Abstract
Antimicrobial photodynamic inactivation (PDI) was shown to be a promising treatment modality for microbial infections. This study explores the effect of chitosan, a polycationic biopolymer, in increasing the PDI efficacy against Gram-positive bacteria, including Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and methicillin-resistant S. aureus (MRSA), as well as the Gram-negative bacteria Pseudomonas aeruginosa and Acinetobacter baumannii. Chitosan at <0.1% was included in the antibacterial process either by coincubation with hematoporphyrin (Hp) and subjection to light exposure to induce the PDI effect or by addition after PDI and further incubation for 30 min. Under conditions in which Hp-PDI killed the microbe on a 2- to 4-log scale, treatment with chitosan at concentrations of as low as 0.025% for a further 30 min completely eradicated the bacteria (which were originally at ∼108 CFU/ml). Similar results were also found with toluidine blue O (TBO)-mediated PDI in planktonic and biofilm cells. However, without PDI treatment, chitosan alone did not exert significant antimicrobial activity with 30 min of incubation, suggesting that the potentiated effect of chitosan worked after the bacterial damage induced by PDI. Further studies indicated that the potentiated PDI effect of chitosan was related to the level of PDI damage and the deacetylation level of the chitosan. These results indicate that the combination of PDI and chitosan is quite promising for eradicating microbial infections.
INTRODUCTION
Antimicrobial photodynamic inactivation (PDI) is a developing therapeutic tool against bacterial infections and has garnered much interest for use in managing drug-resistant bacterial strains (6, 10). PDI as a bactericide employs the combination of a nontoxic photosensitizer (PS) and visible light to generate cytotoxic species. After light irradiation, the activated photosensitizer (triplet photosensitizer) may subsequently react with molecules in its direct environment by an electron transfer process to form radicals, which can further interact with oxygen to produce reactive oxygen species (type I reaction). Alternatively, the triplet photosensitizer can directly transfer energy to oxygen to generate highly reactive singlet oxygen (type II reaction) (7). PDI was shown to be effective against Gram-positive and Gram-negative bacteria as well as antibiotic-resistant strains, and evidence indicates that repeated photosensitization does not induce resistance of the bacteria to this treatment (27). Due to the morphological characteristics of membranes, Gram-positive bacteria are more susceptible to PDI than Gram-negative bacteria for most commonly used PSs (10, 15). Three approaches were developed to increase the efficacy of PDI against Gram-positive and Gram-negative bacteria. First, pretreatment with agents that increase the permeability of the outer membrane, such as the cationic polypeptide polymyxin B (15) or the metal chelator EDTA (1), was shown to increase the effectiveness of PDI in killing Gram-negative bacteria. Furthermore, covalently conjugating poly-l-lysine and PS can produce broad-spectrum antimicrobial photoinactivation (17, 21–23). The third approach is to use PSs with an intrinsic positive charge, which can increase the PDI efficacy in both Gram-positive and Gram-negative bacteria (10, 13).
Chitosan is a natural linear polycationic biopolymer consisting of N-acetyl-d-glucosamine and β-1,4-linked d-glucosamine. Because of its safety and excellent biocompatibility (9), chitosan has received considerable attention for medical and pharmaceutical applications in wound healing (25, 26), tissue engineering (28), and drug delivery (20). Its antimicrobial activity against a wide variety of microorganisms, including fungi, algae, and some bacteria, was reported (18). It is believed that the bacteriostatic/bactericidal activity of chitosan is related to the protonated positive charge number of chitosan and the number of negative charges on the microbial surface (8, 11, 14).
Previously, we showed that pharmaceutical delivery systems can be used to facilitate PDI due to improved photochemical efficiency and the subsequent microbe-killing effect (24). In this study, we further found that one of the most commonly studied biomaterials, chitosan, can potentiate the PDI efficacy in planktonic cells and biofilms. This phenomenon does not exist if there is only chitosan in the culture, either shielded or subjected to light exposure. Simultaneously with or after PDI application, the addition of chitosan can greatly augment the killing of bacteria. Since the safety of chitosan as a biomaterial is well known, the combination of chitosan and PDI for treating bacterial infections is quite encouraging.
MATERIALS AND METHODS
Materials.
Hematoporphyrin dihydrochloride (Hp) was purchased from ChromaDex (Irvine, CA), and used as received. Pluronic F127 (PF127) was purchased from Wei Ming Pharmaceutical (Taipei, Taiwan). Low-molecular-weight chitosans (molecular weight, 25,000 to 35,000) with degrees of deacetylation (DDA) of 75% to 85% were purchased from Shin Era Technology (Taipei, Taiwan). Poly-l-lysine (150 to 300 kDa), toluidine blue O (TBO), and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Except for chitosan 5, the chitosans listed in Table 1 were obtained from Genis Ehf., Reykjavik, Iceland.
Table 1.
