Abstract
Wound infection is a common risk for patients with chronic nonhealing wounds, causing high morbidity and mortality. Currently, systemic antibiotic treatment is the therapy of choice, despite often leading to several side effects and the risk of an insufficient tissue penetration due to impaired blood supply. If systemically delivered, moxifloxacin penetrates well into inflammatory blister fluid, muscle, and subcutaneous adipose tissues and might therefore be a possible option for the topical treatment of skin and infected skin wounds. In this study, topical application of moxifloxacin was investigated in comparison to mupirocin, linezolid, and gentamicin using a porcine wound infection and a rat burn infection model. Both animal models were performed either by an inoculation with methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa. Wound fluid, tissue, and blood samples were taken, and bacterial counts as well as the moxifloxacin concentration were determined for a 14-day follow-up. A histological comparison of the rat burn wound tissues was performed. Both strains were susceptible to moxifloxacin and gentamicin, whereas mupirocin and linezolid were effective only against MRSA. All antibiotics showed efficient reduction of bacterial counts, and except with MRSA, infected burn wounds reached bacterial counts below 105 CFU/g tissue. Additionally, moxifloxacin was observed to promote wound healing as determined by histologic analysis, while no induction of bacterial resistance was observed during the treatment period. The use of topical antibiotics for the treatment of infected wounds confers many benefits. Moxifloxacin is therefore an ideal candidate, due to its broad antibacterial spectrum, its high efficiency, and its potential to promote wound healing.
INTRODUCTION
Antibiotic drug resistance and skin wound infection are a growing concern in all parts of wound management. The risk of wound infection increases as disorders in the local environment (e.g., blood supply and eschar) favor bacterial growth rather than host defense. This can lead to impaired wound healing, bacteremia, or even sepsis and is associated with high morbidity and mortality (23).
Infections, especially those caused by antibiotic-resistant Gram-positive and Gram-negative bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Pseudomonas aeruginosa, are immensely concerning according to the National Nosocomial Infections Surveillance system report from October 2004. MRSA was responsible for an estimated 94,000 life-threatening infections and 18,650 deaths in 2005 in the United States (15).
MRSA can be seen as a continuously evolving pathogen (1). As a result, dramatic changes have occurred in the epidemiology of infections caused by MRSA in the last decade (25). In 2002, the first clinical isolate of vancomycin-resistant S. aureus (VRSA) was identified in patients with diabetic foot ulcers (12). Vancomycin, which is currently the most frequently used antibiotic in the treatment of severe MRSA infections, has become a suboptimal therapeutic agent in selected clinical settings (25).
Today's mainstay for the treatment of wound infection is systemic antibiotic therapy (9), even though it is associated with increased development of antibiotic drug resistance and adverse side effects (16, 17). Moreover, most systemic agents have poor tissue penetration (9); for example, in burn wounds, blood vessels, which normally carry a systemic antibiotic to the wound, are destroyed. Topical antibiotics, however, can be applied directly to the wound site, bypassing the need for an intact circulatory system (18). Therefore, topical treatment has the advantage of avoiding difficulties associated with systemic application while providing increased target site concentration.
Topical antibiotics are also very important but currently limited in their clinical use. Mupirocin ointment is the most widely used topical antibiotic for MRSA decolonization. It is currently the only topical agent with U.S. Food and Drug Administration (FDA) approval, and its use is restricted to the nose (17). However, its resistance to S. aureus has already been identified in several studies (7).
Since 2000, only three antimicrobials of a new class, or subclass, have been approved, two of which are specifically for the treatment of Gram-positive infections (the oxazolidinone linezolid and the cyclic lipopeptide daptomycin) and one with more broad-spectrum activity (the glycylcycline tigecycline) (19). To date, no topical broad-spectrum antibiotic with FDA approval is available for the treatment of skin wound infections.
Therefore, the progressive reduction in therapeutic options of available antibiotics and the need for topical application underlines the urgency for the development of new therapeutic options for the treatment of infected wounds.
Moxifloxacin (Avelox; Bayer, Germany) is a synthetic fluoroquinolone, with broad-spectrum antibiotic activity. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV, an enzyme necessary to separate bacterial DNA strands, thereby inhibiting cell division (6). Moxifloxacin was approved by the FDA in 1999 for intravenous therapy of severe and life-threatening bacterial infections, such as complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal infections (cIAI) (27).
The aim of this study was to investigate the antimicrobial activity of topically delivered moxifloxacin, which was dissolved within a standard gel formulation, against MRSA and Pseudomonas aeruginosa wound infection, using a porcine chronic wound and rat burn infection model.
MATERIALS AND METHODS
Bacteria.
Pseudomonas aeruginosa (ATCC 27853) was grown overnight in standard Luria Bertani (LB) broth medium (IDGLPC, Lancashire, Great Britain). MRSA (CMRSA-4, kindly provided by S. Gatermann, Ruhr University Bochum) was grown overnight in Mueller Hinton (MH) broth (Roth, Karlsruhe, Germany). The resulting stationary-phase cultures were transfused into fresh LB or MH medium and incubated at 37°C until reaching the mid-logarithmic phase. The subculture was centrifuged (10 min, 41°C, 880 × g; Megafuge R 1.0; Haereus, Hanau, Germany), and the resulting bacterial pellet was washed once with phosphate-buffered saline (PBS), pH 7.4, and resuspended in cold PBS. Optical density (OD) was measured at 600 nm (OD600) (Biophotometer, Eppendorf, Hamburg, Germany). Bacterial concentration (number of CFU/ml) was calculated using the following equation: number of CFU/ml = OD600 × 2.5 × 108. A total of 108 CFU were resuspended in 250 μl PBS, and the bacterial suspension was kept on ice until further use.
Determination of MIC and MBC using broth microdilution assays.
Microbroth dilution assays (MDA) were performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). The bacterial solutions were prepared by transferring a single colony into Mueller Hinton broth (Merck GmbH, Hohenbrunn, Germany) and incubated overnight at 37°C. Afterwards, the cultures were diluted, and the absorbance was measured at OD620 to provide the bacterial concentration of 4 × 105 CFU/ml in the microplate wells. A total of 100 μl of the freshly diluted cultures and 11 μl of the serially diluted antibiotics, ranging from 1,000 mg/liter to 1 mg/liter, were dispensed into 96-well polypropylene tissue culture plates (U-shape; Greiner, Solingen, Germany). The plates were covered and incubated at 37°C for 16 to 18 h (Heraeus incubator, Heraeus Holding GmbH, Germany). To determine the MIC, the last well in the series without any visible growth was read. Subcultures were prepared out of each well. The first well in the series without any visible growth indicated the corresponding minimal bactericidal concentration (MBC).
