Abstract
Small-colony variants (SCVs) often are associated with chronic Staphylococcus aureus infections, such as those encountered by cystic fibrosis (CF) patients. We report here that tomatidine, the aglycon form of the plant secondary metabolite tomatine, has a potent growth inhibitory activity against SCVs (MIC of 0.12 μg/ml), whereas the growth of normal S. aureus strains was not significantly altered by tomatidine (MIC, >16 μg/ml). The specific action of tomatidine was bacteriostatic for SCVs and was clearly associated with their dysfunctional electron transport system, as the presence of the electron transport inhibitor 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) caused normal S. aureus strains to become susceptible to tomatidine. Inversely, the complementation of SCVs' respiratory deficiency conferred resistance to tomatidine. Tomatidine provoked a general reduction of macromolecular biosynthesis but more specifically affected the incorporation of radiolabeled leucine in proteins of HQNO-treated S. aureus at a concentration corresponding to the MIC against SCVs. Furthermore, tomatidine inhibited the intracellular replication of a clinical SCV in polarized CF-like epithelial cells. Our results suggest that tomatidine eventually will find some use in combination therapy with other traditional antibiotics to eliminate persistent forms of S. aureus.
INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen that can affect several hosts, organs, and body sites, and it may become both life-threatening and chronic (2, 14). The ability of S. aureus to cause chronic infections is thought to be helped by the formation of biofilms and the persistence of the bacterium within nonphagocytic host cells, which may offer protection against both the host immune system and the action of antibiotics (1, 7, 12, 44).
Small-colony variants (SCVs) are known to form biofilms (27, 29, 42, 43) and to persist within nonphagocytic host cells (39). SCVs have a dysfunctional oxidative metabolism causing an alteration in the expression of virulence factors, a slow growth, and a loss of colony pigmentation (33). This dysfunctional oxidative metabolism was associated with a decreased susceptibility to aminoglycosides, because these antibiotics require the proton-motive force in order to penetrate the bacterium (8). This respiratory deficiency often is caused by mutations affecting the electron transport system, and several SCV isolates are auxotrophs for either hemin or menadione, which are needed to synthesize electron transport system components. SCVs often are isolated from chronic infections, as in the case of lung infections in cystic fibrosis (CF) patients but also from osteomyelitis, septic arthritis, bovine mastitis, and the colonization of orthopedic devices (3, 30, 33). It was shown recently that switching from the normal to the SCV phenotype and then back to the normal phenotype in vivo is an integral part of the pathogenesis of S. aureus, a phenomenon that may be involved in the establishment of chronic infections (46).
Nosocomial and community-acquired infections caused by bacteria that are resistant to antibiotics represent an increasingly important public health concern. One of the reasons explaining the spread of antibiotic resistance resides in the fact that the current arsenal of antibiotics was designed largely on limited chemical scaffolds with only a few innovations since the 1980s, leaving an opportunity for pathogens to develop and spread mechanisms of resistance worldwide (40, 45). There now are numerous reports of plant products that provide antibiotic activities against a wide variety of pathogenic bacteria (10, 15, 34).
Several species of plants accumulate sterol and triterpene antimicrobials termed saponins (31). These molecules are constitutively produced in the plant and play an important role in the host defense against pathogenic insects and microbes.
Among these saponins, tomatine is a steroidal glycoalkaloid that presents some antimicrobial action against yeast and a variety of microbes (4, 11, 31, 37). Many tomato fungal pathogens produce extracellular enzymes, commonly referred to as tomatinases, that are able to detoxify the α-tomatine (23, 35, 36). As an example, Fusarium oxysporum f. sp. lycopersici was reported to cleave α-tomatine into its aglycon (tomatidine) and tetrasaccharide moieties (lycotetraose), the by-products of which have little to no antifungal activity against the pathogen (36, 41). Some of our recent work on bacteria exposed to plant products shed light on the bioactivity of tomatidine against S. aureus and suggested a possible application for this molecule as a virulence attenuator for typical S. aureus strains (6) and, more recently, for SCVs (28). The chemical structures of tomatine and tomatidine are shown in Fig. 1.
Fig. 1.
Structures of tomatidine (TO) and tomatine (TN). For TO, R = H. For TN, R = lycotetraose.
The aim of this study was to evaluate the antibacterial activity of tomatidine against S. aureus SCVs and to investigate its mode of action.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Staphylococcus aureus ATCC 29213, Newbould (ATCC 29740), and cystic fibrosis isolates CF07-L and CF1A-L were used as representatives of the normal phenotype, whereas the strains NewbouldΔhemB and cystic fibrosis isolates CF07-S and CF1D-S were used as SCV representatives. NewbouldΔhemB was generated from Newbould by disrupting the hemB gene with the ermA cassette by homologous recombination (7). CF07-L/CF07-S and CF1A-L/CF1D-S are genetically related pairs of strains coisolated from cystic fibrosis patients (29, 30). The dysfunctional oxidative metabolism of NewbouldΔhemB and CF07-S can be complemented by hemin and menadione, respectively (29). Except where otherwise stated, bacteria were grown in brain heart infusion (BHI) broth (BD, Mississauga, Ontario, Canada).
Chemical reagents and antibiotics.
Menadione (Sigma, Oakville, Ontario, Canada) was solubilized in dimethylsulfoxide (DMSO) at a concentration of 10 mg/ml and used at 1 μg/ml, whereas hemin (Sigma) was solubilized in 1.4 M NH4OH at a concentration of 10 mg/ml and used at 5 μg/ml. The electron transport inhibitor 4-hydroxy-2-heptylquinoline-N-oxide (HQNO; Axxora, San Diego, CA) was solubilized in DMSO at a concentration of 5 mg/ml and used at 20 μg/ml. Tomatidine, gentamicin, vancomycin, erythromycin, ciprofloxacin, oxacillin, rifampin, and norfloxacin were from Sigma, whereas tomatine and chloramphenicol were from ICN Biomedicals (Irvine, CA) and Fisher (Ottawa, Ontario, Canada), respectively. Tomatine and tomatidine were solubilized at 10 and 2 mg/ml in DMSO, respectively. Gentamicin, vancomycin, ciprofloxacin, oxacillin, and norfloxacin were solubilized in water at 10 mg/ml. NaOH solutions at 2.5 and 0.1 N were used during the solubilization of ciprofloxacin and norfloxacin, respectively. Erythromycin and chloramphenicol were solubilized at 10 mg/ml in 1:1 water/ethanol. Rifampin was solubilized at 10 mg/ml in methanol.