Molecular weights and degrees of deacetylation of the chitosans used
| Chitosan | Mean DDA level ± SD (%) | Mwa | Mw/Mn |
|---|---|---|---|
| 1 | 40.0 ± 0.5 | 300,058 | 1.63 |
| 2 | 50.2 ± 0.4 | 2,000 | NAc |
| 3 | 49.6 ± 0.2 | 335,949 | 1.57 |
| 4 | 70.2 ± 0.5 | 440,971 | 1.6 |
| 5b | 80.0 ± 5.0 | 25,000–35,000 | NA |
| 6 | 95.9 ± 0.3 | 45,905 | 1.89 |
| 7 | 96.1 ± 0.7 | 131,958 | 2.41 |
Except for chitosan 5, the Mw represents the weight-average molecular weight, which was analyzed by high-pressure liquid chromatography (HPLC) with a light-scattering detector.
Chitosan 5 was mainly used in this study.
NA, not available.
Microbial strains and growth conditions.
Staphylococcus aureus (BCRC 10780) was purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan). The other microbial strains used in this study were Staphylococcus epidermidis (ATCC 12228), Staphylococcus pyogenes (ATCC 19615), methicillin-resistant S. aureus (MRSA) (ATCC 49476 and ATCC 33592), Pseudomonas aeruginosa (ATCC 27853), and Acinetobacter baumannii (ATCC 19606). Tryptic soy broth (TSB) was used as the liquid medium for S. aureus, S. epidermidis, MRSA, P. aeruginosa, and A. baumannii. S. pyogenes was grown in Todd-Hewitt broth containing yeast extract.
Biofilm preparation.
S. aureus biofilms were cultured on a rotating disk reactor modified from the design of Pitts et al. (16). The reactor consisted of a 500-ml polypropylene container, a Teflon rotor, and 24 removable 316L stainless steel disks inserted on the rotor. One milliliter of S. aureus seed culture was inoculated into 150 ml of 0.1× TSB in the reactor and incubated at room temperature overnight. After incubation, the reactor was continuously fed with 0.01× TSB at a rate of 468 ml/h. The biofilms reached a steady state after 24 h, and the average biofilm density was 1.09 × 107 ± 0.37 × 107 CFU/cm2.
Preparation and characterization of micelles.
Hp was encapsulated in micelles by a reverse-phase evaporation method as described before (24). Briefly, 0.5 mg of Hp and 100 mg of PF127 were dissolved in a cosolvent system (chloroform-methanol at 4:1, vol/vol), which was then removed by rotary evaporation to form a thin film. The film was hydrated using 1 ml of distilled water at room temperature to give a final 10% (wt/vol) micellar solution. To remove the free Hp, the hydrated preparation was passed through a 0.2-μm polyvinylidene difluoride (PVDF) filter to remove the free Hp, since the Hp that is not entrapped within the micelles undergoes aggregation and does not pass through these filters (3). Both UV-visible absorption and fluorescence spectra of the filtrate were used to verify that Hp in the filtrate was in the monomeric form as described before (24).
PDI of planktonic cells.
For PDI, bacterial cells in the stationary phase were harvested by centrifugation of the broth culture, washed three times with phosphate-buffered saline (PBS), and suspended in PBS to produce a cell suspension containing ∼108 CFU/ml. In a typical experiment, 0.1 ml of a cell suspension of the bacteria containing approximately 108 CFU/ml was transferred into a well, and then 0.1 ml of the PBS solution (pH 7.4) containing Hp was added to the well. Samples were incubated in the dark for 30 min unless otherwise specified, and then irradiated at room temperature. The light source used for Hp irradiation consisted of a high-power LED array with the wavelength centered at 635 ± 5 nm, delivered at an irradiance of 60 mW/cm2 (24). Irradiated and nonirradiated bacterial cells were serially diluted 10-fold with PBS, and the colonies formed after 18 h of incubation at 37°C were counted.
TBO-mediated PDI in biofilm cells.
The disks with biofilms were placed in a sterile 48-well microtiter plate, treated with 0, 2, and 4 μM TBO in the dark for 30 min, and then moved to a new plate containing PBS. The disks were irradiated with the LED array at 20 J/cm2. After light irradiation, the disks with biofilms were then put into test tubes containing 10 ml sterile phosphate-buffered saline and vigorously vortexed to remove the biofilm from the disks. The resulting bacterial suspensions were suitably diluted and plated on TSB agar (Difco), and the colonies formed after 18 h of incubation at 37°C were counted. The number of viable cells was determined by averaging the CFU on three plates as described below.
Effect of chitosan on PDI.
A stock solution of chitosan (1%, wt/vol) was prepared in 1% acetic acid (prefiltered through a 0.22-μm PVDF filter) and used within 1 month. Various concentrations of chitosan were prepared by taking aliquots from the chitosan stock and diluting them with the culture medium. Chitosan was added to the bacterial culture concurrently with Hp (coincubation study) or after performing PDI (postincubation study). For the coincubation study, light illumination was administered 30 min after Hp and chitosan incubation. For the postincubation study, chitosan was added after PDI, left for 30 min, and washed out using fresh culture medium. In the presence or absence of chitosan, irradiated and nonirradiated bacterial cells were serially diluted 10-fold with PBS, and the colonies formed after 18 h of incubation at 37°C were counted.