Therapeutic agents.
Standard gel formulation (Bayer Innovation GmbH; batch number N3922A02) functioned as a carrier control in all experiments. Moxifloxacin (0.1%) (Bayer Innovation GmbH; batch number BX01X6E), mupirocin (0.1%) (Sigma Aldrich, St. Louis, M7694; lot number 085K4701), and linezolid (0.1%) (Bayer Innovation GmbH; batch number 19/8746) were each dissolved in the standard gel formulation. Treatments with 2% mupirocin (Infectopyroderm; InfectoPharm, Heppenheim, Germany) and 0.3% gentamicin (MedPhano, Rütersdorf, Germany), which are commercially available ointments, were used as is.
The powdered antibiotics were weighed and placed into a sterile 50-ml tube and dissolved in standard gel. The resulting solution was vortexed until all the components were completely dissolved, centrifuged (4,000 rpm/2 min), and kept at 4°C until use. The solutions were freshly prepared for each treatment.
Animals.
The research protocol described below complied with all regulations related to animal use and other federal statutes. It was conducted in compliance with the Guide for the Care and Use of Laboratory Animals from the German Animal Welfare Act. The animals were housed at the animal facility of the BG University Hospital Bergmannsheil, Ruhr University Bochum, Bochum, Germany.
Göttingen minipigs (n = 3; female, 6 months old, 15 to 20 kg in weight; Fa. Ellegard, Dalmose, Denmark) were allowed to acclimate for at least 2 weeks prior to experiments, fed a standard porcine diet (Ssniff MPig-H; Ssniff, Soest, Germany), and housed at 20 to 30°C in an atmosphere of approximately 65% humidity with a 12-h/12-h dark/light cycle. Spraque-Dawley rats (n = 80; male, pathogen free, 220 to 260 g; Charles River Germany GmbH, Germany) were allowed a resting period of at least 2 weeks prior to experiments. After the first treatment, the rats were placed in individual cages in a temperature-controlled room (22°C) with food and water provided ad libitum and a 12-h/12-h dark/light cycle. After the experiment, all animals were euthanized by intravenous/intraperitoneal injection of T61 (Bayer, Leverkusen, Germany) at a dose of 1 ml/5 kg body weight.
Porcine wound infection model.
On the day of wounding, animals were sedated with 1 mg/kg midazolam (Ratiopharm, Ulm, Germany), 10 mg/kg ketamine (Ratiopharm, Ulm, Germany), and 0.05 mg/kg atropine (Fresenius Kabi, Bad Homburg, Germany) injected intramuscularly. Animals were weighed and transferred to the operating theater. General anesthesia using isoflurane (1 to 1.5%) (Forene, Abbott, Wiesbaden, Germany), oxygen, and nitrous oxide was maintained employing a mask adjusted to the pig's snout. The hair was shaved with an electric clipper (GT104; Aesculap, Melsungen, Germany) and depilated (Veet; Reckitt Benckiser, Mannheim, Germany), and afterwards, pigs were washed thoroughly with water and soap. The implantation of titanium wound chambers (BO-chamber; WiMed, Bochum, Germany) into porcine wounds was described previously (24). Briefly, a full-thickness wound was created using a BO-chamber round scalpel and a no. 11 blade. Titanium wound chambers (n = 12/animal) were inserted and fixed with 3 interrupted sutures. Afterwards a polyvinyl alcohol (PVA) sponge (Coldex; Velo, Reichertshofen, Germany) was placed into each chamber for wound fluid collection, and the closure of the chambers followed. On day 7 after wounding, two pigs were inoculated with 1 × 108 CFU MRSA in 250 μl PBS per wound, and the remaining one was inoculated with 1 × 108 CFU of Pseudomonas aeruginosa. Three days after inoculation, when a stable wound infection had been established, wound fluid was collected by sampling the inserted sponges to determine the bacterial counts on the first day of treatment. The resulting wound fluid was always quantitatively removed before treatment and stored on ice until further preparation.
Afterwards the wound chambers were randomized, and the following treatments were applied. Setup I was conducted to highlight differences between various antibiotics against MRSA under equal conditions. The groups were distributed as follows: carrier control (standard gel formulation; n = 3), 0.1% linezolid (dissolved in standard gel formulation; n = 3), 0.1% mupirocin (dissolved in standard gel formulation; n = 3), or 0.1% moxifloxacin (dissolved in standard gel formulation; n = 3). Setup II was done to confirm the effect of moxifloxacin against a commercially available antibiotic for topical treatment of MRSA-infected tissue, and the groups were distributed as follows: carrier control (standard gel formulation; n = 3), 2% mupirocin (commercially available ointment; n = 4), or 0.1% moxifloxacin (dissolved in standard gel formulation; n = 4) for the two pigs infected with MRSA. Setup III was in comparison to setup II but with the use of a commercially available antibiotic for the treatment of Pseudomonas aeruginosa-infected tissue, and the groups were distributed as follows: carrier control (standard gel formulation; n = 4), 0.1% moxifloxacin (dissolved in standard gel formulation; n = 4), or 0.3% gentamicin (commercially available ointment; n = 4) for the pig infected with Pseudomonas aeruginosa.
A total of 1 ml of gel formulation or ointment was delivered on a PVA sponge (Coldex; Velo, Reichertshofen, Germany) and placed gel side down directly on the infected wound ground. Wound fluid collection and subsequent topical treatment were carried out at days 0, 2, 4, 7, 9, and 11. At day 14 only the wound fluid was taken, since no further treatment was carried out. To monitor wound infection and variances within each group or chamber, wound fluids were analyzed for bacterial counts as described below. Venous blood samples and photographs were taken on each of these days. On day 14 after the treatment start, animals were sacrificed and two punch biopsy specimens (6 mm) were taken out of each wound. All samples were stored at 4°C until further use.
Rat burn infection model.
The infected rat burn model was used as previously described (14, 23). A total of 24 h after depilation, the rats were anesthetized using a gas anesthesia (2% isofluran, 38% O2, and 60% N2O). After immersing two defined skin areas on the back of each rat for 25 s in 65°C water, both areas were dried, marked, and thoroughly disinfected (Softasept N; Braun, Germany). A bacterial solution of 250 μl, containing a definite number of either 1 × 108 CFU of MRSA or 1 × 108 CFU of Pseudomonas aeruginosa, was added topically to both areas. To avoid cross-contamination and to improve growth conditions for the bacteria, occlusive dressings (Tegaderm, 6 × 7 cm; 3 M Health Care, Borken, Germany) were applied immediately after the application of bacteria. The rats were bandaged with Peha-haft (Hartmann, Heidenheim, Germany) for stabilization and protection clipped with VisiStat (Weck Closure Systems, NC).