Antibiotic susceptibility testing.
MICs were determined by a broth microdilution technique by following the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (9), except that the incubation period was extended to 48 h and the medium used was BHI to allow SCVs to reach maximal growth as previously described (3, 29). We confirmed that the MICs obtained against ATCC 29213 for all antibiotics tested were similar in BHI and in cation-adjusted Mueller-Hinton broth (CAMHB) (BD) to ensure that the use of BHI did not influence results. The MICs of the control antibiotics used in the macromolecular synthesis assays were determined in CAMHB.
Time-kill experiments.
Time-kill experiments were performed to determine whether the effect of compounds was bacteriostatic or bactericidal. Bacteria were inoculated at ∼5 × 105 CFU/ml in BHI in the absence or presence of antibiotics at the specified concentrations (see the figure legends). At several time points at 35°C (225 rpm), bacteria were sampled, serially diluted, and plated on tryptic soy agar (TSA) for CFU determinations. Plates were incubated for 24 or 48 h at 35°C for normal and SCV strains, respectively.
Macromolecular biosynthesis assays.
The complete defined medium (CDM) was used for macromolecular biosynthesis assays. CDM was constituted of the following chemicals per liter: 5 g glucose, 50 mg MgSO4, 7 g K2HPO4, 2 g KH2PO4, 0.5 g of Na-citrate dihydrate, 1 g (NH4)2SO4, 1 mg thiamine, 1.2 mg niacin, 0.25 mg calcium pantothenate, 0.005 mg of biotin, 10 mg of l-tryptophan, 5 mg adenine, 5 mg guanine, 5 mg cytosine, 5 mg uracil, 100 mg l-glutamic acid, 90 mg l-aspartic acid, 80 mg l-proline, 50 mg l-arginine, 50 g glycine, 50 mg l-lysine, 60 mg l-alanine, 30 mg l-serine, 20 mg l-cysteine, 10 mg l-methionine, 50 mg l-tyrosine, 40 mg l-phenylalanine, 20 mg l-histidine, 30 mg l-threonine, 30 mg l-isoleucine, 80 mg l-valine, 90 mg l-leucine, and 20 mg thymine. The medium CDM-LEU had 22.5 mg/liter of l-leucine instead of 90 mg/liter, whereas the medium CDM-ALA had 15 mg/liter of l-alanine instead of 60 mg/liter.
Protein, DNA, RNA, and cell wall peptidoglycan biosynthesis were evaluated by measuring the incorporation of the appropriate radiolabeled precursors into bacteria prior to treatment with trichloroacetic acid (TCA). Inocula were prepared by incubating bacteria overnight at 35°C (225 rpm) in the CDM medium. Cultures then were adjusted to an absorbance at 600 nm (A600) of 0.1 and grown until an A600 of 0.3 in CDM, CDM-LEU (protein), or CDM-ALA (cell wall) was achieved. Three μCi/ml [3H]leucine, 1 μCi/ml [3H]thymine, 1μCi/ml [3H]uridine, or 2μCi/ml [3H]d-alanine was added to aliquots of cultures in the presence of the different antimicrobial compounds to evaluate protein, DNA, RNA, or cell wall peptidoglycan synthesis, respectively. The incorporation of [3H] molecules into macromolecules was allowed for 45 min for the protein and cell wall assays and for 35 min for the DNA and RNA assays. Cold 10% TCA was added to all samples to stop the incorporation and precipitate macromolecules for 1 h on ice. All samples were filtered through a glass microfiber filter (GE Healthcare Biosciences, Piscataway, NJ) by using a dot blot filtration system. Each filter was washed with 100 μl of 10% TCA containing 1.5 M NaCl and 100 μl of 10% TCA. Filters were dried overnight, and their radioactivity was measured in a liquid scintillation counter. MICs of the control antibiotics chloramphenicol (protein), norfloxacin (DNA), rifampin (RNA), and vancomycin (cell wall) against S. aureus ATCC 29213 were 8 to 16, 1, 0.008 to 0.015, and 0.5 to 1 μg/ml, respectively.
Cell culture.
The CF-like human airway epithelial cells shCFTR (32), derived from the Calu-3 cell line (ATCC HTB 55), were cultured in Eagle's minimum essential medium (EMEM) supplemented with 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2.5 μg/ml fungizone, and 10% fetal bovine serum (FBS) at 37°C in 5% CO2. For routine culture, 4 μg/ml of puromycin was added to culture media. All cell culture reagents were purchased from Wisent (St-Bruno, Quebec, Canada).
Cell infection assays.
Cell infection assays were performed as previously described, with a few adaptations for the Transwell system (26, 30). Cells were seeded at 2.5 × 105 cells/insert on 12-well Transwell plates and cultured for 9 to 10 days in an air-liquid system. The complete medium in the basal compartment was replaced by the invasion medium (1% FBS and no antibiotics) 18 h before assays. Inocula were prepared by suspending bacteria grown for 20 h on BHI agar in ice-cold phosphate-buffered saline (PBS). Bacteria then were washed three times in ice-cold PBS and suspended in the invasion medium supplemented with 0.5% bovine serum albumin (BSA) at a density of approximately 4 × 108 CFU/ml. Cells were washed twice with PBS, and 250 μl of bacterial suspension was added apically to each insert. Invasion was allowed for 3 h, and inserts were emptied and washed three times with PBS. Invasion medium supplemented with 20 μg/ml of lysostaphin (Sigma) then was added to kill extracellular bacteria, and the cells were further incubated for 24 or 48 h in the presence of lysostaphin. DMSO or the different concentrations of tomatidine were added after invasion. At 24 h postinternalization for the 48-h assays, cells were washed once with PBS and the invasion medium supplemented with lysostaphin, DMSO, and/or tomatidine was replaced. Fresh invasion medium supplemented with lysostaphin also was added 1 h before cell lysis to ensure that only intracellular bacteria were counted. Following three washes with PBS, cells were detached with 100 μl of trypsin 0.25% and lysed for 10 min by the addition of 400 μl of water containing 0.05% Triton X-100. Lysates were serially diluted 10-fold and plated on agar for CFU determination. Plates were incubated for 24 or 48 h at 35°C for normal and SCV strains, respectively.