Bacterial cell survival assay.
The CFU of a bacterial suspension were counted using the following standard protocol. Aliquots (10 μl) of appropriate dilutions (from 10−1 to 10−5) were plated on TSB agar plates and incubated at 37°C in the dark for 18 h. The surviving fraction was calculated as NPDI/N0, where NPDI is the CFU/ml (planktonic cells) or CFU/cm2 (biofilm) after antimicrobial photodynamic therapy and N0 is the CFU/ml or CFU/cm2 in the initial sample. The dark toxicity of the substrates, defined as the intrinsic toxicity of the compounds in the absence of light, was monitored by evaluating the surviving fraction of nonilluminated bacterial samples and calculated as NDARK/N0, where NDARK is the CFU/ml of the nonilluminated samples. All results are expressed as the mean ± standard deviation. Differences between two means were assessed for significance by the two-tailed Student t test, and a P value of <0.05 was considered significant.
RESULTS
PDI effect of chitosan coincubation with Hp or micellar Hp on S. aureus.
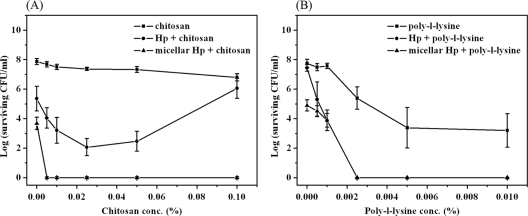
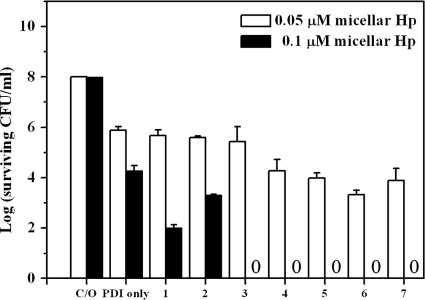
To examine the possible potentiation of PDI by chitosan, wild-type S. aureus was incubated with 0.1 μM free-form Hp or micellar Hp for 30 min in the presence of a low-molecular-weight chitosan (molecular weight, 25,000 to 35,000) and then irradiated with 25 J/cm2 of light. As shown in Fig. 1A, there was a dramatic difference in the lethality of PDI against S. aureus with or without chitosan. In the absence of chitosan, about a 4-log bacterial killing was observed when using Hp encapsulated in micelles (micellar Hp), compared to a 2-log killing with free-form Hp. This result is consistent with our previous finding that micellar Hp exerted better antimicrobial PDI efficacy than free-form Hp (24). In the presence of chitosan (0.005% to 0.1%, wt/vol), micellar Hp-mediated PDI resulted in a complete bacterial killing. Compared to micellar Hp, free-form Hp caused only 2- to 4-log-increased bacterial killing, and the PDI efficacy decreased when the concentration of chitosan was increased to 0.1% (wt/vol), most likely due to light scattering caused by the increased chitosan concentration. Although chitosan has an intrinsic antimicrobial effect, 0.1% chitosan alone only slightly decreased bacterial survival with an increasing chitosan concentration (Fig. 1A). These results indicate that this low-molecular-weight chitosan can potentiate PDI efficacy against S. aureus. The potentiated effect of chitosan in PDI was further verified by isobologram representation of the statistical modeling (see Fig. S1 in the supplemental material).
Fig. 1.
Cell survival fraction of S. aureus after PDI in the presence of chitosan or polylysine. Hp at 0.1 μM and various concentrations of chitosan (A) or polylysine (B) were coincubated with the bacteria for 30 min and then subjected to illumination at 25 J/cm2. Each point is the mean from three independent experiments ± standard deviation.
Soukos et al. showed that a mixture of poly-l-lysine and chlorin e6 resulted in a pronounced antimicrobial effect (∼40% survival for chlorin e6 alone and ∼8% for the mixture) (21). Therefore, we also examined whether a mixture of poly-l-lysine and Hp resulted in a pattern of PDI similar to that caused by chitosan. When poly-l-lysine was mixed with Hp for 30 min and subjected to light irradiation, a reduced bacterial survival rate was observed (Fig. 1B). The enhanced PDI effect of poly-l-lysine coincubated with Hp was found to be similar to that of chitosan. However, a significant dose-dependent dark toxicity was observed when the concentration of poly-l-lysine was >0.0025% (wt/vol), suggesting that the complete eradication of bacteria using poly-l-lysine seemed to be an additive effect.
Combined effect of PDI and chitosan on MRSA, S. epidermidis, and S. pyogenes.
