mammalian Pgp, including xenobiotics with various chemical structures and modes of action (3), such as ivermectin (45, 57), rhodamine 123 (44), and verapamil (16, 26, 54). In addition to ivermectin, several other macrocyclic lactones were also demonstrated to affect R123 transport, using two vertebrate cell lines transfected with murine or human Pgp genes (35). We thus showed that anthelmintics can also be substrates for nematode Pgp.
Nevertheless, our findings showed some major differences from vertebrate cells, in which ivermectin strongly inhibits Pgp transport and increases R123 accumulation (45). In nematodes, the transport of R123 was stimulated by most of the MLs tested, except for ivermectin. The differences between ivermectin and other MLs seemed to be attributable to the chemical structure of MLs, as discussed below. The stimulatory effect in nematodes may be due either to an upregulation resulting in an increase in the number of active Pgp proteins recruited to the surfaces of the parasites (for a review, see reference 59) or to an increased rate of efflux by Pgp already present at the time of addition of R123 coupled with anthelmintics or other compounds. Several chemically unrelated compounds have been identified as “inducers” of Pgp synthesis, such as colchicine (24, 26, 66), daunorubicin (8, 57), vinblastine (1, 54, 57), and vincristine (54, 66). However, our study of the dynamics of R123 efflux, observed within a few minutes after addition of anthelmintics, is not compatible with such upregulation but rather points to Pgp already present near or at the surface of the parasite. Under the latter conditions, the affinity of anthelmintics for Pgp could be a determinant of the modulatory effect, as described for a large number of compounds for vertebrate Pgp (19, 33, 45, 58).
Affinity depends on both the structure of the substrate and the nature of the binding sites. For nematodes, these sites have not yet been identified. Comparison of Pgp sequences from mammalian Pgp and nematode Pgp showed that except for the ATP binding cassette, the overall sequence similarity is low. Nevertheless, one can expect that the binding sites are localized in the same part of the molecule. The transmembrane domains TM5-TM6 and TM11-TM12 have been shown to be part of the drug-binding domains in vertebrate Pgp (18, 59, 66). The sequences of these putative TMs in H. contortus, O. volvulus, and C. elegans present very low similarities with those in mammalian Pgp. Different patterns of resistance have been attributed to a single amino acid substitution (10, 20, 27, 53). Thus, since many residues differ between nematode and mammalian Pgp, it seems likely that the binding properties of the drug recognition pocket, and thus the effects of this binding on Pgp activity, also differ.
The mechanisms underlying the increase in R123 efflux in nematodes remain to be elucidated. Shapiro and Ling (61) suggested a functional model for Pgp containing at least two cooperative sites, the “H” site for Hoechst 33342 and the “R” site for rhodamine 123. They showed that drugs binding the H site, for example, Hoechst 33342, colchicine, and quercetin, activate the release of R123 and that drugs that bind the R site activate the release of Hoechst 33342. By comparison, the effects of the compounds tested here which activated R123 efflux could be explained through binding to an H site. The results obtained for Hoechst 33342 and colchicine are consistent with this assumption. Daunorubicin and vinblastine, which bind the R site in mammalian Pgp, activated R123 efflux in nematodes and thus probably bound an H site in this model. This could also be explained by more complex interactions with three or four binding sites, some of which could be “regulatory” sites, some with a “regulatory” function allowing activation of the efflux of Hoechst 33342 and R123 (60). Considering this model, anthelmintics may thus also bind “regulatory” sites due to the specific composition of Pgp in nematodes.
The stimulatory effect also depends on the chemical group and the structures of the drugs. Levamisole and macrocyclic lactones, except for ivermectin, were the most stimulatory anthelmintics. Ivermectin and some other MLs are good substrates for mammalian Pgp (34, 45), whereas levamisole has not been identified clearly as a Pgp substrate (14, 25). However, several imidazothiazole drugs have been shown to be reversing agents for MDR, suggesting that they can interact with Pgp (38, 43). Of the two benzimidazole derivatives tested, only one, albendazole, showed a very slight (but nonsignificant) stimulatory effect.