At 2 days postinfection, five animals were sacrificed without any treatment to measure bacterial counts and verify MRSA infection as a baseline at day 0 of the corresponding time course. A baseline bacterial count was not obtained for the P. aeruginosa group.
In the MRSA group, animals received a topical application of 500 μl of either carrier control (standard gel formulation; n = 13 rats), 0.1% moxifloxacin (standard gel formulation; n = 17 rats), 0.1% linezolid (standard gel formulation; n = 3 rats), 0.1% mupirocin (standard gel formulation; n = 4 rats), or 2% mupirocin (commercially available ointment; n = 11 rats). In the Pseudomonas aeruginosa group, animals were treated with the same volume of the standard gel formulation (n = 6 rats), 0.1% moxifloxacin (standard gel formulation; n = 7 rats), or 0.3% gentamicin (commercially available ointment; n = 5 rats).
After application, the wounds were again occlusively dressed and bandaged as described above. Treatment was carried out every day for the duration of 2 weeks.
A total of 24 h after the first treatment, a subset of animals was sacrificed, and two cross-sectional biopsy specimens were taken aseptically out of each infected wound area for the following: 0.1% moxifloxacin (n = 5 rats), 0.1% linezolid (n = 3 rats), 0.1% mupirocin (n = 4 rats), or the carrier control (n = 5). A further subset of animals was sacrificed on day 7 after the first treatment, and the corresponding biopsy specimens were taken: 0.1% moxifloxacin (n = 5 rats), 2% mupirocin (n = 6 rats), or the carrier control (n = 4 rats). At day 14 after the treatment start, the following groups were sacrificed: the carrier control (n = 4 rats), 0.1% moxifloxacin (n = 7 rats), and 2% mupirocin (n = 5 rats). One sample of each wound was fixed in 4% buffered paraformaldehyde for further histological analysis, and the other one was stored at 4°C until further use.
Quantification of bacteria.
To determine the bacterial counts in the tissue samples, biopsy specimens were individually weighed and homogenized in 2 ml of PBS using a Polytron homogenizer (T3100; Kinematika, Luzern, Switzerland). The homogenates and the collected wound fluids of each wound were then serially diluted in PBS (1:10, 1:100, 1:1,000, and 1:10,000) and plated on mannitol agar plates in triplicates, MRSA selection agar (Oxoid, Wesel, Germany), Pseudomonas isolation agar, and MH agar plates containing 5% sheep blood (Becton Dickinson, Heidelberg, Germany). Plates were then incubated for at least 18 h at 37°C under a humidified atmosphere. All colony counts were expressed as log10 CFU per gram tissue or milliliter wound fluid. Bacterial counts of >1 × 105 were considered to indicate bacterial infection (20, 22).
Peripheral blood analysis.
At appropriate time points, venous EDTA anticoagulated blood was drawn. The blood counts for whole-blood cells (WBC), red blood cells (RBC), hemoglobin, and hematocrit (HCT) were determined using blood Vet abc (scil Animal Care Company GmbH, Viernheim, Germany). Furthermore, 200 μl of plasma was collected and stored at −20°C for further analysis.
Quantification of moxifloxacin.
Blood serum, wound fluid, or homogenized tissue samples were taken to determine the corresponding concentration of moxifloxacin. Therefore, a Sirocco protein precipitation plate (Waters, Eschborn, Germany) was placed on the top of a 1-ml 96-well collection plate, and 250 μl of internal standard (sparfloxacin; Fluka, Germany) and 100 μl of each sample were added. After shaking for 1 min (Vibrax VXR; IKA, Staufen, Germany), the precipitation plate was placed on a vacuum manifold (Büchi Vacuum System B-178; Büchi, Flavil, Switzerland), and the mixture was filtrated into the collection plate by applying a vacuum of 130 mbar for approximately 2 min. The filtrate was diluted by adding 200 μl of aqueous formic acid (5%) into the cavities of the collection plate, and the collection plate was sealed by foil and was shaken for at least 2 min. Samples were transferred to a liquid chromatography-mass spectrometry (LC-MS) autosampler (HTC PAL; CTC Analytics, Zwingen, Switzerland) and were injected into the high-pressure LC tandem MS (HPLC-MS/MS) system (TSQ Quantum Ultra with H-ESI ionization interface; Thermo Scientific, Bonn, Germany). The HPLC column (C18 SunFire; Waters, Eschborn, Germany) was performed with a water-methanol gradient containing 0.1% formic acid. The amount of moxifloxacin was determined as micrograms/liter, and the calibration range of the procedure was from 10 μg/liter (lower limit of quantification [LLOQ]) to 5,000 μg/liter (upper limit of quantification [ULOQ]).
Histological assessment.
Punch biopsy specimens from the porcine wound infection model were obtained on day 14, and cross-sectional biopsy specimens from the rat burn infection model on days 0, 1, 7, and 14. All samples were fixed in 4% buffered paraformaldehyde and routinely processed for hematoxylin and eosin (H&E) staining. The H&E stains of all slides were analyzed and verified by two different pathologists in a blinded fashion using the following criteria: the thickness of granulation tissue, the numbers of capillaries per 10 high-power fields (HPF) representing a ×400 magnification level, re-epithelization, and lymphocyte infiltration. The entire gamut of slides was taken into consideration, and a scale was generated, scoring each slide according to the above-mentioned criteria.
Determination of antimicrobial susceptibility by agar disk diffusion method.
The collected wound fluids of day 14 (groups treated with 0.1% moxifloxacin and 2% mupirocin) and the pure culture of MRSA were separately transferred to MH agar and incubated for 24 h. With a sterile loop, the tops of four or five colonies out of each plate were picked up. The colonies were separately suspended in 5 ml of sterile saline. The inoculum turbidity was standardized to the equivalent of a 0.5 McFarland standard. Then the entire surface of an MH agar plate was inoculated using a sterile swab. Disks containing 5 μg of moxifloxacin and 5 μg of mupirocin were placed using sterile forceps onto each of the three agar surfaces and gently pressed down to ensure contact. Plates were incubated at 35°C for 20 h. Subsequently, the diameter of the zone of inhibition around each disk was measured. This procedure conforms with the Clinical and Laboratory Standards Institute (3) method.
Statistical analysis.
Results underwent analysis of variance and independent t tests when data were normally distributed (SPSS, Chicago, IL). Differences were considered to be statistically significant at a P value of <0.05. Wound fluid evaluation was performed in at least triplicate for each chamber, dilution, and time point. Data are presented as the mean ± standard error of the mean (SEM).
RESULTS
Antimicrobial susceptibility.