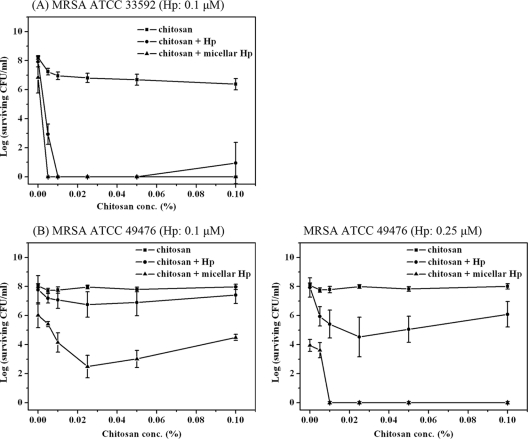
The possible potentiated effect of chitosan in PDI was further examined against two methicillin-resistant strains of S. aureus (MRSA ATCC 33592 and MRSA ATCC 49476). As shown in Fig. 2A, there was an approximately 1- to 2-log reduction in viable counts caused by 0.1 μM free-form or micellar Hp-mediated PDI against MRSA ATCC 33592 in the absence of chitosan. However, the presence of chitosan potentiated the PDI effect and resulted in the complete eradication of ATCC 33592 at a very low concentration range, 0.005% (wt/vol) for the micellar Hp and 0.01% (wt/vol) for free-form Hp. Although the combination of chitosan and 0.1 μM micellar Hp did not achieve complete eradication, chitosan still potentiated the PDI efficacy against the ATCC 49476 MRSA strain (left panel of Fig. 2B). A further increase in the Hp concentration to 0.25 μM achieved complete eradication of the bacteria when micellar Hp was cotreated with chitosan at >0.01% (wt/vol), as shown in the right panel of Fig. 2B.
Fig. 2.
Effect of chitosan on Hp-mediated PDI against MRSA ATCC 33592 (0.1 μM Hp) (A) and MRSA ATCC 49476 (B). Hp (0.1 and 0.25 μM) was incubated with the bacteria for 30 min in the presence of chitosan and subjected to illumination at 25 J/cm2.
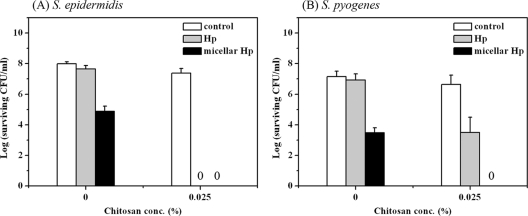
To test the generality of the potentiated PDI effect of chitosan, we used two other Gram-positive species, S. epidermidis and S. pyogenes, which are important pathogens. As shown in Fig. 3, the potentiated PDI effects of 0.025% (wt/vol) chitosan on S. epidermidis and S. pyogenes were similar to that on S. aureus. Micellar Hp at 0.1 μM was able to produce a stronger PDI effect than the free form, and the presence of chitosan at 0.025% (wt/vol) further resulted in the complete eradication of the bacteria.
Fig. 3.
Cell survival fractions of S. epidermidis (A) and S. pyogenes (B) after incubation with 0.1 μM Hp and 0.025% chitosan for 30 min followed by light exposure of 25 J/cm2 and then plate count. Each value is the mean from three independent experiments ± standard deviation.
Chitosan postincubation with free-form Hp or micellar Hp.
As shown in Fig. 1 and 2, 0.1% (wt/vol) chitosan reduced the free-form Hp-mediated PDI effect. To rule out the possibility of light scattering by chitosan during light irradiation, we incubated wild-type S. aureus with 0.1 μM free-form Hp or micellar Hp for 30 min, followed by illumination under a light dose of 25 J/cm2 and a 30-min incubation with different concentrations of chitosan. As shown in Fig. 4, without chitosan incubation, there were only approximately 2- to 4-log reductions in viable counts with 0.1 μM free-form Hp or micellar Hp. However, S. aureus could be completely killed by incubation with micellar Hp-mediated PDI followed by a 30-min incubation with chitosan (0.025%, wt/vol), which is consistent with results found for the coincubation of chitosan and Hp. Although free-form Hp exhibited only an ∼4- to 5-log reduction in the presence of chitosan (0.025%, wt/vol), complete destruction was achieved by increasing the chitosan concentration. These results indicate that the potentiated effect of chitosan might work after the bacterial damage induced by PDI.
Fig. 4.