The effects produced by MLs differed according to the molecule, suggesting a role of the peripheral substituents. The number of drugs studied here was not sufficient to determine the structure activity relationship (SAR) in nematodes. However, the results can help to identify differences compared to mammalian Pgp. A distinguishing feature of MLs is the presence or absence of a sugar moiety known to influence the drug activity against parasites (42) or drug transport by Pgp in the host. Using mammalian cell models, Lespine et al. (35) observed a large inhibitory effect on Pgp activity for ivermectin (containing a sugar moiety), in contrast to a very small inhibitory effect of moxidectin (not containing a sugar moiety). In nematodes, near all MLs tested had a stimulatory effect on Pgp activity, and no direct relationship was observed with the presence or absence of a sugar moiety. Most of the MLs used here were disaccharide compounds but nevertheless showed significant differences, from no effect for ivermectin to a very large inducing effect for doramectin. Moreover, emamectin, a monosaccharide compound, showed the highest level of activity. Moxidectin, with no sugar moiety, had an intermediate effect. Thus, other chemical features or minor differences in the sugar moieties seemed to play a more determinant role in the stimulatory effect. This is illustrated by the largest inhibitory effect correlating with the presence of two different saccharides in eprinomectin and emamectin (α-l-lyxo-hexapyranosyl and α-l-arabino-hexapyranosyl), in contrast to the two identical saccharides (α-l-arabino-hexapyranosyl) in ivermectin, abamectin, and doramectin, which showed the lowest activities.
Our results also highlighted other chemical features associated with high inducing activity, such as an oxime substituent at C-5, a cyclohexyl substituent at C-25, and a double bond at C-22–C-23. In the brain barrier, Pgp have been associated with ivermectin elimination. A mutation in Pgp sequence in collie dogs resulted in higher toxicity (39). However, when the hydroxyl group in C-5 was replaced by a ketoxime, the toxicity was reduced (63). This seems compatible with the inducing activity of selamectin, with a ketoxime at C-5, compared to no effect with ivermectin, which has a hydroxyl group at C-5.
Other biophysical properties may also play a significant role in the drug-Pgp interaction within the cell membrane. Pgp transport lipophilic or amphiphilic compounds. Polar substituents at C-13 or cyclohexyl at C-25 could favor the insertion of drugs into the lipid membranes, and thus binding to Pgp. These substituents have been shown to diminish anthelmintic activity in mammals (62). More generally, the entry of drugs into the membrane depends on the partition coefficient log P, as demonstrated for phenothiazine-type multidrug resistance modifiers (22). In nematodes, the MLs with the highest log P values were associated with the largest increases in the stimulatory effect as a function of drug concentration. This could be interpreted as a “log P-response” relationship, as previously described for Pgp inhibitors (22), but attributing an inverse effect to the biological model. Pgp recognizes its substrates in the inner region of the cell membrane (4). The lipid environment may thus regulate Pgp transport (9). Nematode envelopes (eggshells and cuticles) differ from cell membranes in their lipid composition, in sterol and phospholipid (PL) content in particular, which plays a key role in drug resistance (47). In particular, the interactions of drugs with PLs influence drug absorption, tissue distribution, subcellular distribution, and protein activity (2). The amount of xenobiotics embedded within the bilayer may depend on the presence of anionic PLs (15). In nematodes, phosphatidic acid is more abundant in resistant parasites (47). A number of features suggest a possible a role of this PL in drug transport. Indeed, this molecule can bind to xenobiotics and can help to capture proteins by forming recruitment sites for cytosolic molecules and translocating them to the plasma membrane. Such interactions may be dependent on the partitioning of xenobiotic molecules in membranes and envelopes. The level of resistance to anthelmintics has also been shown to depend on the free cholesterol concentration in nematodes (47). Cholesterol acts as a modulator of Pgp localization and a promoter of Pgp function (32, 51).
Conclusions.