Fluorescence microscopy.
Cells on inserts were fixed for 120 min in 4% paraformaldehyde in PBS. They then were permeabilized using a 5-min treatment with 50% methanol at −20°C, followed by a 10-min incubation at room temperature in PBS supplemented with 50 mM glycine, 0.06% saponin from quillaja bark (Sigma), 0.06% Tween 20, 0.5% NP-40, and 0.5% Triton X-100 (PBSP). The permeabilized cells then were incubated for 30 min with Image-iT FX signal enhancer (Invitrogen, Burlington, Ontario, Canada). S. aureus was probed with the rabbit antibody AB20920 (1:250; Abcam), and F actin was stained with Alexa Fluor 488 phalloidin (1:20; Invitrogen) in PBS with 2% normal goat serum, 2% BSA, and 0.45% fish gelatin (PBSB) for 180 min at room temperature. After five washes in PBS with 0.01% saponin and 0.01% Tween 20 (PBSD), primary antibodies were detected by an incubation of 90 min at room temperature with Alexa Fluor 555 goat anti-rabbit IgG (Invitrogen) at a dilution of 1:1,000 in PBSB. The DNA was stained for 10 min with Hoechst (Invitrogen) at 1:5,000 after five washes in PBSD and five washes in water. Inserts were washed five additional times in water and were mounted in Prolong Gold anti-fade reagent (Invitrogen). Pictures were taken using an Olympus Fluoview FV 300 confocal system or a Zeiss microscope with the Apotome system.
Effect of the combination of tomatidine and gentamicin.
Bacteria were inoculated at ∼5 × 105 CFU/ml in BHI in the absence or presence of gentamicin and/or tomatidine at 4 and 0.12 μg/ml, respectively. Cultures were incubated for 48 h at 35°C and 225 rpm, and pictures were taken. The A600 of the different cultures also were measured.
Statistical analysis.
Statistical analyses were carried out with the GraphPad Prism software (v.5.00). Statistical tests used for the analysis of each experiment are specified in the figure legends.
RESULTS
Tomatidine is a potent inhibitor of SCVs.
Our previous works showed that although tomatidine (TO) (Fig. 1) alters the expression of some virulence factors in normal S. aureus strains, it allows the growth of those bacteria at concentrations of up to 128 μg/ml (6). After having observed that tomatidine also had the ability to inhibit the production of biofilms by S. aureus SCVs (28), we were interested in determining whether this result was attributable to a direct antimicrobial effect of tomatidine on SCVs. Table 1 shows the MICs of tomatidine, tomatine, and control antibiotics (gentamicin, vancomycin, erythromycin, ciprofloxacin, and oxacillin) against normal (ATCC 29213, Newbould, CF07-L, and CF1A-L) and SCV (NewbouldΔhemB, CF07-S, and CF1D-S) strains. Remarkably, the MIC of tomatidine against all SCVs was 0.12 μg/ml, whereas no MIC was measurable for normal strains. Also, no MIC was observed for tomatine, the lycotetraose-substituted derivative of tomatidine, against SCVs. MICs of gentamicin for the different strains were in accordance with the known decreased susceptibility of SCVs to aminoglycosides (33). The MIC of erythromycin against NewbouldΔhemB (>16 μg/ml) is explained by the insertion of ermA in the genome of this strain (see Materials and Methods). MICs obtained for the other control antibiotics were in the expected CLSI ranges and did not seem to vary significantly among strains, except maybe for ciprofloxacin MICs, which were slightly lower for SCV strains. These results demonstrated that tomatidine specifically inhibits the growth of SCV strains.
Table 1.
Susceptibility of normal and SCV S. aureus strains to tomatidine, tomatine, and control antibiotics with or without the presence of HQNO
| Straina | MICb (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| HQNO | TO | TN | GEN | VAN | ERY | CIP | OXA | |
| ATCC 29213 | − | >16 | >16 | 1 | 2 | 0.12–0.25 | 0.5 | 0.12–0.25 |
| + | 0.12–0.25 | >16 | 4 | 2 | 0.25 | 0.25 | 0.12 | |
| Newbould | − | >16 | >16 | 0.5–1 | 1 | 0.25 | 0.25–0.5 | 0.06–0.12 |
| NewbouldΔhemB | − | 0.12 | >16 | 4–8 | 2 | >16 | 0.12–0.25 | 0.03–0.06 |
| CF07-L | − | >16 | >16 | 1–2 | 2 | 0.25 | 0.5 | 0.06–0.12 |
| + | 0.5 | >16 | 4 | 2 | 0.25 | 0.25 | 0.06–0.12 | |
| CF07-S | − | 0.12 | >16 | 8 | 2 | 0.12 | 0.12 | 0.06–0.12 |
| + | 0.12 | >16 | 4–8 | 2 | 0.06–0.12 | 0.12 | 0.06–0.12 | |
| CF1A-L | − | >16 | >16 | 1–2 | 1–2 | 0.25 | 0.5 | 0.25 |
| CF1D-S | − | 0.12 | >16 | 8 | 2 | 0.12 | 0.12 | 0.06–0.12 |
ATCC 29213, Newbould, CF07-L, and CF1A-L are normal strains, whereas NewbouldΔhemB, CF07-S, and CF1D-S are SCVs.