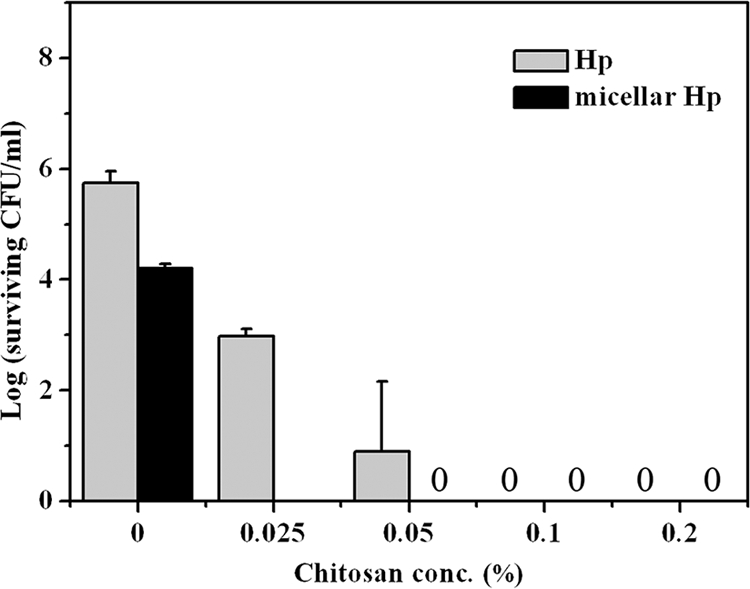
Cell survival fraction of S. aureus after PDI (0.1 μM Hp and 25 J/cm2 irradiation) followed by treatment with various concentrations of chitosan for 30 min and then plate count. Each value is the mean from three independent experiments ± standard deviation.
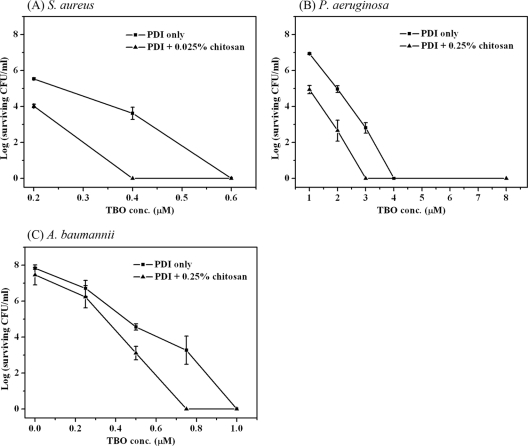
Chitosan augments TBO-mediated PDI against Gram-positive and Gram-negative bacteria.
It was shown that an anionic photosensitizer such as Hp cannot effectively exert its PDI effect against Gram-negative bacteria (12, 15). Our studies also found that Hp-mediated PDI exerted limited antimicrobial efficacy against P. aeruginosa (data not shown). To examine whether the potentiated PDI effect of chitosan occurred with other photosensitizers as well as in Gram-negative bacteria, we carried out PDI mediated by TBO against S. aureus, P. aeruginosa, and A. baumannii followed by a 30-min incubation with chitosan. Figure 5 shows the results of PDI mediated by TBO against S. aureus, P. aeruginosa, and A. baumannii. Under a light irradiation of 20 J/cm2, ∼4-log killing was obtained for S. aureus with 0.4 μM TBO, for P. aeruginosa with 3 μM TBO, and for A. baumannii with 0.75 μM TBO. In the presence of chitosan, complete bacterial killing was achieved. Interestingly, the concentration of chitosan needed for the potentiated effect was higher for P. aeruginosa and A. baumannii than for S. aureus. In addition, under the same PDI conditions, the augmented effect of chitosan was not found in mammalian cells (see Fig. S2 in the supplemental material).
Fig. 5.
Cell survival fractions of S. aureus (A), P. aeruginosa (B), and A. baumannii (C) after PDI. Cells were incubated with different concentrations of TBO for 30 min, followed by light exposure at 20 J/cm2. After PDI, different concentrations of chitosan were added and further incubated for 30 min, and then a plate count was performed. Each point is the mean from three independent experiments ± standard deviation.
Finally, we examined the potentiated PDI of chitosan in a biofilm with S. aureus. A concentration of 4 μM TBO and a light energy dose of 20 J/cm2 resulted only in ∼2-log killing of S. aureus in the biofilm (Fig. 6). However, complete killing of S. aureus in the biofilm was found after the addition of chitosan (0.025%, wt/vol) following PDI. The potentiated effect of chitosan in TBO-mediated PDI against biofilm was further confirmed by isobologram representation of the statistical modeling (see Fig. S3 in the supplemental material). These results clearly indicate that chitosan can potentiate the PDI efficacy against biofilm cells as well as planktonic cells.
Fig. 6.
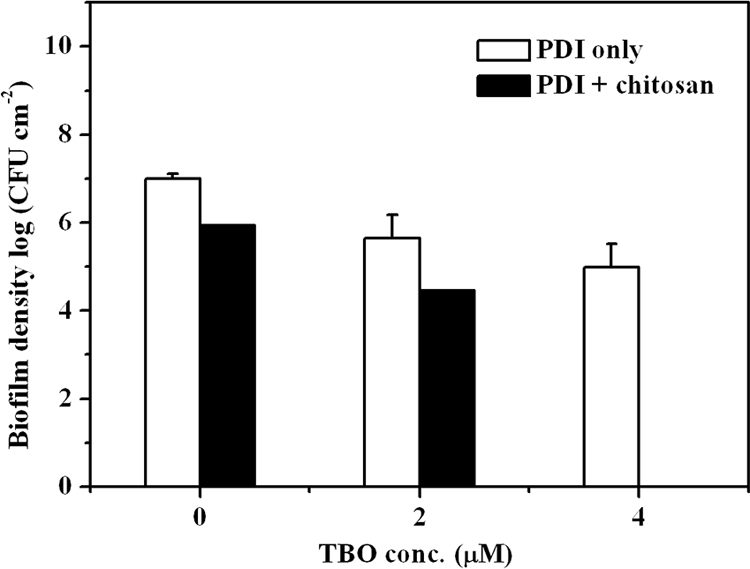
Effect of TBO-mediated PDI followed by chitosan treatment on biofilm of S. aureus. Biofilms were treated with 2 or 4 μM TBO for 30 min, followed by light exposure at 20 J/cm2. After PDI, biofilms were further treated with 0.025% chitosan and then subjected to a plate count. Error bars indicate the standard errors of the means from three separate experiments.