The present experiments did not directly assess the changes in drug activity against nematodes resulting from the interaction between drugs and nematode Pgp. Nevertheless, previous results clearly demonstrated the relationships between nematode Pgp activity and drug efficacy against parasites (7, 30, 31, 48). The obtained results should thus be useful in finding structural refinements to enhance the effectiveness of MLs as anthelmintic agents while limiting drug resistance mechanisms.
Free-living worms, which are not the clinically relevant stage, were chosen for study here. Even if the results remain to be confirmed with parasitic organisms, previous results showing the presence of active Pgp in eggshells and cuticles of both free-living and parasitic worms (49) allow us to expect similar effects in adult worms.
H. contortus, a sheep parasite, was chosen as a model for parasitic nematodes of ruminants. However, Pgp have also been identified in human nematode parasites, such as Onchocerca volvulus. Mass treatment with ivermectin is a primary part of the Onchocercosis Control Program (OCP). Intensive use of ivermectin has restricted the genetic variation of the H. contortus-homologous O. volvulus Pgp (OvPGP) gene (5). As a consequence, selection for ivermectin resistance is presently occurring in O. volvulus populations isolated in West Africa (6). The use of livestock nematodes, which are more appropriate for experimental purposes, can result in useful information for identifying mechanisms which are expected to be close considering the molecular similarity of Pgp genes in nematodes (H. contortus, O. volvulus, and C. elegans) to those in mammals.
ACKNOWLEDGMENTS
This work was financially supported by INRA and by the FP6 PARASOL (FOOD-CT-2005-022851) project.
We thank Gilles Aumont for kindly providing the H. contortus isolate and Bertrand Schwartz, Claude Limouzin, Thierry Chaumeil, and Maud Renouard, from the Experimental Platform for Infectious Diseases (PFIE), for the careful maintenance of the animals.
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Akiyama S., Cornwell M. M., Kuwano M., Pastan I., Gottesman M. M. 1988. Most drugs that reverse multidrug resistance also inhibit photoaffinity labeling of P-glycoprotein by a vinblastine analog. Mol. Pharmacol. 33:144–147 [PubMed] [Google Scholar]
- 2. Alakoskela J. M., Vitovic P., Kinnunen P. K. 2009. Screening for the drug-phospholipid interaction: correlation to phospholipidosis. ChemMedChem 4:1224–1251 [DOI] [PubMed] [Google Scholar]
- 3. Ambudkar S. V., et al. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39:361–398 [DOI] [PubMed] [Google Scholar]
- 4. Ambudkar S. V., Kim I. W., Sauna Z. E. 2006. The power of the pump: mechanisms of action of P-glycoprotein (ABCB1). Eur. J. Pharm. Sci. 27:392–400 [DOI] [PubMed] [Google Scholar]
- 5. Ardelli B. F., Guerriero S. B., Prichard R. K. 2006. Ivermectin imposes selection pressure on P-glycoprotein from Onchocerca volvulus: linkage disequilibrium and genotype diversity. Parasitology 132:375–386 [DOI] [PubMed] [Google Scholar]
- 6. Ardelli B. F., Prichard R. K. 2007. Reduced genetic variation of an Onchocerca volvulus ABC transporter gene following treatment with ivermectin. Trans. R. Soc. Trop. Med. Hyg. 101:1223–1232 [DOI] [PubMed] [Google Scholar]
- 7. Bartley D. J., et al. 2009. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology 136:1081–1088 [DOI] [PubMed] [Google Scholar]
- 8. Beck W. T., Cirtain M. C., Glover C. J., Felsted R. L., Safa A. R. 1988. Effects of indole alkaloids on multidrug resistance and labeling of P-glycoprotein by a photoaffinity analog of vinblastine. Biochem. Biophys. Res. Commun. 153:959–966 [DOI] [PubMed] [Google Scholar]