The MIC and MBC were measured for all used antibiotics, with a serial dilution of 0.2 mg/liter to 100 mg/liter against the used bacterial strains. Testing linezolid and mupirocin revealed similar results. Both showed the same MIC (1.25 mg/liter) and MBC (25 mg/liter) against the MRSA strain but were ineffective against Pseudomonas aeruginosa (MIC and MBC of >100 mg/liter). Moxifloxacin and gentamicin were active against both strains. The MIC for gentamicin against both strains was 0.78 mg/liter, while the MBC was 0.78 mg/liter against MRSA and 1.56 mg/liter against Pseudomonas aeruginosa. Moxifloxacin demonstrated a MIC of 3.13 mg/liter against MRSA and 1.25 mg/liter against Pseudomonas aeruginosa, with the MBC being 25 mg/liter for MRSA and 6.25 mg/liter for Pseudomonas aeruginosa (Table 1).
Table 1.
MIC and MBC for all antibiotics used against both bacterial strains in the study
| Antibiotic | MRSA |
P. aeruginosa |
||
|---|---|---|---|---|
| MIC (mg/liter) | MBC (mg/liter) | MIC (mg/liter) | MBC (mg/liter) | |
| Moxifloxacin | 3.13 | 25 | 1.25 | 6.25 |
| Mupirocin | 1.25 | 25 | >100 | >100 |
| Linezolid | 1.25 | 25 | >100 | >100 |
| Gentamicin | 0.78 | 0.78 | 0.78 | 1.56 |
Strains of the chosen MRSA seeding culture prior to the application were classified as being resistant against 0.1% moxifloxacin according to the zone-of-inhibition diameters (18 mm) and susceptible to 2% mupirocin (28 mm). Strains from the wounds treated with 0.1% moxifloxacin for at least 14 days were resistant against 0.1% moxifloxacin (18 mm) and susceptible to 2% mupirocin (30 mm). Finally, strains from wounds treated with 2% mupirocin for the same follow-up times were resistant against 0.1% moxifloxacin (16 mm) and susceptible to 2% mupirocin (25 mm). The existing resistance against moxifloxacin but not mupirocin before inoculation has to be taken into account when assessing the experimental results.
Porcine wound infection.
The entire porcine infection model proceeded without any adverse effect. Animals were only temporarily affected by trauma and anesthesia and recovered quickly after surgery. WBC, hemoglobin, and HCT revealed no signs of systemic infection and remained within a standard range during the course of the experiment (data not shown).
(i) Pseudomonas aeruginosa.
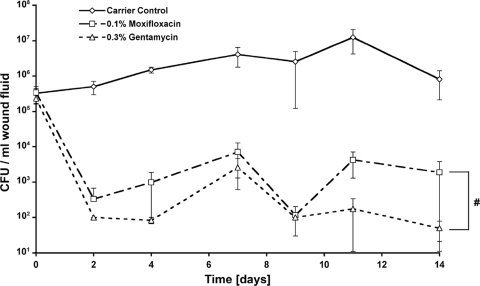
Before initiation of treatment, wounds showed an infection ranging between 2.3 × 105 and 3.3 × 105 CFU/ml wound fluid. Within the time course of 14 days, bacterial counts continuously increased within the carrier control group up to a peak at day 11 (1.2 × 107 CFU/ml wound fluid). Bacterial counts in the 0.1% moxifloxacin and 0.3% gentamicin groups were significantly reduced during the entire time course (P ≤ 0.001, 0.1% moxifloxacin and 0.3% gentamicin versus carrier control). While bacterial counts for 0.3% gentamicin (5 × 101 CFU/ml wound fluid) reached background levels on days 11 and 14, the differences were not significant compared to bacterial counts for 0.1% moxifloxacin (1.8 × 103 CFU/ml wound fluid) (Fig. 1).
Fig. 1.
Bacterial counts of Pseudomonas aeruginosa-infected porcine wounds. Effect of topically administered moxifloxacin and gentamicin against Pseudomonas aeruginosa wound infection. Reduction of the number of CFU below detectability was observed with the treatment groups compared to the carrier control during the entire time course. For both moxifloxacin and gentamicin treatments, the total bacterial counts within the tissue remained below the infection level (105 CFU/g of tissue) during the whole follow-up. Detection limit was 102 CFU/g of tissue. #, P < 0.001, 0.1% moxifloxacin and 0.3% gentamicin versus carrier control. The values are shown as mean ± SEM.
(ii) MRSA.
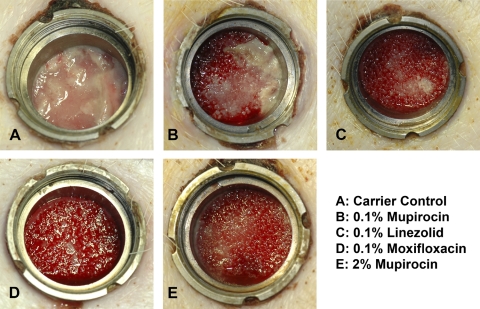
While the macroscopic photographs, which were taken on every day of treatment, reveal strong signs of infection in the carrier control group, the 0.1% mupirocin group showed only little purulence. In all treatment groups, a formation of granulation tissue with only minimal or even no macroscopic signs of infection was observed (Fig. 2).
Fig. 2.
Wound infection. This figure documents the macroscopic signs of infection taken on day 17 postinfection and 14 days after treatment start. Strong or medium purulence is shown (A and B); no or minimal signs of infection are visible (C, D, and E). Additionally, the granulation tissue of the wounds treated with 0.1% linezolid, 0.1% moxifloxacin, or 2% mupirocin showed efficient blood supply to the periphery, as indicated by the dark red color of the tissue.