Characterization of chitosan's properties in augmenting PDI efficacy.
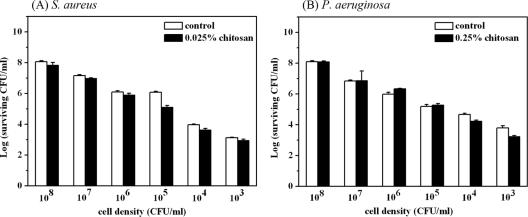
Although chitosan alone was shown to be an antimicrobial agent, the concentration of chitosan needed for it to exert its antimicrobial activity varies depending on the species and concentration of microorganism. In this regard, it is possible that the potentiated effect of chitosan might be due to the lower numbers of surviving bacteria after PDI. To examine this, various concentrations of S. aureus and P. aeruginosa were incubated with 0.025% and 0.25% (wt/vol) chitosan for 30 min. As shown above, chitosan further resulted in complete bacterial killing under conditions of an ∼4-log reduction by PDI. However, without PDI treatment, chitosan alone did not exert significant antimicrobial activity with a 30-min incubation (Fig. 7).
Fig. 7.
Cell survival fraction of S. aureus (A) and P. aeruginosa (B) treated with 0.025% and 0.25% chitosan, respectively. Various concentrations of S. aureus and P. aeruginosa were incubated with chitosan for 30 min and then subjected to a plate count for the viability analysis. Each value is the mean from three independent experiments ± standard deviation.
It was shown that the molecular weight and the degree of deacetylation (DDA) of chitosan play important roles in its biological and physicochemical properties (18). The chitosan used in this study had a molecular weight of about 25,000 to 35,000, and its DDA was about 75 to 85%. To further address the effect of molecular weight and DDA in augmenting PDI, we used chitosans with different DDA levels and molecular weights (Table 1). As shown in Fig. 8, chitosan with a DDA of <70% could increase bacterial killing by only 2 logs at a concentration of 0.05 μM TBO and a light energy dose of 20 J/cm2. However, chitosan with a DDA of >70% resulted in complete bacterial killing under the conditions of a 4-log reduction by PDI (Fig. 8). Interestingly, in the same molecular weight range (300,000 to 400,000), chitosans with different DDA levels showed different potentiation of PDI effects, of ∼1- to 2-log increases and complete killing for DDA levels of 50% and 70%, respectively. These results indicate that the potentiated PDI effects of chitosan are related to the PDI damage level and the DDA of the chitosan. In the future, it is necessary to further address the relationships between the initial PDI damage level and the DDA and concentration of chitosan required to induce complete bacterial lethality.
Fig. 8.
Effect of chitosans with different molecular weights and deacetylation levels in augmenting the PDI against S. aureus. Cells were incubated with 0.1 μM Hp for 30 min, followed by light exposure at 25 J/cm2. After PDI, cells were further incubated with 0.025% of various chitosans as shown in Table 1 for 30 min and then subjected to a plate count. Each value is the mean from three independent experiments ± standard deviation.
DISCUSSION
Antimicrobial PDI has provided new hope for controlling microbial infections. The main advantages of PDI are that bacteria can be eradicated almost instantly and that damage to adjacent host tissues can be avoided. A number of PSs showed PDI efficacy against microbial pathogens (10). However, those PSs were found to easily form aggregates in aqueous medium, which can lead to a self-quenching effect on the excited state, thus reducing the yield of singlet oxygen (1O2) formation (2). In addition, many commonly used PSs, which can produce significant phototoxicity against Gram-positive bacteria, are not effective against Gram-negative bacteria due to their outer membranes. Previously, we showed that encapsulation of PSs in micelles can improve their photochemical efficiency and subsequently enhance the PDI efficacy in bacterial killing (24). In this study, we further found that one of the most commonly studied biomaterials, chitosan, can potentiate the PDI efficacy against Gram-positive (S. aureus, S. epidermidis, and S. pyogenes) and Gram-negative (P. aeruginosa and A. baumannii) bacteria, including antibiotic-resistant strains. The augmentation effect of chitosan in PDI was effective in planktonic cells and in biofilm. Although chitosan alone was shown to have antimicrobial activity, the complete inactivation against microbes was observed after 18 to 24 h of incubation with chitosan. In addition, not all microbes can be inactivated by chitosan; some are more susceptible, and some are quite resistant. However, in the present study, a 30-min incubation with chitosan following PDI was sufficient to completely eradicate Gram-positive as well as Gram-negative bacteria. In addition, we found that the mode of action for the PDI-chitosan combination was a potentiated effect, and chitosan alone had no antimicrobial activity (Fig. 7).