- 9. Belli S., Elsener P. M., Wunderli-Allenspach H., Kramer S. D. 2009. Cholesterol-mediated activation of P-glycoprotein: distinct effects on basal and drug-induced ATPase activities. J. Pharm. Sci. 98:1905–1918 [DOI] [PubMed] [Google Scholar]
- 10. Choi K. H., Chen C. J., Kriegler M., Roninson I. B. 1988. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell 53:519–529 [DOI] [PubMed] [Google Scholar]
- 11. Coles G. C. 1990. Recent advances in laboratory models for evaluation of helminth chemotherapy. Br. Vet. J. 146:113–119 [DOI] [PubMed] [Google Scholar]
- 12. Coles G. C., et al. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136:167–185 [DOI] [PubMed] [Google Scholar]
- 13. Didier A., Loor F. 1996. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer Drugs 7:745–751 [DOI] [PubMed] [Google Scholar]
- 14. Efferth T., Volm M. 1993. Reversal of doxorubicin-resistance in sarcoma 180 tumor cells by inhibition of different resistance mechanisms. Cancer Lett. 70:197–202 [DOI] [PubMed] [Google Scholar]
- 15. Gallois L., Fiallo M., Laigle A., Priebe W., Garnier-Suillerot A. 1996. The overall partitioning of anthracyclines into phosphatidyl-containing model membranes depends neither on the drug charge nor the presence of anionic phospholipids. Eur. J. Biochem. 241:879–887 [DOI] [PubMed] [Google Scholar]
- 16. Genne P., et al. 1992. Cinchonine, a potent efflux inhibitor to circumvent anthracycline resistance in vivo. Cancer Res. 52:2797–2801 [PubMed] [Google Scholar]
- 17. Germann U. A., et al. 1996. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. J. Biol. Chem. 271:1708–1716 [DOI] [PubMed] [Google Scholar]
- 18. Gottesman M. M., Pastan I. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385–427 [DOI] [PubMed] [Google Scholar]
- 19. Griffin J., Fletcher N., Clemence R., Blanchflower S., Brayden D. J. 2005. Selamectin is a potent substrate and inhibitor of human and canine P-glycoprotein. J. Vet. Pharmacol. Ther. 28:257–265 [DOI] [PubMed] [Google Scholar]
- 20. Gros P., Dhir R., Croop J., Talbot F. 1991. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc. Natl. Acad. Sci. U. S. A. 88:7289–7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayeshi R., Masimirembwa C., Mukanganyama S., Ungell A. L. 2006. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur. J. Pharm. Sci. 29:70–81 [DOI] [PubMed] [Google Scholar]
- 22. Hendrich A. B., Wesolowska O., Motohashi N., Molnar J., Michalak K. 2003. New phenothiazine-type multidrug resistance modifiers: anti-MDR activity versus membrane perturbing potency. Biochem. Biophys. Res. Commun. 304:260–265 [DOI] [PubMed] [Google Scholar]
- 23. Higgins C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67–113 [DOI] [PubMed] [Google Scholar]
- 24. Ichikawa M., et al. 1991. Interaction of organic chemicals with P-glycoprotein in the adrenal gland, kidney, and a multidrug-resistant KB cell. J. Biol. Chem. 266:903–908 [PubMed] [Google Scholar]
- 25. James C. E., Davey M. W. 2009. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 39:213–220 [DOI] [PubMed] [Google Scholar]
- 26. Kajiji S., Dreslin J. A., Grizzuti K., Gros P. 1994. Structurally distinct MDR modulators show specific patterns of reversal against P-glycoproteins bearing unique mutations at serine939/941. Biochemistry 33:5041–5048 [DOI] [PubMed] [Google Scholar]
- 27. Kajiji S., et al. 1993. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry 32:4185–4194 [DOI] [PubMed] [Google Scholar]
- 28. Kerboeuf D., Aycardi J., Soubieux D. 1996. Flow-cytometry analysis of sheep-nematode egg populations. Parasitol. Res. 82:358–363 [DOI] [PubMed] [Google Scholar]