4-Hydroxy-2-heptylquinoline-N-oxide (HQNO) was used at 20 μg/ml. TO, tomatidine; TN, tomatine; GEN, gentamicin; VAN, vancomycin; ERY, erythromycin; CIP, ciprofloxacin; OXA, oxacillin.
Inhibition of the electron transport system by HQNO sensitizes normal strains to tomatidine.
We used 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) to inhibit the electron transport system of normal strains and to generate the SCV phenotype (17, 20, 29). Table 1 shows MICs of tomatidine, tomatine, and control antibiotics (gentamicin, vancomycin, erythromycin, ciprofloxacin, and oxacillin) against the normal strains ATCC 29213 and CF07-L as well as the SCV CF07-S in the presence of 20 μg HQNO/ml. HQNO allowed tomatidine to generate a growth-inhibitory effect against normal strains similar to that observed against SCVs. HQNO did not, however, alter the susceptibility of SCVs to any other antibiotics. HQNO also decreased the susceptibility of normal strains to the aminoglycoside gentamicin, further supporting that the effect of HQNO on normal strains generates the SCV phenotype. Accordingly, subinhibitory concentrations of the proton-motive force uncoupler carbonyl cyanide m-chlorophenylhydrazone also caused ATCC 29213 to become susceptible to the growth-inhibitory activity of tomatidine (data not shown) and decreased the susceptibility to gentamicin as previously reported (25). These results confirm that tomatidine possesses a specific antibacterial activity against SCVs because of their defective electron transport system.
Tomatidine is bacteriostatic against SCVs.
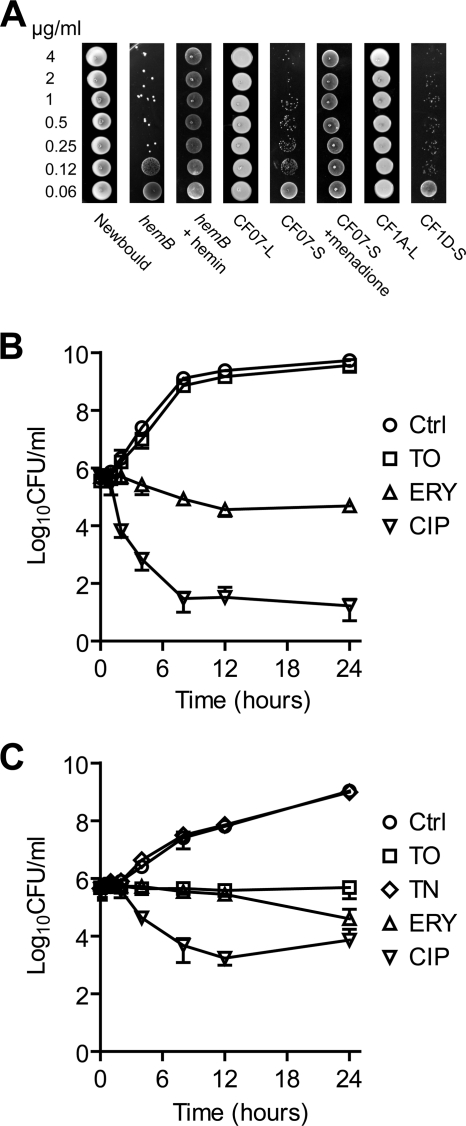
We performed experiments to determine whether tomatidine is bactericidal or bacteriostatic against SCVs. Figure 2A shows that tomatidine at concentrations of up to 4 μg/ml is not able to completely kill SCVs (NewbouldΔhemB, CF07-S, and CF1D-S) in culture but clearly inhibited their growth compared to that of the normal strains Newbould, CF07-L, and CF1A-L. Of note, the susceptibility of the hemin-dependent SCV NewbouldΔhemB and menadione-dependent SCV CF07-S to tomatidine was abolished in the presence of hemin and menadione, respectively (Fig. 2A), which again confirmed that a defective electron transport is required for the antibacterial activity of tomatidine. The antibacterial activities of tomatidine and control antibiotics (erythromycin and ciprofloxacin) against normal and SCV strains as a function of time are presented in Fig. 2B and C, respectively. The antibacterial activity of tomatine against the SCV strain also was evaluated (TN in Fig. 2C). Figure 2C clearly demonstrates that the presence of tomatidine at 0.250 μg/ml (2×MIC) induced bacteriostasis in SCVs, whereas it does not affect the growth of normal strains (Fig. 2B). Overall, our results demonstrate that tomatidine (and not tomatine) has a specific bacteriostatic activity against SCVs.
Fig. 2.
Tomatidine is bacteriostatic against SCVs but not against normal strains. (A) Samples (10 μl) from cultures treated with various concentrations of tomatidine following the antibiotic susceptibility testing procedure (see Materials and Methods) were spotted on agar plates and photographed. Time-kill experiments showing the effect of tomatidine (TO), tomatine (TN), erythromycin (ERY), or ciprofloxacin (CIP) on the growth of the normal strain CF07-L (B) and of the SCV CF07-S (C). Concentrations of 16 μg/ml of TO (n = 3), 0.5 μg/ml of ERY (n = 3), and 1.0 μg/ml of CIP (n = 3) were used against CF07-L, whereas concentrations of 0.25 μg/ml of TO (n = 4), 16 μg/ml of TN (n = 3), 0.25 μg/ml of ERY (n = 3), and 0.5 μg/ml of CIP (n = 2) were used against CF07-S. The no-drug control experiments are from four independent experiments.
Effect of tomatidine on the biosynthesis of macromolecules in HQNO-treated S. aureus.