For clinical applications, an ideal PDI should exhibit extensive killing of the pathogen population with minimal damage to host tissues in the area of infection. Although PSs can exert phototoxic effects on the microbes that take them up or adhere to them, it is still possible to damage neighboring healthy cells if the PS dose is too high. It would be ideal if a lower PS dose that is safe for human tissues can efficiently function as a microbial killing agent. Previously, we showed that 0.25 μM micellar Hp was required to completely eradicate S. aureus under a light dose of 50 J/cm2 (24). However, in the presence of chitosan, complete eradication was found at lower PDI conditions (0.1 μM micellar Hp and 25 J/cm2). Similar results were also found for the MRSA strains (Fig. 2). The potentiating effect of chitosan on Hp- or TBO-mediated PDI was also found with other Gram-positive bacteria (S. epidermidis and S. pyogenes) and two Gram-negative bacteria (P. aeruginosa and A. baumannii). These results clearly indicate that combined with chitosan, a lower dose of PS and light can efficiently function as a microbial killing agent while causing less damage to human tissues.
The potentiated effect of chitosan in PDI was not specific to the bacterial strain. However, sufficient damage to the bacterial walls or membranes could be the response-limiting point and the major criterion for introducing a subsequent potentiated response by chitosan. The minimal bactericidal concentration (MBC) which can induce a 4-log drop in survival was proposed for the efficacy of a PS as an antimicrobial agent under a given set of irradiation parameters (10). Interestingly, according to our present studies, an initial 2- to 4-log reduction by PDI was required to result in the complete killing augmented by chitosan in this study. Depending on the bacterial strains and damage level induced by PDI, the minimum concentration of chitosan might vary. MRSA ATCC 33592 seemed to be the most sensitive strain, because only 0.005% chitosan was required to augment the complete killing after an initial 2-log reduction by PDI, compared to a 4-log reduction in wild-type S. aureus. However, a higher concentration of chitosan (0.01%) was required for the potentiated effect of PDI against MRSA ATCC 49476, which was previously found to be less susceptible to PDI (24). Cui et al. reported that the reduced susceptibilities of multidrug-resistant MRSA to daptomycin and vancomycin were related to cell wall thickening (4). Whether the reduced susceptibility of MRSA strains to PDI and chitosan is related to cell wall thickening deserves further investigation.
To induce an approximately 4-log reduction in viable counts, different concentrations of TBO were required for S. aureus (0.4 μM), A. baumannii (0.75 μM), and P. aeruginosa (3 μM) (Fig. 5). In addition, at a concentration of 0.025%, chitosan was able to completely eradicate S. aureus, while a higher concentration of chitosan (0.25%) was required for P. aeruginosa and A. baumannii to achieve the same result. The higher concentrations of TBO and chitosan required for Gram-negative bacteria might be related to the outer membrane of the Gram-negative bacterium. The outer membrane has a very heterogeneous composition, including lipopolysaccharides and lipoproteins, and the outer wall of Gram-negative bacteria possesses an additional 10- to 15-nm-thick structural element. It was shown that the outer membrane acts as a physical and chemical barrier to prevent PDI-induced cytotoxic agents from interacting with vital targets, such as the membrane or cytoplasmic components (5, 19). In addition, lipopolysaccharides on the surface of the outer membrane give it densely packed negative charges that make it impermeable to neutral and anionic compounds. In this regard, it is not surprising that a higher TBO concentration was required to exert a 4-log reduction in Gram-negative bacteria and a higher concentration of chitosan to exert a complete bactericidal effect following PDI.
For the first time, this study demonstrated that inexpensive and nontoxic chitosan can augment the antibacterial PDI efficacy against Gram-positive and -negative bacteria in planktonic form as well as biofilm cells. Compared to photodynamic therapy (PDT) for cancer treatment, PDI is more easily carried out for localized infections. A PS can be administrated to infected tissues by local delivery such as topical application. Chitosan can be further formulated as a hydrogel or film to apply to PDI-treated tissues to augment the bactericidal effects. The combination of PDI and chitosan was shown to be a promising antimicrobial approach against infectious disease.
Supplementary Material
ACKNOWLEDGMENTS
Chitosans with different molecular weights and deacetylation levels were kindly provided by Johannes Gislason, Jon M. Einarsson, and Chuen-How Ng (Genis Ehf., Reykjavik, Iceland). We thank Telma T. Franco (University Estadual de Campinas, UNICAMP, Brazil) for determining the molecular weights of chitosans provided by Genis Ehf. We also thank Michael Jay for critical reading of the manuscript.