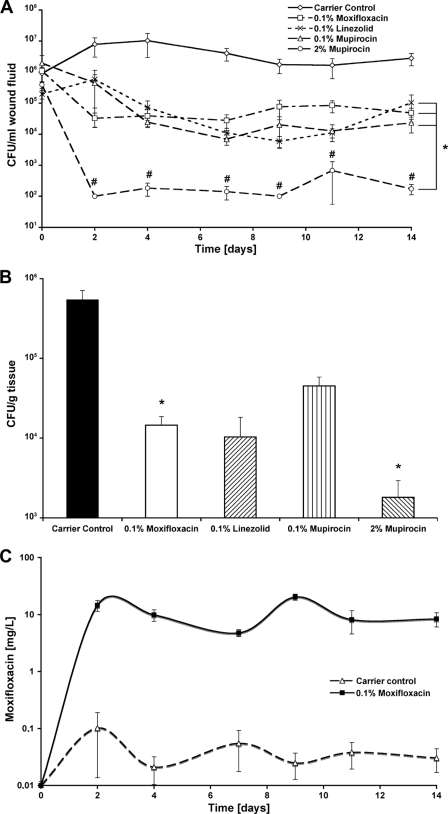
Before treatment and after inoculation with 108 CFU of MRSA in each chamber, all groups showed an active infection ranging between 3.9 × 105 CFU/ml wound fluid for the 2% mupirocin chamber (lowest) and 1.9 × 106 CFU/ml wound fluid for the 0.1% mupirocin chamber (highest). The initial value in the 0.1% moxifloxacin chamber was slightly higher (1.1 × 106 CFU/ml wound fluid) than in the carrier control chamber (9 × 105 CFU/ml wound fluid). On day 2, 0.1% moxifloxacin significantly decreased infection compared to the carrier control (3.2 × 104 CFU/ml wound fluid; P = 0.00018). In comparison to 0.1% moxifloxacin, the quantified CFU were 1 log higher for 0.1% mupirocin (4.4 × 105 CFU/ml wound fluid) and 0.1% linezolid (5.8 × 105 CFU/ml wound fluid), whereas both values were significant compared to the carrier control. Two percent mupirocin, however, strongly decreased bacterial counts down to 1 × 102 CFU/ml wound fluid. Except for day 11 (6.7 × 102 CFU/ml wound fluid), 2% mupirocin gradually kept infection levels (P ≤ 0.05, 2% mupirocin versus 0.1% moxifloxacin, 0.1% linezolid, and 0.1% mupirocin) below even the detection limit. From day 4 onwards, bacterial reductions of 98.26 (day 7) to 99.8% (day 9) for 0.1% moxifloxacin, 96.78 (day 2) to 99.81% (day 9) for 0.1% linezolid, and 97.52 (day 2) to 99.88% (day 4) for 0.1% mupirocin were determined, compared to the control (P ≤ 0.05, 0.1% moxifloxacin, 0.1% linezolid, and 0.1% mupirocin versus the carrier control) (Fig. 3A). Tissue samples were taken on day 14 after the treatment start. The carrier control showed the highest bacterial counts (5.4 × 105 CFU/g tissue). Bacterial counts were significantly reduced within the 0.1% moxifloxacin (1.4 × 104 CFU/g tissue; P = 0.012) and 2% mupirocin (1.8 × 103 CFU/g tissue; P = 0.047) groups compared to the carrier control. However, chambers with 0.1% moxifloxacin and 2% mupirocin, as well as 0.1% linezolid (1.1 × 104 CFU/g tissue) and 0.1% mupirocin (4.4 × 104 CFU/g tissue), stayed below 1 × 105 CFU/g tissue (Fig. 3B). The overall moxifloxacin content was determined for each sample of collected wound fluid using an LC-MS/MS analyzer system. The values obtained for the control group were between 101.8 μg/liter at day 2 and the detection limit of 10 μg/liter. As expected, the values for the moxifloxacin-treated wounds were significantly higher and ranged nearly constantly at a level between 10 (day 7) and 29 mg/liter (day 9). This correlates to 3.2- to 9.3-fold the MIC of 3.13 mg/liter (Fig. 3C). At the therapy start, the value for all wounds showed background levels. However, no moxifloxacin was detectable within the porcine tissue biopsy samples. All serum samples taken throughout this study were negative for moxifloxacin (data not shown).
Fig. 3.
Bacterial counts of MRSA-infected porcine wounds. (A) The time-dependent change of bacterial counts within the sampled wound fluids is shown. All treatment groups showed reduced bacterial levels over time that remained beneath the infection level during the complete follow-up. Complete bacterial clearance was seen only when 2% mupirocin was applied to the wounds. Bacterial counts were significantly reduced in all treatment groups compared to carrier control. *, P < 0.05, 0.1% moxifloxacin, 0.1% linezolid, 0.1% mupirocin, and 2% mupirocin versus carrier control; #, P < 0.05, 2% mupirocin versus 0.1% moxifloxacin, 0.1% linezolid, or 0.1% mupirocin. Endpoint measurements of MRSA tissue counts are shown (B). Therefore, punch biopsy specimens were taken, homogenized, and serially plated 14 days after treatment start. Bacterial counts were significantly reduced only after 0.1% moxifloxacin or 2% mupirocin treatment, while only 2% mupirocin showed a partial remission of bacterial counts (values below detection limit of 102). All treatment groups demonstrated bacterial reduction below infection level (105 CFU/g of tissue). *, P < 0.05, 0.1% moxifloxacin and 2% mupirocin versus carrier control. (C) The ratio between the bacterial counts within the wound fluid and the corresponding amount of moxifloxacin, which was detected by LC-MS/MS analysis, for the complete follow-up of 14 days. These correlations discriminate the treatment group from the control group and demonstrate the relation between moxifloxacin concentration and bacterial reduction. The values are shown as mean ± SEM. The moxifloxacin concentration at the level of the x axis indicates the detection limit of the measurement.
Murine burn wound infection. (i) Pseudomonas aeruginosa.
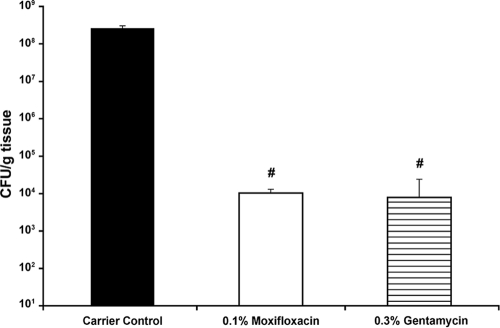
To test the efficacy in Pseudomonas aeruginosa-infected wounds, test animals were sacrificed on day 7 after treatment with either 0.1% moxifloxacin or 0.3% gentamicin. The carrier control group showed the highest bacterial counts up to 2.6 × 108 CFU/g tissue. Bacterial counts were significantly reduced within the 0.1% moxifloxacin group (1 × 104 CFU/g tissue) and the 0.3% gentamicin group (7.8 × 103 CFU/g tissue) (P ≤ 0.001, 0.1% moxifloxacin and 0.3% gentamicin versus the carrier control) (Fig. 4).
Fig. 4.
Treatment of Pseudomonas aeruginosa-infected burn wounds. Graph shows the bacterial reduction in tissue counts of Pseudomonas aeruginosa-infected rat burn wounds on day 7 after treatment start. Bacterial counts were significantly reduced within both treatment groups and remained below the infection level (105 CFU/g of tissue), while carrier control tissue demonstrated massive infection levels. #, P < 0.001, 0.1% moxifloxacin and 0.3% gentamicin versus carrier control. The values are shown as mean ± SEM.
(ii) MRSA.