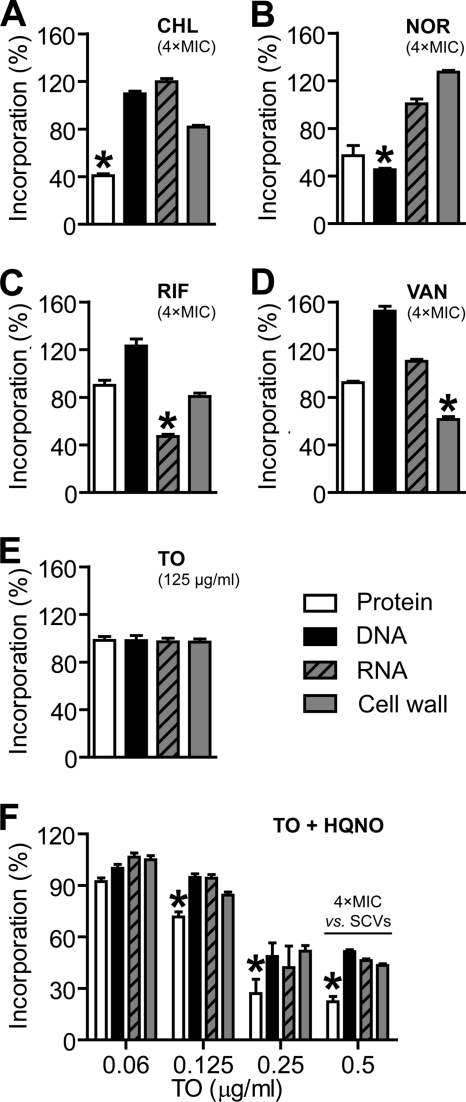
To get insight into the mechanism of action of tomatidine on SCVs, we performed macromolecular biosynthesis assays with the normal strain ATCC 29313 in the presence or absence of 20 μg HQNO/ml. We used HQNO-treated bacteria instead of SCVs, because it was more reliable and allowed a reproducible production of elevated cell densities until the addition of HQNO. Figure 3A to D shows the results for the control antibiotics chloramphenicol, norfloxacin, rifampin, and vancomycin, which are well known to target protein synthesis, DNA replication, RNA transcription, and cell wall peptidoglycan synthesis, respectively. As expected, each of those antibiotics used at 4× MIC preferentially and significantly inhibited the incorporation of radiolabeled precursors into the targeted macromolecules, although the inhibition of other or all macromolecular biosynthesis also occurs at concentrations of >4× MIC, which is consistent with a collapse of cellular functions (data not shown). Tomatidine diluted in DMSO at a concentration of up to 125 μg/ml did not alter the synthesis of any macromolecule in ATCC 29213 compared to that of the DMSO-treated control (Fig. 3E). However, in the presence of 20 μg HQNO/ml, tomatidine decreased the biosynthesis of all macromolecules at concentrations equal to or greater than 0.12 μg/ml (i.e., the MIC recorded against all SCV strains tested) compared to that of the HQNO-treated control (Fig. 3F). In the presence of HQNO, the inhibition of protein synthesis was significantly more affected by tomatidine (P < 0.05) than was the biosynthesis of all other macromolecules (Fig. 3F). This indicates that the primary cellular target of tomatidine is the bacterial protein biosynthesis machinery.
Fig. 3.
Effect of tomatidine on the biosynthesis of macromolecules in HQNO-treated S. aureus. (A to D) The effect of control antibiotics at approximately four times the MIC on the biosynthesis of proteins (chloramphenicol [CHL], 64 μg/ml), DNA (norfloxacin [NOR], 4 μg/ml), RNA (rifampin [RIF], 0.06 μg/ml), and cell wall peptidoglycan synthesis (vancomycin [VAN], 4 μg/ml) was evaluated for strain ATCC 29213. (E) Effect of TO at 125 μg/ml on the biosynthesis of macromolecules in ATCC 29213. (F) Effect of different concentrations of TO on the biosynthesis of macromolecules in ATCC 29213 in the presence of HQNO at 20 μg/ml. Significant decreases of the biosynthesis of one macromolecule compared to that of all three others are indicated (*, P < 0.05 by one-way analysis of variance [ANOVA] with Dunnett's post test for A to E and two-way ANOVA with a Bonferroni's post test for F). Results are from three independent experiments and are expressed as percentages of the incorporation of radiolabeled molecules by untreated (A to D), DMSO-treated (E), or HQNO-treated bacteria (F).
Tomatidine inhibits the replication of a clinical small-colony variant of S. aureus in polarized CF-like airway epithelial cells.
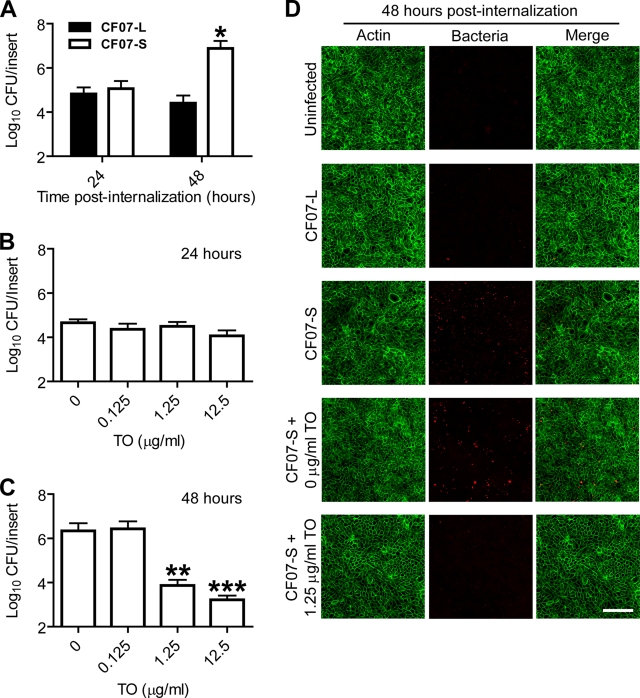
SCVs are known to possess an increased ability to persist within nonphagocytic host cells (39), as confirmed by us for polarized CF-like airway epithelial cells (26). Figure 4A shows that although both strains caused similar levels of infection at 24 h postinternalization, the intracellular load of SCV CF07-S at 48 h postinternalization clearly is larger than that resulting from the normal strain CF07-L. These differences in cellular infection levels are likely to be explained by the ability of SCVs to persist and replicate within epithelial cells.
