TEXT
After 6 decades during which it was almost completely neglected, malaria workers have begun to acknowledge that vivax malaria poses a serious threat to human health. Nearly 3 billion people live at risk of the infection, and 100 to 400 million suffer clinical attacks each year (21, 23). Recent studies challenge the notion of Plasmodium vivax as a benign infection. A spectrum of severe disease syndromes historically considered the reserve of Plasmodium falciparum have been demonstrated in vivax malaria (4, 20, 25, 28, 31). Realization of the threat posed by this parasite, along with acknowledgment of the need to eliminate all of the malarias (19), draws attention to several important problems in the chemotherapeutic management of vivax malaria.
The treatment of P. vivax requires a blood schizontocide against the acute attack and a hypnozoitocide against the dormant forms in the liver that are responsible for relapse. Over the past 60 years, chloroquine and primaquine have been the companion therapies of choice for radical cure of vivax malaria. Resistance to chloroquine by the asexual blood stages of P. vivax emerged on the Indonesian archipelago and is now spreading through Southeast Asia (5). In 2009, the Ministry of Health of Indonesia abandoned chloroquine as the blood schizontocide component of radical cure and adopted artemisinin-combined therapy (ACT) for use with primaquine (22). This commentary explains how that decision, regardless of where made, comes with no assurance of the safety or efficacy of primaquine against relapse.
WARTIME CRISIS AND CLINICAL DEVELOPMENT
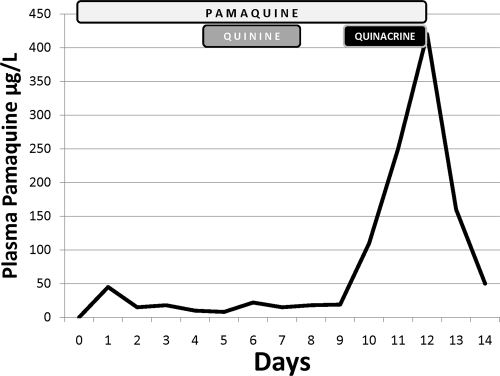
Understanding the difficulty in replacing chloroquine in radical cure requires examining the genesis of chloroquine and primaquine as companion therapies in radical cure. In March 1942, Japanese forces occupied Java, thereby securing 95% of the world supply of quinine. Allied forces checked the Japanese advance at Guadalcanal in the Solomon Islands later that year and suffered malaria attack rates of 1,700/1,000 person-years despite chemoprophylaxis with quinacrine (also called atebrine or mepacrine) (24). Troops evacuated from Guadalcanal to nonmalarious zones were withdrawn from chemoprophylaxis and suffered vivax malaria attack rates of 3,700/1,000 man-years (16). This partly explains how in the Pacific theater of World War II, 5 of 6 cases of malaria were caused by P. vivax. The Allies deployed pamaquine, a then commercially available 8-aminoquinoline known to be active against relapse and to be relatively toxic (13). However, the plasma levels of this drug increased 10-fold when administered with quinacrine (Fig. 1), and serious toxicity problems occurred among American forces (9, 10). Lacking alternatives to quinacrine, the U.S. Surgeon General ordered the withdrawal of pamaquine against relapse (27) and precipitated a hastily executed antirelapse drug discovery effort by the American government.
Fig. 1.
Drug-drug interaction between pamaquine and quinacrine, 1942. These data are for a single human subject characterized as typical of many others in the original confidential report (9). The boxes indicate days of dosing of pamaquine (10 mg every 4 h), quinine (600 mg every 8 h), or quinacrine (100 mg every 8 h). The administration of quinacrine but not of quinine caused a 10-fold elevation in plasma concentrations of pamaquine.
The Board for the Coordination of Malaria Studies under the National Research Council oversaw a vast network of clinics and laboratories managing 14,000 compounds from synthesis to clinical trials (12, 29, 32). That board directed a search for new antirelapse therapies that focused solely on the 8-aminoquinolines despite their relatively high toxicities because these were the only compounds with known activity against relapse. Replacing pamaquine was viewed as a high-priority national security matter significantly impacting the likelihood and speed of victory in the Pacific, and yet the task would require 8 years. With screening in rats and monkeys for safety, 22 candidate 8-aminoquinolines advanced to clinical trials conducted by Alf Alving and his colleagues at the University of Chicago. Those trials commenced just as the Second World War came to a close, and primaquine did not emerge as the drug of choice until about 1950. Earlier, in 1946, chloroquine had been identified as a first-line blood schizontocide for vivax malaria (26). The trials of Alving and colleagues nonetheless routinely applied quinine as the companion therapy because their experimental work had already been rigorously standardized to quinine therapy against the blood stages in assessing the activities of 8-aminoquinolines against relapse (1, 8, 11).