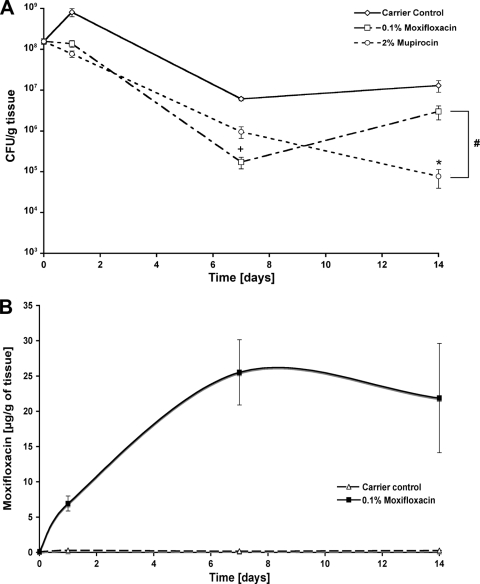
Twenty-four hours after the first treatment, tissue biopsy specimens were obtained to test the short-time efficacy of the different, topically applied antibiotics. The carrier control group (n = 10) showed the highest bacterial counts (with 8 × 108 CFU/g tissue). With 0.1% moxifloxacin (1.3 × 108 CFU/g tissue) and 0.1% mupirocin (7.7 × 107 CFU/g tissue), bacterial counts were significantly reduced (P ≤ 0.001, 0.1% moxifloxacin and 0.1% mupirocin versus the carrier control). With 0.1% linezolid, bacterial counts were significantly reduced down to 2.7 × 108 CFU/g tissue (P ≤ 0.05, 0.1% linezolid versus carrier control). In order to test the long-term efficacy, tissue biopsy specimens were obtained at different time points. On day 2 postinfection, 10 wounds were analyzed, and the initial infection was quantified to be 1.5 × 108 CFU/g of tissue. For the 24-h time point, the values were used as described in the legend to Fig. 5A. On day 7, bacterial counts decreased within the carrier control group (6 × 106 CFU/g tissue). Also, 0.1% moxifloxacin (1.7 × 105 CFU/g tissue) was more efficient than 2% mupirocin (6.9 × 105 CFU/g tissue), On day 14, however, 2% mupirocin (7.6 × 104 CFU/g tissue) reduced the CFU count further, whereas the values of the 0.1% moxifloxacin group (2.9 × 106 CFU/g tissue) were increasing again. Over the entire time course, 0.1% moxifloxacin and 2% mupirocin significantly reduced bacterial counts (P ≤ 0.001, 0.1% moxifloxacin and 2% mupirocin versus carrier control) (Fig. 5A). The final concentration of moxifloxacin within the burn wound tissue ranged between 6.93 μg/g of tissue at day 1 and 25.51 or 21.88 μg/g of tissue at day 7 or 14, respectively. The values for the carrier control group remained at background level during the complete experiment. Additionally, no moxifloxacin was detectable in the serum of either the moxifloxacin or the carrier control group. The ratio between remaining bacterial counts and the amount of moxifloxacin, which was measured in the tissue, showed levels comparable to those of the wound fluid samples from the porcine model described in the legend to Fig. 3B (Fig. 5B).
Fig. 5.
MRSA reduction in infected burn wounds. Biopsy specimens were obtained at 24 h and 7 and 14 days after the first treatment. (A) Bacterial counts were significantly reduced within all treatment groups, whereas the use of mupirocin was most efficient. All tissues remained at infected levels except the mupirocin-treated sample at day 14, which reached the noninfected borderline. #, P < 0.001, 0.1% moxifloxacin and 2% mupirocin versus carrier control; +, P < 0.05, 0.1% moxifloxacin versus 2% mupirocin at day 7; *, P < 0.01, 2% mupirocin versus 0.1% moxifloxacin at day 14. The ratio between the bacterial counts of the tissue and the corresponding moxifloxacin amount (B) is shown, with values comparable to those seen for the porcine wound fluids. The values are shown as mean ± SEM.
Histological analysis.
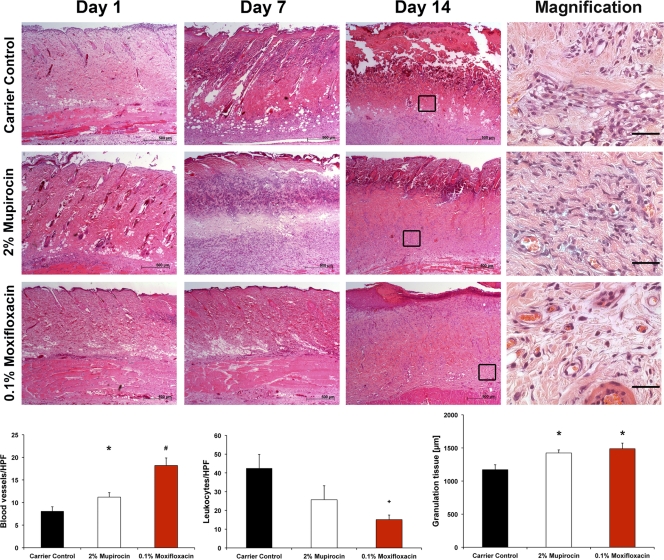
Histological analysis confirmed a second-degree (2°) burn in all groups after a scald burn (carrier control, 0.1% moxifloxacin, and 2% mupirocin). All slides showed a loss of the entire epidermis and inflammation of the dermis, all the way down to the subcutaneous connective tissue. Necrotic tissue and inflammation, as characterized by neutrophil infiltration, progressed in intensity and were most evident on the final day. On day 14, edema and an extensive necrosis within the deep muscular and dermal layers could be seen. According to our set criteria as mentioned above, treatment with 0.1% moxifloxacin showed reduced leukocyte infiltration (15.1/HPF), increased blood vessel counts (18.2/HPF) per 10 HPF, and wide granulation tissue (1,488 μm) with re-epithelization compared to the carrier control (42.5 leukocytes, 8.1 blood vessels, and 1,172 μm granulation tissue). The entire gamut of slides showed comparable scores in all criteria and can therefore be seen as significant in reduced inflammation and necrosis. In the 2% mupirocin group, deep and superficial inflammation was reduced but remains, however, more intense (25.7 leukocytes/HPF), while the number of blood vessels (11.2 blood vessels/HPF) was significantly (P = 0.0057) decreased compared to that of the 0.1% moxifloxacin group (Fig. 6).
Fig. 6.