Fig. 4.
Tomatidine (TO) inhibits the replication of a clinical small-colony variant of S. aureus in polarized CF-like airway epithelial cells. (A) Infection levels of the polarized CF-like airway epithelial cells (the shCFTR cell line) with CF07-L and CF07-S 24 and 48 h postinternalization (from two to three independent experiments performed in duplicate). A significant difference between cells infected with CF07-L and CF07-S 48 h postinternalization is shown (*, P < 0.05 by two-way ANOVA with Bonferroni's post test). (B) Effect of different concentrations of TO on the intracellular load of CF07-S 24 h postinternalization. Data are from two independent experiments performed in duplicate. (C) Effect of different concentrations of TO on the intracellular load of CF07-S 48 h postinternalization. Data are from three independent experiments performed in duplicate. Significant differences compared to results for the control are shown (**, P < 0.01; ***, P < 0.001; one-way analysis of variance with a Dunnett's post test). Data are presented as means with standard deviations. (D) Fluorescence microscopy confirmed the capacity of tomatidine (1.25 μg/ml) to decrease the number of SCVs within cells compared to that with DMSO (0 μg/ml of tomatidine) 48 h postinternalization. S. aureus bacteria and cellular F-actin are stained red and green, respectively. Pictures are from stacked images acquired with a Zeiss microscope with an ApoTome attachment. The scale bar is 100 μm.
We then evaluated the impact of tomatidine on the infection of epithelial cells by SCVs. We speculated that the bacteriostatic agent tomatidine had a greater impact at 48 h postinternalization, since the replication of SCVs is most evident within these cells between 24 and 48 h. Indeed, Fig. 4B demonstrated that tomatidine did not have any effect on the infection of cells by the SCV CF07-S 24 h postinternalization. However, cells treated with 1.25 and 12.5 μg/ml tomatidine contained significantly less SCVs than DMSO-treated cells 48 h postinternalization (Fig. 4C). Figure 4D shows images obtained by fluorescence microscopy that confirmed the capacity of tomatidine to decrease the number of SCVs within cells 48 h postinternalization. Of note, there were no differences in the number of nuclei in each observed area between cells treated for 48 h with DMSO or tomatidine at 12.5 μg/ml (data not shown), suggesting that the effect of tomatidine on the level of infection was not caused by a possible toxicity toward cells. These results demonstrated that tomatidine can significantly decrease the infection of polarized CF-like airway epithelial cells by SCVs.
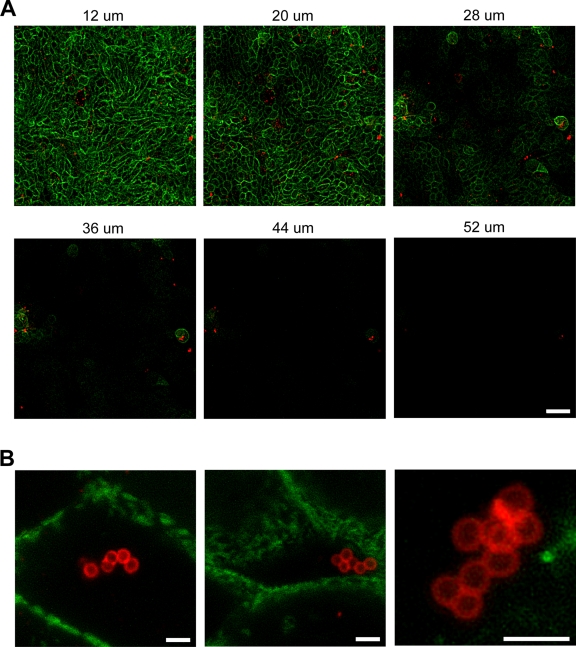
To confirm that the SCV CF07-S still was intracellular 48 h postinternalization, we performed confocal microscopy. Figure 5A shows an example of z-series images of shCFTR cells infected with CF07-S 48 h postinternalization. These images clearly suggest that almost all bacteria are inside the cell layers. Figure 5B shows images acquired at higher magnification and in which bacteria are clearly seen inside cells. Some antigenic materials also were detected at lower levels in the extracellular medium but are likely to represent dead bacteria or bacterial debris resulting from the action of the added lysostaphin. Overall, these results suggest that tomatidine inhibits the intracellular replication of SCVs in polarized CF-like airway epithelial cells.
Fig. 5.
Confocal microscopy of polarized CF-like airway epithelial cells infected by the SCV CF07-S. (A) Z-series images of cells infected with CF07-S 48 h postinternalization. Distances from the bottom of the insert are indicated in microns above each picture. The scale bar is 40 μm. (B) Stacked images of selected layers obtained with confocal microscopy showing S. aureus bacteria. The scale bars are 2 μm. S. aureus bacteria and cellular F-actin are stained red and green, respectively.
Synergistic effect of tomatidine and gentamicin against a heterogeneous S. aureus population composed of both normal and SCV strains.
We think that tomatidine eventually could be used in combination with classical antibiotics during therapies, especially considering that phenotypic switching between the normal and SCV phenotypes seems an integral part of S. aureus pathogenesis (46). We therefore were interested to evaluate if tomatidine could complete the antibacterial effect of the aminoglycoside gentamicin against a bacterial population composed of both normal and SCV strains (Table 2). Gentamicin at 4 μg/ml inhibits the growth of the normal strain CF07-L, whereas tomatidine at 0.12 μg/ml does not. On the other hand, gentamicin at 4 μg/ml does not inhibit the growth of the SCV CF07-S, while tomatidine at 0.12 μg/ml does. Hence, a combination of gentamicin at 4 μg/ml and tomatidine at 0.12 μg/ml inhibits the growth of a heterogeneous population composed of both the normal strain CF07-L and the SCV CF07-S, whereas neither molecule alone can. These results support the hypothesis that tomatidine can be used in combination with classical antibiotics and, more particularly, in the context of chronic infections, where the normal and SCV phenotypes coexist.
Table 2.