SYNERGY
Alving and his colleagues noticed an important potential confounding factor and executed a clinical trial designed to gauge its impact. In 1948 they wrote, “Quinine was administered concurrently with the drugs [candidate 8-aminoquinolines against relapse]… the synergistic effect of quinine on pamaquin (sic) may also extend to pamaquin analogs…” (3). Today this statement would not be understood by most workers in malaria chemotherapeutics. Quinine, like most other blood schizontocides, given even at very high doses consistently and completely fails to prevent relapse (32). Convention segregates the antiparasitic activity of blood schizontocides and that of hypnozoitocides: one does not depend upon the other in achieving the elimination of parasites from the respective chemotherapeutic compartments. This commentary explores how this may be a fundamentally flawed assumption and, later, examines the implications with respect to replacing chloroquine for treatment of vivax malaria.
As early as the 1920s, at least one report described the first 8-aminoquinoline, pamaquine, failing against relapse when not administered with quinine (30). The early clinical trials by Alving's colleagues examined pamaquine against relapse and observed the same phenomenon: pamaquine administered concurrently with quinine consistently achieved much higher cure rates than the two drugs administered consecutively (7, 8, 15, 17). Alving et al. (2) referred to these early experiments when explaining the rationale for a clinical trial published in 1955: “These experiments, however, cannot be considered definitive… The possibility of potentiation of the action of 8-aminoquinolines against tissue stages by concurrent administration of quinine, therefore, still remains a matter of uncertainty.” Their clinical trial, as designed, largely resolved that uncertainty.
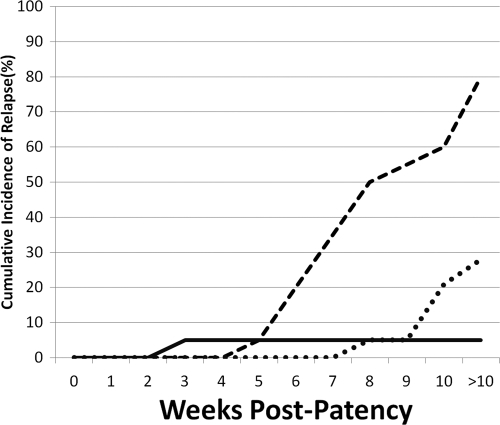
They randomized 57 Caucasian prisoners at the Illinois State Penitentiary to 3 groups of 19 men each. The experimental challenge followed an elaborate protocol for biting mosquitoes that ensured roughly equal numbers of sporozoites of Chesson strain P. vivax from New Guinea among treatment groups (14, 18). All men became parasitemic and febrile within 14 days. One group received 0.6 g and 0.4 g chloroquine base on the same day that primaquine commenced (15 mg base in six daily divided doses over 14 days). The two other groups received precisely the same dose of primaquine along with identical doses of quinine (2 g quinine in six daily divided doses over 14 days). However, one group received the quinine and primaquine on the same days (concurrent therapy) and the other received quinine first and, after a 2-day pause, primaquine (consecutive therapy). Figure 2 illustrates the cumulative incidence of recurrent parasitemia among all three groups with follow-up of nonrelapsing subjects lasting 114 to 373 days (mean 319 days) postpatency. The concurrent administration of quinine and primaquine achieved good efficacy; only a single recurrence appeared, at 31 days postpatency (and 17 days after cessation of therapy). Consecutive dosing, however, almost completely failed; 15 of 19 subjects had recurrent parasitemias between 62 and 122 days postpatency. The chloroquine arm had 5 recurrences between 58 and 198 days. Alving and colleagues concluded that good efficacy of primaquine against relapse required the coadministration of quinine or chloroquine.
Fig. 2.
Relapse among 57 Caucasian subjects (19/group) in the United States who were challenged with sporozoites of the Chesson strain of P. vivax and randomized to three treatment arms represented by each line in the graph, as follows: 14 days of 2 g quinine daily followed by a 2-day pause and then 14 days of 15 mg primaquine daily (dashed line); precisely the same quinine and primaquine therapies except administered on the same days (solid line); and 1 g of chloroquine administered on the first of 14 days of 15 mg primaquine daily (dotted line). Data are from a study by Alving et al. (2).
The superior efficacy of quinine to chloroquine with primaquine against relapse (see Fig. 2) hints at chemical class-specific synergistic effects. Alving and colleagues later corrected this deficiency by increasing the daily dose of primaquine to 30 mg over 14 days (for the Chesson strain of P. vivax only) (6). Chloroquine and primaquine thus became the companion therapies of choice for vivax malaria, and drug discovery for this infection lapsed into a 60-year quiescence.