Financial support for the authors' laboratories was provided by grants (NSC95-2314-B-002-319-MY3 and NSC98-2320-B038-012-MY3) from the National Science Council of Taiwan.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 31 January 2011.
REFERENCES
- 1. Bertoloni G., Rossi F., Valduga G., Jori G., van Lier J. 1990. Photosensitizing activity of water- and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol. Lett. 59:149–155 [DOI] [PubMed] [Google Scholar]
- 2. Boyle R. W., Dolphin D. 1996. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 64:469–485 [DOI] [PubMed] [Google Scholar]
- 3. Chowdhary R. K., Chansarkar N., Sharif I., Hioka N., Dolphin D. 2003. Formulation of benzoporphyrin derivatives in Pluronics. Photochem. Photobiol. 77:299–303 [DOI] [PubMed] [Google Scholar]
- 4. Cui L., Tominaga E., Neoh H. M., Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahl T. A., Midden W. R., Hartman P. E. 1987. Pure singlet oxygen cytotoxicity for bacteria. Photochem. Photobiol. 46:345–352 [DOI] [PubMed] [Google Scholar]
- 6. Hamblin M. R., Hasan T. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasan T., Parrish J. A. 1996. Photodynamic therapy of cancer, p. 739–751 In Holland J. F., Frei E. I., Bast R. C. J., Kufe D. W., Morton D. L., Weichselbaum R. R. (ed.), Cancer medicine. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 8. Helander I. M., Nurmiaho-Lassila E. L., Ahvenainen R., Rhoades J., Roller S. 2001. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 71:235–244 [DOI] [PubMed] [Google Scholar]
- 9. Hirano S., Seino H., Akiyama Y., Nonaka I. 1988. Biocompatibility of chitosan by oral and intravenous administrations. Polym. Eng. Sci. 59:897–901 [Google Scholar]
- 10. Jori G., et al. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468–481 [DOI] [PubMed] [Google Scholar]
- 11. Leuba J., Stössel P. 1986. Chitosan and other polyamines: antifungal activity and interaction with biological membranes, p. 215–221 In Muzzarelli R., Jeuniaux C., Gooday G. (ed.). Chitin in nature and technology. Plenum Press, New York, NY [Google Scholar]
- 12. Malik Z., Ladan H., Nitzan Y. 1992. Photodynamic inactivation of gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262–266 [DOI] [PubMed] [Google Scholar]
- 13. Merchat M., Bertolini G., Giacomini P., Villanueva A., Jori G. 1996. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 32:153–157 [DOI] [PubMed] [Google Scholar]
- 14. Muzzarelli R., et al. 1990. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 34:2019–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitzan Y., Gutterman M., Malik Z., Ehrenberg B. 1992. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 55:89–96 [DOI] [PubMed] [Google Scholar]
- 16. Pitts B., et al. 2001. A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms. J. Appl. Microbiol. 91:110–117 [DOI] [PubMed] [Google Scholar]
- 17. Polo L., et al. 2000. Polylysine-porphycene conjugates as efficient photosensitizers for the inactivation of microbial pathogens. J. Photochem. Photobiol. B 59:152–158 [DOI] [PubMed] [Google Scholar]
- 18. Rabea E. I., Badawy M. E., Stevens C. V., Smagghe G., Steurbaut W. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465 [DOI] [PubMed] [Google Scholar]
- 19. Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. 1974. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can. J. Microbiol. 20:1135–1145 [DOI] [PubMed] [Google Scholar]
- 20. Shimono N., et al. 2002. Chitosan dispersed system for colon-specific drug delivery. Int. J. Pharm. 245:45–54 [DOI] [PubMed] [Google Scholar]
- 21. Soukos N. S., Ximenez-Fyvie L. A., Hamblin M. R., Socransky S. S., Hasan T. 1998. Targeted antimicrobial photochemotherapy. Antimicrob. Agents Chemother. 42:2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tegos G. P., et al. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tome J. P., et al. 2004. Synthesis and antibacterial activity of new poly-S-lysine-porphyrin conjugates. J. Med. Chem. 47:6649–6652 [DOI] [PubMed] [Google Scholar]
- 24. Tsai T., Yang Y. T., Wang T. H., Chien H. F., Chen C. T. 2009. Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg. Med. 41:316–322 [DOI] [PubMed] [Google Scholar]
- 25. Ueno H., Mori T., Fujinaga T. 2001. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 52:105–115 [DOI] [PubMed] [Google Scholar]
- 26. Ueno H., et al. 1999. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 20:1407–1414 [DOI] [PubMed] [Google Scholar]
- 27. Wainwright M., Crossley K. 2004. Photosensitizing agents—circumventing resistance and breaking down biofilms: a review. Int. Biodeterior. Biodegrad. 53:119–126 [Google Scholar]
- 28. Wang X. H., et al. 2003. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 24:3213–3220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.