Histological examinations of burn wounds. Cross-sectional biopsy specimens were obtained on days 1, 7, and 14 from MRSA-infected rat burn wounds, and H&E staining was performed (50-fold magnification). A strong tissue degeneration, which demonstrates a 2° superficial burn, was observed 24 h postburn. At day 7, the tissue started to regenerate only within the treatment groups, whereas the tissue of the control group showed ongoing degeneration. The corresponding graphs (bottom) demonstrate the number of blood vessels or leukocytes per HPF, or the width of the granulation tissue. Finally, distinct re-epithelialization was observed only for wounds treated with 0.1% moxifloxacin, while neither mupirocin- nor carrier control-treated wounds showed comparable results. The scale bar is illustrated at the bottom right of each image. Scale bars equal 500 μm for the overview images or 50 μm for the magnifications on the right. *, P ≤ 0.05, treatment versus control; #, P ≤ 0.01, 0.1% moxifloxacin versus control; +, P ≤ 0.05, 0.1% moxifloxacin versus both other groups.
DISCUSSION
Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus infection constitute a major therapeutic challenge in the management of burn wounds and chronic wounds. A study performed by Tammelin et al., which included 656 patients with chronic wounds, showed that more than 25% received antibiotics at some time. Approximately 60% of those had been treated systemically with antibiotics within an earlier 6-month period (26).
However, currently used systemic antibiotics are often ineffective due to reduced tissue availability, most often due to the limited peripheral blood supply found in patients with chronic wounds. Additionally, the systemic application of antibiotics to treat peripheral wound infection must be critically examined, considering the high dose that needs to be administered and the resulting risks for adverse events.
Thus, topically administered antibiotics are a helpful adjunct in the treatment of wound infection and are indicated for wounds with necrotic tissue, decreased blood supply, or granulation tissue formation (8). Topical agents may be more appealing since systemic side effects, like nephropathy, allergic reactions, and disturbances within the intestinal flora may be avoided. In addition, application of the antibiotic agent directly to the infected wound site results in higher local concentration, bypassing the necessity for sufficient vascularization. If a wound is not severely infected, so that a systemic administration would not be necessarily indicated, smaller local doses should be used in order to prevent systemic infection or sepsis. In this case the organism does not need to be flooded with high concentrations of antibiotics because smaller local doses will achieve the same effect.
Clinical studies investigating the efficiency of systemically delivered moxifloxacin for the treatment of skin or soft tissue infections demonstrated clinical cure rates between 79 and 90% (10, 21). Therefore, the daily dose for a systemic application of moxifloxacin added up to 400 mg, while the final amount of moxifloxacin used in our study was only 0.5 mg per wound per day, which aggregates to a maximum load of 2 mg moxifloxacin per animal and day. During the time course of at least 14 days, including 6 applications, the total need of antibiotics was very low compared to a systemic application. Desrosiers et al. demonstrated by an in vitro study that elevated concentrations of moxifloxacin (0.1 to 0.2 mg/ml) are able to reduce viable bacteria by 2 to 2.5 log scales of biofilm-forming Staphylococcus aureus or Pseudomonas aeruginosa (5). However, the drug amount used in our study should also be sufficient to eliminate biofilm-forming bacteria.
The concerted use of topical antimicrobial agents in the treatment of chronic and burn wound infection is of major importance for a successful therapy. Therefore, our main topic was the analysis of the efficacy of topically administered moxifloxacin against MRSA. In this context we used moxifloxacin in comparison to linezolid as a systemically used antibiotic and mupirocin as a topically used antibiotic against MRSA. All antibiotics were diluted in the same concentration with the same standard gel formulation to ensure high reproducibility. Additionally we used a further setup to verify the efficacy of moxifloxacin in comparison to that of a commercially available topical ointment; wherefore, 2% mupirocin ointment was used. While moxifloxacin has traditionally been used against Gram-negative bacteria, we also used Pseudomonas aeruginosa within the same models to demonstrate its broad antimicrobial activity and also compared it to a commercially available ointment containing 0.3% gentamicin.
The bacteria used were tested for susceptibility to the antibiotics used, and the respective MICs and MBCs were determined. All strains were susceptible to the antibiotics used, and the measured values for moxifloxacin are consistent with those in the literature (11).
In this study we were able to show that moxifloxacin has activity comparable to that of clinically used antibiotics, such as mupirocin and linezolid, resulting in an efficient bacterial reduction in MRSA-infected wounds, if the same drug amount and the same conditions are used. While wounds are generally colonized or infected by various pathogens and a pathogen determination is unfortunately not always possible, a broad-spectrum antibiotic should be used for the corresponding treatment. In contrast to mupirocin and linezolid, moxifloxacin has broad-spectrum antibacterial activity. The general concern that topical administration of antibiotics would lead to increased resistance could not be confirmed in our experiment. Bacterial susceptibility to moxifloxacin did not change over the 14 days of therapy in this study.
Within the Pseudomonas aeruginosa trial, we used a commercially available ointment containing 0.3% gentamicin as a positive control to demonstrate clinical relevance. However, gentamicin was highly effective against both bacterial strains used in this study and furthermore was well dissolvable within the standard gel formulation. Therefore, it may be used as a monotherapy or in combination with other antimicrobial drugs, such as, for example, moxifloxacin, to combine their positive properties and to reduce their weaknesses for a standardized topical treatment. This should be explored in further studies.
Compared to gentamicin, moxifloxacin has the advantage of better tissue penetration due to its molecular structure, as it was shown as highly available in peripheral tissues after intravenous administration (11). In this context, the values that were measured for rat burn wound tissue samples showed magnitudes comparable to those observed directly for the porcine wound fluid, indicating good tissue penetration of the antibiotic. The measured concentration of moxifloxacin in porcine wound fluid showed values that were above the MIC but below the MBC, both determined in vitro. This fact might explain the steady state of the corresponding curve visualizing the number of CFU/milliliter of wound fluid after day 2 of treatment. On the other hand, the tissue concentrations of moxifloxacin in the rat model show values that are on the level of the MBC. Given the significantly lower volume of distribution of moxifloxacin in the tissues, the therapeutic concentration of moxifloxacin in the tissues should be significantly higher than that in the wound fluid. Even more surprising is the fact that the average bacterial counts in the tissue of rat burn wounds were significantly higher than those in comparable wound fluids and tissue samples of the pig model. The reason why no moxifloxacin could be detected in the porcine tissue samples may be associated with the method of sample collection, because punch biopsy specimens were taken in pigs with greater tissue depth than that in the rats, which negatively influenced the surface-to-volume ratio of the sample. This also shows that the penetration behavior of moxifloxacin in the wound tissue needs to be further explored.