REPLACING CHLOROQUINE-PRIMAQUINE
The erosion to collapse of chloroquine efficacy in Indonesia and the spread of the problem into South and Southeast Asia, where the vast majority of vivax malaria occurs, commands attention to the observations of Alving and colleagues (2). The strategy of replacing chloroquine on the basis of efficacy against the asexual blood stages alone requires reassessment. The synergistic effect of quinine or chloroquine upon primaquine efficacy documented by Alving cannot be assumed for any given new blood schizontocide: the safety and efficacy of primaquine against relapse, when combined with another drug for radical cure of vivax malaria, must be established for each candidate blood schizontocide or combination thereof. This imposes serious obstacles to safe and effective radical cure.
The developers of chloroquine-primaquine had virtually unlimited access to infected soldiers and, especially, domestic prisoners for their experimental challenge trials. This was a key advantage because assessing the efficacy of primaquine against relapse requires long-term follow-up in the absence of risk of reinfection. Unlike falciparum malaria, biological ambiguities imposed by hypnozoites prohibit distinguishing therapeutic failures from reinfections, i.e., genotypes mismatched between primary and secondary parasitemias may originate from either reinfection or relapse. While it may be possible to measure likely reinfection rates among treated cohorts and estimate the risk attributable to relapse, statistical certainty with this approach would likely require relatively vast sample sizes. Heavily exposed populations returned to areas where the parasite is not endemic could provide the required analytical leverage and practicality, but such populations may be very rare. The most practical solution, despite high costs and difficult ethical issues, may be experimental challenge of human and nonhuman (using Plasmodium cynomolgi in Macaca mulatta) primate subjects in areas where the parasite is not endemic. That difficult task is compounded by the inability to culture this parasite in continuous in vitro systems and, thus, the requirement of regular access to patients with vivax malaria. This in turn requires inspired international cooperation and trust with fair sharing of biological materials and the intellectual property derived from them.
Indonesia has lost chloroquine-primaquine for radical cure, and other nations in South and Southeast Asia may soon follow. The selection of new blood schizontocide companions to primaquine in the absence of evidence demonstrating the safety and efficacy of primaquine against relapse could be a reckless course. The prudent course demands clinical trials of new blood schizontocides combined with primaquine that include credible estimates of efficacy against relapse. As Alving, his colleagues, and sponsors did successfully, we need only accept the difficulty and necessity of this task.
CONCLUSIONS
The loss of chloroquine to resistance in vivax malaria impels the selection of new companion drugs to primaquine for radical cure of this infection. If the hypnozoitocidal activity of primaquine requires an appropriate companion drug, the efficacy of primaquine against relapse must be reestablished with each new blood schizontocide paired with it. This imposes serious difficulties because such trials must be conducted in either rare special populations or experimental challenge trials. Countries like Indonesia, having neither therapeutic options nor evidence, must field a radical cure having no proven clinical benefit. In the absence of deliberate action by donors and sponsors to resolve this quandary, other nations in the region and beyond will face the same predicament. Resistance to chloroquine unhinges vivax malaria therapeutics.
ACKNOWLEDGMENT
The author thanks Colin Ohrt for his helpful review of the manuscript.
The author is supported by the Wellcome Trust.
Footnotes
Published ahead of print on 7 March 2011.