Combination of tomatidine and gentamicin inhibits the growth of a mixed population of normal and SCV strains coisolated from a CF patient
| Straina | Growth in the presence of: |
||
|---|---|---|---|
| GEN | TO | GEN+TO | |
| CF07-L | − | + | − |
| CF07-S | + | − | − |
| CF07-L and CF07-S | + | + | − |
CF07-L is a normal strain, whereas CF07-S is an SCV. The presence (+) or the absence (−) of growth is indicated. TO, tomatidine (0.12 μg/ml); GEN, gentamicin (4 μg/ml).
DISCUSSION
There now are numerous examples of plant secondary metabolites active against clinically relevant pathogens, and their use as antimicrobial agents, antibiotic potentiators, or virulence attenuators for the control of infectious diseases is promising (15). Our preliminary work demonstrated that tomatidine has the ability to decrease the hemolytic activity and toxin production of normal S. aureus strains while also affecting the expression of virulence factors and biofilm formation in SCVs (6, 28). SCVs often are isolated from difficult-to-treat chronic infections (3, 30, 33), but no specific therapeutic approach against these variants had been considered to date. The present study demonstrated that tomatidine has a specific growth-inhibitory activity against SCVs, whereas it does not significantly affect the growth of normal strains. Tomatidine thus can be seen as both a virulence attenuator and an anti-SCV agent.
While the fundamental reason for the intrinsic resistance of normal strains to tomatidine still is unknown, our results suggest that the specific effect of tomatidine on SCVs is linked to their electron transport deficiency. Indeed, the metabolic complementation of either hemin- or menadione-dependent SCVs abolished the susceptibility of SCVs to tomatidine. Furthermore, we demonstrated that the electron transport inhibitor HQNO allows tomatidine to inhibit the growth of normal S. aureus strains. Interestingly, HQNO is an exoproduct of Pseudomonas aeruginosa recently associated with the formation of S. aureus SCVs (17, 29). This effect of P. aeruginosa on the phenotype of S. aureus could be especially relevant to cystic fibrosis (CF), as both organisms are commonly coisolated from the airways of CF patients (16, 17). It therefore is possible that the presence of tomatidine prevents the development of the SCV phenotype induced by interspecies interactions, thus reducing the likelihood of chronic S. aureus infections.
Although further investigations remain to be performed to completely understand the mechanism of action of tomatidine on S. aureus, our work suggests that tomatidine induces bacteriostasis in SCVs at a concentration as low as 0.12 μg/ml by inhibiting the biosynthesis of macromolecules, with a pronounced effect on protein synthesis. Other approaches, such as ribosome binding experiments, will need to be performed to verify this hypothesis. Based on our results, tomatidine may represent a new tool to combat SCV infections without exerting a selection pressure on S. aureus isolates of the normal phenotype. Moreover, the reversion of SCVs to the normal phenotype, as observed during some of our experiments (data not shown), would provide resistance to the growth-inhibitory activity of tomatidine but not necessarily to its antivirulence effect. Furthermore, a combination therapy targeting both normal and SCV phenotypes presumably would better control reversion and resistance. The toxicological and pharmacological properties of tomatidine have not yet been fully investigated, and consequently, the real potential of tomatidine as a therapeutic agent remains to be explored.
As mentioned before, SCVs often are isolated from chronic infections, in particular from infections of the airways of cystic fibrosis patients. This propensity to cause chronic infections is thought to be related, at least in part, to the persistence of SCVs within nonphagocytic host cells (39). Antibiotic treatments often are ineffective against S. aureus infecting CF lungs, and relapsing infections are often observed (13, 18). These relapsing infections are thought to be caused by bacteria persisting inside host cells in the lungs (21). In fact, it is now thought that switching from the normal to the SCV phenotype and then back to the normal phenotype is an integral part of the pathogenesis of S. aureus in vivo, and that novel therapeutic strategies targeting SCVs are needed to combat infections caused by bacterial species capable of generating such transitory variants (46). Our results show that the antimicrobial activity of tomatidine is effective against intracellular SCVs, which suggest that the clinical use of tomatidine may help to defeat difficult-to-treat and relapsing S. aureus infections caused by SCVs.
The use of tomatidine in combination with a classical antibiotic, such as an aminoglycoside antibiotic, is promising. Aminoglycosides often are used in the treatment of CF patients (22). However, it is known that SCVs are less susceptible to this class of antibiotics and are frequently isolated from the airways of CF patients (5, 19, 30). Moreover, it was shown by us and others that aminoglycosides can in fact induce the formation of SCVs (24, 27, 38). Thus, as suggested by our results, the combination of tomatidine with an aminoglycoside antibiotic in the treatment of CF patients should efficiently eradicate a population of S. aureus consisting of both the normal and SCV phenotypes. We presently are working at better characterizing the proprieties of tomatidine and also are exploring derivatives to optimize their physicochemical, antibacterial, and antivirulence properties.
ACKNOWLEDGMENTS
This study was supported by a grant from the Canadian Cystic Fibrosis Foundation to F.M. and by a team grant from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT). G.M. is a recipient of an Alexander-Graham-Bell graduate studentship from the Natural Science and Engineering Research Council of Canada and a doctoral research studentship from FQRNT.