- 29. Kerboeuf D., Chambrier P., Le Vern Y., Aycardi J. 1999. Flow cytometry analysis of drug transport mechanisms in Haemonchus contortus susceptible or resistant to anthelmintics. Parasitol. Res. 85:118–123 [DOI] [PubMed] [Google Scholar]
- 30. Kerboeuf D., Guegnard F., Le Vern Y. 2002. Analysis and partial reversal of multidrug resistance to anthelmintics due to P-glycoprotein in Haemonchus contortus eggs using Lens culinaris lectin. Parasitol. Res. 88:816–821 [DOI] [PubMed] [Google Scholar]
- 31. Kerboeuf D., Guegnard F., Vern Y. L. 2003. Detection of P-glycoprotein-mediated multidrug resistance against anthelmintics in Haemonchus contortus using anti-human mdr1 monoclonal antibodies. Parasitol. Res. 91:79–85 [DOI] [PubMed] [Google Scholar]
- 32. Kimura Y., Kioka N., Kato H., Matsuo M., Ueda K. 2007. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 401:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J. S., et al. 1994. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol. 46:627–638 [PubMed] [Google Scholar]
- 34. Lespine A., Alvinerie M., Vercruysse J., Prichard R. K., Geldhof P. 2008. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 24:293–298 [DOI] [PubMed] [Google Scholar]
- 35. Lespine A., et al. 2007. Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship. Eur. J. Pharm. Sci. 30:84–94 [DOI] [PubMed] [Google Scholar]
- 36. Ludescher C., et al. 1992. Detection of activity of P-glycoprotein in human tumour samples using rhodamine 123. Br. J. Haematol. 82:161–168 [DOI] [PubMed] [Google Scholar]
- 37. Maingi N., Bjorn H., Dangolla A. 1998. The relationship between faecal egg count reduction and the lethal dose 50% in the egg hatch assay and larval development assay. Vet. Parasitol. 77:133–145 [DOI] [PubMed] [Google Scholar]
- 38. Martins M. J., Negrao M. R., Hipolito-Reis C. 2001. Alkaline phosphatase from rat liver and kidney is differentially modulated. Clin. Biochem. 34:463–468 [DOI] [PubMed] [Google Scholar]
- 39. Mealey K. L., Bentjen S. A., Gay J. M., Cantor G. H. 2001. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics 11:727–733 [DOI] [PubMed] [Google Scholar]
- 40. Mechetner E. B., et al. 1997. P-glycoprotein function involves conformational transitions detectable by differential immunoreactivity. Proc. Natl. Acad. Sci. U. S. A. 94:12908–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merino G., Alvarez A. I., Prieto J. G., Kim R. B. 2002. The anthelminthic agent albendazole does not interact with P-glycoprotein. Drug Metab. Dispos. 30:365–369 [DOI] [PubMed] [Google Scholar]
- 42. Michael B., Meinke P. T., Shoop W. 2001. Comparison of ivermectin, doramectin, selamectin, and eleven intermediates in a nematode larval development assay. J. Parasitol. 87:692–696 [DOI] [PubMed] [Google Scholar]
- 43. Naito S., et al. 1998. Development of novel reversal agents, imidazothiazole derivatives, targeting MDR1- and MRP-mediated multidrug resistance. Oncol. Res. 10:123–132 [PubMed] [Google Scholar]
- 44. Nare B., Prichard R. K., Georges E. 1994. Characterization of rhodamine 123 binding to P-glycoprotein in human multidrug-resistant cells. Mol. Pharmacol. 45:1145–1152 [PubMed] [Google Scholar]
- 45. Pouliot J. F., L'Heureux F., Liu Z., Prichard R. K., Georges E. 1997. Reversal of P-glycoprotein-associated multidrug resistance by ivermectin. Biochem. Pharmacol. 53:17–25 [DOI] [PubMed] [Google Scholar]
- 46. Prichard R. K., Roulet A. 2007. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology 134:1123–1132 [DOI] [PubMed] [Google Scholar]
- 47. Riou M., Grasseau I., Blesbois E., Kerboeuf D. 2007. Relationships between sterol/phospholipid composition and xenobiotic transport in nematodes. Parasitol. Res. 100:1125–1134 [DOI] [PubMed] [Google Scholar]