The author has paid a fee to allow immediate free access to this article.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Alving A. S., Craige B., Jr., Pullman T. N., Whorton C. M., Jones R., Jr., Eichelberger L. 1948. Procedures used at Stateville Penitentiary for the testing of potential antimalarial drugs. J. Clin. Invest. 27:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alving A. S., et al. 1955. Potentiation of the curative action of primaquine in vivax malaria by quinine and chloroquine. J. Lab. Clin. Med. 46:301–306 [PubMed] [Google Scholar]
- 3. Alving A. S., et al. 1948. The clinical trial of eighteen analogues of pamaquin (plasmochin) in vivax malaria (Chesson strain). J. Clin. Invest. 27:34–45 [PubMed] [Google Scholar]
- 4. Andrade B. B., et al. 2010. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malaria J. 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baird J. K. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 22:508–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baird J. K., Hoffman S. L. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336–1345 [DOI] [PubMed] [Google Scholar]
- 7. Berliner R. W., Taggart J. V., Welch W. J., Earle D. P., Jr., Shannon J. A. 1946. Pamaquin. 1. Curative antimalarial activity in vivax malaria. Fed. Proc. 5:165. [PubMed] [Google Scholar]
- 8. Berliner R. W., et al. 1948. Studies on the chemotherapy of the human malarias. VII. The antimalarial activity of pamaquine. J. Clin. Invest. 27:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Board for the Coordination of Malaria Studies 1943. Results of study of plasmochin toxicity: cases occurring in large scale use of the drug for suppression and treatment of malaria. Malaria report no. 290 Archive of the National Academy of Sciences, Washington, DC [Google Scholar]
- 10. Board for the Coordination of Malaria Studies 1945. Study of the curative action of plasmochin in vivax malaria: studies in Army and Navy installations. Malaria report no. 358 Archive of the National Academy of Sciences, Washington, DC [Google Scholar]
- 11. Coatney G. R., Cooper W. C., Ruhe D. S. 1948. Studies on human malaria. VI. The organization of program for testing potential antimalarial drugs in prisoner volunteers. Am. J. Epidemiol. 47:113–119 [PubMed] [Google Scholar]
- 12. Condon-Rall M. E. 1994. The Army's war against malaria: collaboration in drug research during World War II. Armed Forces Soc. 21:129–143 [Google Scholar]
- 13. Cordes W. 1927. Observations on the toxic effect of plasmochin, p. 62-67 16th Annual Report, United Fruit Company, Medical Department United Fruit Company, Boston, MA [Google Scholar]
- 14. Craige B., Jr., et al. 1947. The Chesson strain of Plasmodium vivax. II. Relationship between prepatent period, latent period, and relapse rate. J. Infect. Dis. 80:228–236 [DOI] [PubMed] [Google Scholar]
- 15. Craige B., Jr., et al. 1947. Clinical standardization of pamaquine (plasmochin) in mosquito-induced vivax malaria, Chesson strain. Am. J. Trop. Med. 27:309–315 [Google Scholar]
- 16. Downs W. G., Harper P. A., Lisansky E. T. 1947. Malaria and other insect-borne diseases in the South Pacific campaign. 1942-1945. II. Epidemiology of insect-borne diseases in Army troops. Am. J. Trop. Med. 27:69–89 [Google Scholar]
- 17. Edgecomb J. H., et al. 1950. Primaquine, SN13272, a new curative agent in vivax malaria. A preliminary report. J. Natl. Malar. Soc. 9:285–292 [PubMed] [Google Scholar]
- 18. Ehrman F. C., Ellis J. M., Young M. D. 1945. Plasmodium vivax Chesson strain. Science 101:377. [DOI] [PubMed] [Google Scholar]
- 19. Feachem R. G. A., et al. 2010. Shrinking the malaria map: progress and prospects. Lancet 376:1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genton, et al. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS. Med. 5:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerra C. A., et al. 2010. The international limits and population at risk of Plasmodium vivax in 2009. PLoS Negl. Trop. Dis. 4:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harijanto P. N. 2010. Malaria treatment using artemisinin in Indonesia. Acta Med. Indones. 42:51–56 [PubMed] [Google Scholar]
- 23. Hay S. I., Guerra C. A., Tatem A. J., Noor A. M., Snow R. W. 2004. Mapping the global extent of malaria past, present and future. Lancet Infect. Dis. 4:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joy R. J. T. 1999. Malaria in American troops in the South and Southwest Pacific in World War II. Med. Hist. 43:192–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kochar D. K., et al. 2009. Severe Plasmodium vivax malaria: report of serial cases from Bikaner in northwestern India. Am. J. Trop. Med. Hyg. 80:194–198 [PubMed] [Google Scholar]
- 26. Most H., et al. 1946. Chloroquine for the treatment of acute attacks of vivax malaria. JAMA 131:963–967 [DOI] [PubMed] [Google Scholar]
- 27. Office of the Surgeon General 1943. The drug treatment of malaria, suppressive and clinical. Circular letter no. 153. JAMA 123:205–208 [Google Scholar]
- 28. Price R. N., Douglas N. M., Anstey N. M. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435 [DOI] [PubMed] [Google Scholar]
- 29. Shannon J. A. 1946. Chemotherapy in malaria. Bull. N. Y. Acad. Med. 22:345–357 [PubMed] [Google Scholar]
- 30. Sinton J. A., Smith S., Pottinger D. 1929. Studies in malaria with special reference to treatment; further researches into the treatment of chronic benign malaria with plasmoquine and quinine. Indian J. Med. Res. 17:793–795 [Google Scholar]
- 31. Valecha N., et al. 2009. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 81:758–762 [DOI] [PubMed] [Google Scholar]
- 32. Wiselogle F. Y. 1946. A survey of antimalarial drugs, 1941-1945. J. W. Edwards, Ann Arbor, MI [Google Scholar]