The antimicrobial activity of topically delivered moxifloxacin against MRSA and Pseudomonas aeruginosa wound infection was investigated using two different animal models. We used a porcine wound infection model, because pig skin resembles human skin anatomically and physiologically (29) and porcine wound healing has been found to be significantly similar to that in humans (28). The porcine BO-chamber model prevented contraction and cross-contamination and therefore simulated separate chronic wound conditions (24). On the other hand, we decided that the rat burn infection model would be a favorable study design, because MRSA and Pseudomonas aeruginosa infection tend to affect patients with burn wounds, whose local innate immune system is impaired due to the loss of the protective barrier function of the epithelia (13). On the resulting H&E stainings of the tissue, moxifloxacin clearly showed better wound healing results than those of the mupirocin- or carrier control-treated wounds. This improved efficiency could not be explained alone by the reduction of bacterial counts, as the progress of wound healing was significantly poorer in wounds treated with mupirocin, which showed the same or even slightly lower CFU values. In this context, moxifloxacin, like other fluoroquinolones, was hypothesized to reduce the production of proinflammatory cytokines, like interleukin 8 (IL-8), tumor necrosis factor alpha (TNF-α), or IL-1β, in activated monocytes or the accumulation of cytokines in activated leukocytes (2, 4, 30). This may reduce inflammation and promote tissue regeneration, which could explain the observed results.
The results of the in vivo study demonstrated that the use of moxifloxacin is beneficial for the topical treatment of infected wounds, because of a significant reduction in Gram-positive or -negative bacteria and an acceleration of the wound repair process. There was no change observed in the susceptibility of the bacteria to moxifloxacin between the beginning and the end of the therapy. However, this aspect, together with the tissue penetration and wound healing properties of moxifloxacin, should be investigated in greater detail in further studies.
ACKNOWLEDGMENTS
We sincerely thank Andrea Rittig and Susanne Friedrich for expert technical assistance.
This work was in part financially supported by the German Research Foundation (DFG, Ste-1099) and by Bayer Innovation GmbH.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Boucher H. W., Corey G. R. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 2. Choi J. H., et al. 2003. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 47:3704–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CLSI. 2006. Performance standards for antimicrobial disk susceptibility tests, 9th ed. Approved standard M2-A9. CLSI, Wayne, PA [Google Scholar]
- 4. Dalhoff A. 2005. Immunomodulatory activities of fluoroquinolones. Infection 33(Suppl. 2):55–70 [DOI] [PubMed] [Google Scholar]
- 5. Desrosiers M., Bendouah Z., Barbeau J. 2007. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am. J. Rhinol. 21:149–153 [DOI] [PubMed] [Google Scholar]
- 6. Drlica K., Zhao X. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fawley W. N., Parnell P., Hall J., Wilcox M. H. 2006. Surveillance for mupirocin resistance following introduction of routine peri-operative prophylaxis with nasal mupirocin. J. Hosp. Infect. 62:327–332 [DOI] [PubMed] [Google Scholar]
- 8. Franz M. G., et al. 2008. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 16:723–748 [DOI] [PubMed] [Google Scholar]
- 9. Garau J., Bouza E., Chastre J., Gudiol F., Harbarth S. 2009. Management of methicillin-resistant Staphylococcus aureus infections. Clin. Microbiol. Infect. 15:125–136 [DOI] [PubMed] [Google Scholar]
- 10. Giordano P., Song J., Pertel P., Herrington J., Kowalsky S. 2005. Sequential intravenous/oral moxifloxacin versus intravenous piperacillin-tazobactam followed by oral amoxicillin-clavulanate for the treatment of complicated skin and skin structure infection. Int. J. Antimicrob. Agents 26:357–365 [DOI] [PubMed] [Google Scholar]
- 11. Guay D. R. 2006. Moxifloxacin in the treatment of skin and skin structure infections. Ther. Clin. Risk Manag. 2:417–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirsch T., et al. 2008. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobsen F., et al. 2005. Activity of histone H1.2 in infected burn wounds. J. Antimicrob. Chemother. 55:735–741 [DOI] [PubMed] [Google Scholar]
- 14. Jacobsen F., et al. 2005. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 12:1494–1502 [DOI] [PubMed] [Google Scholar]
- 15. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 16. Lentino J. R., Narita M., Yu V. L. 2008. New antimicrobial agents as therapy for resistant gram-positive cocci. Eur. J. Clin. Microbiol. Infect. Dis. 27:3–15 [DOI] [PubMed] [Google Scholar]
- 17. McConeghy K. W., Mikolich D. J., LaPlante K. L. 2009. Agents for the decolonization of methicillin-resistant Staphylococcus aureus. Pharmacotherapy 29:263–280 [DOI] [PubMed] [Google Scholar]
- 18. Neely A. N., et al. 2009. Are topical antimicrobials effective against bacteria that are highly resistant to systemic antibiotics? J. Burn Care Res. 30:19–29 [DOI] [PubMed] [Google Scholar]
- 19. Norrby S. R., Nord C. E., Finch R. 2005. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 5:115–119 [DOI] [PubMed] [Google Scholar]
- 20. O'Meara S., et al. 2006. Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabet. Med. 23:341–347 [DOI] [PubMed] [Google Scholar]
- 21. Parish L. C., et al. 2000. Moxifloxacin versus cephalexin in the treatment of uncomplicated skin infections. Int. J. Clin. Pract. 54:497–503 [PubMed] [Google Scholar]
- 22. Robson M. C., Mannari R. J., Smith P. D., Payne W. G. 1999. Maintenance of wound bacterial balance. Am. J. Surg. 178:399–402 [DOI] [PubMed] [Google Scholar]
- 23. Steinstraesser L., et al. 2002. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob. Agents Chemother. 46:1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steinstraesser L., et al. 2006. A novel titanium wound chamber for the study of wound infections in pigs. Comp. Med. 56:279–285 [PubMed] [Google Scholar]
- 25. Stryjewski M. E., Corey G. R. 2009. New treatments for methicillin-resistant Staphylococcus aureus. Curr. Opin. Crit. Care 15:403–412 [DOI] [PubMed] [Google Scholar]
- 26. Tammelin A., Lindholm C., Hambraeus A. 1998. Chronic ulcers and antibiotic treatment. J. Wound Care 7:435–437 [DOI] [PubMed] [Google Scholar]
- 27. Tuberculosis. 2008. Moxifloxacin. Tuberculosis 88:127–131 [DOI] [PubMed] [Google Scholar]
- 28. Vardaxis N. J., Brans T. A., Boon M. E., Kreis R. W., Marres L. M. 1997. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J. Anat. 190(4):601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J. F., Olson M. E., Reno C. R., Wright J. B., Hart D. A. 2001. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp. Med. 51:341–348 [PubMed] [Google Scholar]
- 30. Weiss T., et al. 2004. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob. Agents Chemother. 48:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]