We thank S. M. O'Grady (University of Minnesota) for the kind gift of the shCFTR cell line.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Alexander E. H., Hudson M. C. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl. Microbiol. Biotechnol. 56:361–366 [DOI] [PubMed] [Google Scholar]
- 2. Archer G. L. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 3. Atalla H., et al. 2008. Characterization of a Staphylococcus aureus small colony variant (SCV) associated with persistent bovine mastitis. Foodborne Pathog. Dis. 5:785–799 [DOI] [PubMed] [Google Scholar]
- 4. Bednarek P., Osbourn A. 2009. Plant-microbe interactions: chemical diversity in plant defense. Science 324:746–748 [DOI] [PubMed] [Google Scholar]
- 5. Besier S., et al. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45:168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouarab K., Ordi E., Gattuso M. M., Moisan H., Malouin F. 2007. Plant stress response agents affect Staphylococcus aureus virulence genes. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1483. [Google Scholar]
- 7. Brouillette E., Martinez A., Boyll B. J., Allen N. E., Malouin F. 2004. Persistence of a Staphylococcus aureus small-colony variant under antibiotic pressure in vivo. FEMS Immunol. Med. Microbiol. 41:35–41 [DOI] [PubMed] [Google Scholar]
- 8. Bryan L. E., Kwan S. 1981. Aminoglycoside-resistant mutants of Pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob. Agents Chemother. 19:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Cowan M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman M. 2002. Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 50:5751–5780 [DOI] [PubMed] [Google Scholar]
- 12. Galli J., et al. 2007. Recurrent upper airway infections and bacterial biofilms. J. Laryngol. Otol. 121:341–344 [DOI] [PubMed] [Google Scholar]
- 13. Goering R. V., Bauernfeind A., Lenz W., Przyklenk B. 1990. Staphylococcus aureus in patients with cystic fibrosis: an epidemiological analysis using a combination of traditional and molecular methods. Infection 18:57–60 [DOI] [PubMed] [Google Scholar]
- 14. Goerke C., Wolz C. 2004. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int. J. Med. Microbiol. 294:195–202 [DOI] [PubMed] [Google Scholar]
- 15. González-Lamothe R., et al. 2009. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 10:3400–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrison F. 2007. Microbial ecology of the cystic fibrosis lung. Microbiology 153:917–923 [DOI] [PubMed] [Google Scholar]
- 17. Hoffman L. R., et al. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 103:19890–19895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahl B., et al. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029 [DOI] [PubMed] [Google Scholar]
- 19. Kahl B. C., et al. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41:4424–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lightbown J. W., Jackson F. L. 1956. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem. J. 63:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowy F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341–343 [DOI] [PubMed] [Google Scholar]
- 22. Lyczak J. B., Cannon C. L., Pier G. B. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin-Hernandez A. M., Dufresne M., Hugouvieux V., Melton R., Osbourn A. 2000. Effects of targeted replacement of the tomatinase gene on the interaction of Septoria lycopersici with tomato plants. Mol. Plant Microbe Interact. 13:1301–1311 [DOI] [PubMed] [Google Scholar]
- 24. Massey R. C., Buckling A., Peacock S. J. 2001. Phenotypic switching of antibiotic resistance circumvents permanent costs in Staphylococcus aureus. Curr. Biol. 11:1810–1814 [DOI] [PubMed] [Google Scholar]
- 25. Miller M. H., Edberg S. C., Mandel L. J., Behar C. F., Steigbigel N. H. 1980. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 18:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell G., Bilodeau G., Grondin G., Cantin A., Malouin F. 2010. Defects in the cystic fibrosis transmembrane conductance regulator (CFTR) increase Staphylococcus aureus intracellular infection of human pulmonary Cells. Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. D-1179. [Google Scholar]
- 27. Mitchell G., et al. 2010. A role for sigma factor B in the emergence of Staphylococcus aureus small-colony variants and elevated biofilm production resulting from an exposure to aminoglycosides. Microb. Pathog. 48:18–27 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell G., Gattuso M., Bouarab K., Malouin F. 2009. Tomatidine (TO) affects virulence regulators of prototypical Staphylococcus aureus (SA) and small colony variants (SCV) of cystic fibrosis (CF) patients. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1341 [Google Scholar]
- 29. Mitchell G., et al. 2010. Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide. BMC Microbiol. 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moisan H., et al. 2006. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osbourn A. E. 1996. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8:1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer M. L., et al. 2006. Protease-activated receptor regulation of Cl-secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am. J. Physiol. Cell Physiol. 290:C1189–C1198 [DOI] [PubMed] [Google Scholar]
- 33. Proctor R. A., et al. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305 [DOI] [PubMed] [Google Scholar]
- 34. Ríos J. L., Recio M. C. 2005. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 100:80–84 [DOI] [PubMed] [Google Scholar]
- 35. Roddick J. G. 1974. The steroidal glycoalkaloid α-tomatine. Phytochemistry 13:9–25 [Google Scholar]
- 36. Ruiz-Rubio M., et al. 2001. Metabolism of the tomato saponin α-tomatine by phytopathogenic fungi, p. 293–326 In Rahman A. (ed.), Studies in natural products chemistry, vol. 25 Elsevier, Oxford, United Kingdom [Google Scholar]
- 37. Sandrock R. W., Vanetten H. D. 1998. Fungal sensitivity to and enzymatic degradation of the phytoanticipin α-tomatine. Phytopathology 88:137–143 [DOI] [PubMed] [Google Scholar]
- 38. Schaaff F., Bierbaum G., Baumert N., Bartmann P., Sahl H. G. 2003. Mutations are involved in emergence of aminoglycoside-induced small colony variants of Staphylococcus aureus. Int. J. Med. Microbiol. 293:427–435 [DOI] [PubMed] [Google Scholar]
- 39. Sendi P., Proctor R. A. 2009. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 17:54–58 [DOI] [PubMed] [Google Scholar]
- 40. Shah P. M. 2005. The need for new therapeutic agents: what is the pipeline? Clin. Microbiol. Infect. 11 Suppl. 3:36–42 [DOI] [PubMed] [Google Scholar]
- 41. Simons V., et al. 2006. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 50:2732–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh R., Ray P., Das A., Sharma M. 2010. Enhanced production of exopolysaccharide matrix and biofilm by a menadione-auxotrophic Staphylococcus aureus small-colony variant. J. Med. Microbiol. 59:521–527 [DOI] [PubMed] [Google Scholar]
- 43. Singh R., Ray P., Das A., Sharma M. 2009. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J. Med. Microbiol. 58:1067–1073 [DOI] [PubMed] [Google Scholar]
- 44. Stewart P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107–113 [DOI] [PubMed] [Google Scholar]
- 45. Talbot G. H., et al. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668 [DOI] [PubMed] [Google Scholar]
- 46. Tuchscherr L., et al. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]