Abstract
Mutant forms of the Plasmodium falciparum chloroquine resistance transporter (PfCRT) mediate chloroquine resistance by effluxing the drug from the parasite's digestive vacuole, the acidic organelle in which chloroquine exerts its parasiticidal effect. However, different parasites bearing the same mutant form of PfCRT can vary substantially in their chloroquine susceptibility. Here, we have investigated the biochemical basis for the difference in chloroquine response among transfectant parasite lines having different genetic backgrounds but bearing the same mutant form of PfCRT. Despite showing significant differences in their chloroquine susceptibility, all lines with the mutant PfCRT showed a similar chloroquine-induced H+ leak from the digestive vacuole, indicative of similar rates of PfCRT-mediated chloroquine efflux. Furthermore, all lines showed similarly reduced levels of drug accumulation. Factors other than chloroquine efflux and accumulation therefore influence the susceptibility to this drug in parasites expressing mutant PfCRT. Furthermore, in some but not all strains bearing mutant PfCRT, the 50% inhibitory concentration (IC50) for chloroquine and the degree of resistance compared to that of recombinant control parasites varied with the length of the parasite growth assays. In these parasites, the 50% inhibitory concentration for chloroquine measured in 72- or 96-h assays was significantly lower than that measured in 48-h assays. This highlights the importance of considering the first- and second-cycle activities of chloroquine in future studies of parasite susceptibility to this drug.
INTRODUCTION
In malaria parasite strains throughout the tropics, chloroquine (CQ) resistance is associated with point mutations in the gene encoding the Plasmodium falciparum chloroquine resistance transporter (PfCRT) (13). PfCRT is localized to the membrane of the parasite's internal digestive vacuole (DV) (9), the organelle in which CQ is thought to exert its toxicity (1, 14). Allelic exchange of the pfcrt allele in CQ-sensitive (CQS) GC03 parasites with mutant alleles found in CQ-resistant (CQR) parasites from Asia and Africa (the Dd2 allele) or South America and Papua New Guinea (the 7G8 allele) gave rise to CQR parasites (36). This showed that mutations in PfCRT are sufficient to confer CQ resistance to at least some CQS strains.
CQR parasites accumulate less CQ than their CQS counterparts (15, 19). The majority of the CQ accumulated by CQS parasites is within the DV (5), and DVs isolated from CQR parasites accumulate less CQ than those from CQS parasites (32), consistent with the view that a reduction in the intravacuolar CQ concentration is central to the phenomenon of CQ resistance (4). Verapamil, a weak base, increases the amount of CQ accumulated by CQR parasites, thus chemosensitizing the parasites to CQ action (19, 26, 41). It was recently reported that under conditions in which CQS HB3 and CQR Dd2 parasites accumulated CQ to similar (total) intracellular concentrations (as occurred when exposing the parasitized erythrocytes to external CQ concentrations of 250 nM and 750 nM, respectively), fewer CQR parasites were killed than were CQS parasites (7). Thus, a reduction in CQ accumulation may not be the only way by which CQR parasites avoid the toxic effects of CQ.
Upon expression of the wild-type and Dd2 mutant forms of PfCRT in Xenopus laevis oocytes, the mutant protein mediates verapamil-sensitive CQ transport, whereas the wild-type protein does not (25). This is consistent with the hypothesis that mutant PfCRT imparts CQ resistance by mediating the efflux of CQ from the DV, thereby reducing the accumulation of CQ in this compartment (4). However, PfCRT is not the sole determinant of parasite response to CQ. Several studies involving large numbers of P. falciparum strains, either field isolates from different geographic regions (8, 27) or the progeny of genetic crosses (12, 30), have shown that the CQ responses of strains with mutant PfCRT (including those with identical mutant forms of this protein) can vary widely.
In a recent study, Valderramos et al. (40) provided direct evidence that the degree of CQ resistance imparted by mutations in pfcrt is dependent on additional genetic factors. Wild-type pfcrt was replaced with the mutant South American 7G8 allele in three different CQS strains: GC03 (a progeny of the cross between the CQS HB3 clone and the CQR Dd2 clone), 3D7 (isolated in the Netherlands and thought to be of West African origin), and D10 (isolated in Papua New Guinea). The effect of introducing mutant pfcrt on parasite susceptibility to CQ varied between the three strains (40). Introduction of mutant pfcrt into the GC03 strain (generating the recombinant GC037G8 lines) resulted in a 4.7- to 4.8-fold increase in the 50% inhibitory concentration (IC50) for CQ in GC037G8 parasites compared to the recombinant control (GC03C) parasites. With 3D7, introduction of mutant pfcrt (generating the 3D77G8 lines) caused a smaller 2.7- to 2.9-fold increase in the IC50 for CQ relative to the recombinant control (3D7C) line. In contrast, when mutant pfcrt was introduced into the D10 strain, the CQ IC50s for the resulting (D107G8) lines were not significantly different from that of the recombinant control (D10C) line and were lower than those of the 3D77G8 and GC037G8 lines.
In all three genetic backgrounds, the introduction of 7G8 pfcrt was found to decrease the slope of the CQ dose-response curve, such that the CQ concentrations needed to prevent parasite proliferation by 90% (i.e., the IC90 values) were increased (40). In vitro tests with the CQ metabolite monodesethyl-CQ also indicated significantly decreased susceptibility in parasites expressing the 7G8 pfcrt allele in all three genetic backgrounds. Furthermore, recrudescence was observed for all lines expressing the 7G8 form of PfCRT after a 6-day treatment with CQ concentrations (50 nM and 80 nM) that were 100% lethal to all parasites expressing wild-type PfCRT. Thus, while not CQR, the D107G8 lines were deemed CQ tolerant (40).