- 48. Riou M., Guegnard F., Sizaret P. Y., Le Vern Y., Kerboeuf D. 2010. Drug resistance is affected by colocalization of P-glycoproteins in raft-like structures unexpected in eggshells of the nematode Haemonchus contortus. Biochem. Cell Biol. 88:459–467 [DOI] [PubMed] [Google Scholar]
- 49. Riou M., Koch C., Delaleu B., Berthon P., Kerboeuf D. 2005. Immunolocalisation of an ABC transporter, P-glycoprotein, in the eggshells and cuticles of free-living and parasitic stages of Haemonchus contortus. Parasitol. Res. 96:142–148 [DOI] [PubMed] [Google Scholar]
- 50. Riou M., Koch C., Kerboeuf D. 2005. Increased resistance to anthelmintics of Haemonchus contortus eggs associated with changes in membrane fluidity of eggshells during embryonation. Parasitol. Res. 95:266–272 [DOI] [PubMed] [Google Scholar]
- 51. Rothnie A., et al. 2001. The importance of cholesterol in maintenance of P-glycoprotein activity and its membrane perturbing influence. Eur. Biophys. J. 30:430–442 [DOI] [PubMed] [Google Scholar]
- 52. Roulet A., et al. 2003. MDR1-deficient genotype in collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur. J. Pharmacol. 460:85–91 [DOI] [PubMed] [Google Scholar]
- 53. Safa A. R., Agresti M., Tamai I., Mehta N. D., Vahabi S. 1990. The alpha 1-adrenergic photoaffinity probe [125I]arylazidoprazosin binds to a specific peptide of P-glycoprotein in multidrug-resistant cells. Biochem. Biophys. Res. Commun. 166:259–266 [DOI] [PubMed] [Google Scholar]
- 54. Safa A. R., Roberts S., Agresti M., Fine R. L. 1994. Tamoxifen aziridine, a novel affinity probe for P-glycoprotein in multidrug resistant cells. Biochem. Biophys. Res. Commun. 202:606–612 [DOI] [PubMed] [Google Scholar]
- 55. Sangster N. C. 1994. P-glycoproteins in nematodes. Parasitol. Today 10:319–322 [DOI] [PubMed] [Google Scholar]
- 56. Schinkel A. H., et al. 1994. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502 [DOI] [PubMed] [Google Scholar]
- 57. Schinkel A. H., Wagenaar E., Mol C. A., van Deemter L. 1996. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97:2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schinkel A. H., Wagenaar E., van Deemter L., Mol C. A., Borst P. 1995. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 96:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seelig A. 1998. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 251:252–261 [DOI] [PubMed] [Google Scholar]
- 60. Shapiro A. B., Fox K., Lam P., Ling V. 1999. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur. J. Biochem. 259:841–850 [DOI] [PubMed] [Google Scholar]
- 61. Shapiro A. B., Ling V. 1997. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 250:130–137 [DOI] [PubMed] [Google Scholar]
- 62. Shoop W. L., Mrozik H., Fisher M. H. 1995. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 59:139–156 [DOI] [PubMed] [Google Scholar]
- 63. Tranquilli W. J., Paul A. J., Todd K. S. 1991. Assessment of toxicosis induced by high-dose administration of milbemycin oxime in collies. Am. J. Vet. Res. 52:1170–1172 [PubMed] [Google Scholar]
- 64. Varady M., Cernanska D., Corba J. 2006. Use of two in vitro methods for the detection of anthelmintic resistant nematode parasites on Slovak sheep farms. Vet. Parasitol. 135:325–331 [DOI] [PubMed] [Google Scholar]
- 65. Xu M., et al. 1998. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 91:327–335 [DOI] [PubMed] [Google Scholar]
- 66. Zhang L., Sachs C. W., Fu H. W., Fine R. L., Casey P. J. 1995. Characterization of prenylcysteines that interact with P-glycoprotein and inhibit drug transport in tumor cells. J. Biol. Chem. 270:22859–22865 [DOI] [PubMed] [Google Scholar]