In this study we investigated the biochemical basis for the variation in the CQ response among the pfcrt transfectant lines bearing the 7G8 form of PfCRT. In all three genetic backgrounds, the presence of CQ induced a verapamil-sensitive H+ leak from the DV, indicative of a PfCRT-mediated efflux of CQ from the DV. The magnitude of the H+ leak was similar in each case. Furthermore, all three recombinant mutant lines showed a similarly low level of CQ accumulation. Thus, differences in CQ susceptibility between these lines cannot be attributed to differences in PfCRT-mediated CQ efflux from the DV or to differences in steady-state CQ accumulation.
In several of the lines expressing 7G8 (but not wild-type) PfCRT, the degree of resistance was found to vary with the period over which the assay was conducted. In these lines, the CQ IC50 obtained when proliferation was measured within 48 h of the start of CQ exposure was significantly higher than when proliferation was measured over longer periods. Thus, investigations into the degree of CQ resistance must take into account both the first- and second-cycle actions of the drug.
MATERIALS AND METHODS
Parasite lines and culture.
The C2GC03 and C67G8 lines used in this study were generated by Sidhu et al. (36) using GC03, a CQS progeny of the HB3 × Dd2 cross (43). The C67G8 line was generated by replacing the wild-type pfcrt allele in GC03 with the CQ resistance-conferring mutant allele of pfcrt from the CQR 7G8 strain. The C2GC03 line is a CQS recombinant control line that expresses the wild-type pfcrt coding sequence. The D10C, D107G8, 3D7C, 3D77G8, GC03C, and GC037G8 lines were generated by Valderramos et al. (40). The superscripts “C” and “7G8” denote that the parasite line indicated (D10, 3D7, or GC03) expresses either the control wild-type or the mutant (7G8) pfcrt allele, respectively. The transfection strategies employed by Valderramos et al. (40) and Sidhu et al. (36) differed in terms of the genetic organization of the recombinant locus. Nevertheless, the GC03C and C2GC03 lines both express wild-type pfcrt in the GC03 background, and the GC037G8 and C67G8 lines both express the 7G8 form of pfcrt in the GC03 background.
It should be noted that the C67G8 line generated by Sidhu et al. (36) and the D107G8, 3D77G8, and GC037G8 lines generated by Valderramos et al. (40) contain an additional I351M mutation in PfCRT that does not occur in 7G8 parasites. The functional significance of this, if any, is unclear. Sidhu et al. (36) reported similar CQ IC50s for C67G8 parasites and 7G8 parasites, suggesting that this mutation does not influence the CQ response. Efforts are under way in the Fidock laboratory to remake the C67G8 line without the I351M mutation.
The parasites were cultured as described previously (2) and synchronized by sorbitol treatment (21). The pfcrt transfectant lines of Sidhu et al. (36) were maintained in the presence of the selection agents blasticidin (5 μM; Invitrogen, Australia) and WR99210 (5 nM; Jacobus Pharmaceuticals, Princeton, NJ). The pfcrt transfectant lines of Valderramos et al. (40) were maintained in the presence of 5 μM blasticidin. Selection agents were not present during experiments.
Measurement of the DV H+ leak.
The rate of H+ leakage from the parasite DV was measured using the membrane-impermeant pH-sensitive dye fluorescein-dextran (pKa ∼6.4; ∼10 × 103 Mr; Invitrogen, Australia). Uninfected erythrocytes were loaded with fluorescein-dextran (55 μM) and inoculated with trophozoite-infected erythrocytes, as described previously (20, 22–24, 31). As the parasites developed within the dye-loaded cells, they ingested the fluorescent dye, depositing it in their DV. In some experiments the trophozoite-infected erythrocytes used for inoculation were separated from uninfected erythrocytes by the use of a Miltenyi Biotec VarioMACS magnet (28, 37), in which case experiments were performed approximately 48 h (approximately one complete asexual cycle) after the inoculation. Alternatively, 1 to 2 ml of packed fluorescein-dextran-loaded erythrocytes were inoculated with an equal volume of an approximately 10% parasitemia and 4% hematocrit culture of trophozoite-infected erythrocytes, in which case experiments were performed after two complete cycles.
Fluorometry experiments were performed at 37°C with suspensions of dye-loaded, trophozoite-stage parasites functionally isolated from their host erythrocytes by saponin permeabilization of the erythrocyte membrane, as described elsewhere (23). Changes in the pH of the DV (pHDV) were tracked by monitoring the ratio of the fluorescence intensity at 520 nm using excitation wavelengths of 490 nm and 450 nm. Concanamycin A, CQ diphosphate, and verapamil hydrochloride were purchased from Sigma (Australia).
To measure the leakage of H+ from the DV, the V-type H+ pump inhibitor concanamycin A was added to parasite suspensions and the rate of the consequent alkalinization was monitored (22–24). DV alkalinization half-times were determined by fitting the following sigmoidal curve to the data by regression analysis using SigmaPlot 11.0 (Systat Software, Inc.): F = F0 + Fmax/[1 + (t/t1/2)c], where F is the fluorescence ratio, F0 is the initial fluorescence ratio (set to the resting fluorescence ratio averaged over the 20 s immediately prior to the opening of the fluorometer chamber to add concanamycin A), Fmax is the maximal change in the fluorescence ratio, and c is a fitted constant.
CQ accumulation assays.
Mature (intact) trophozoite-infected erythrocytes (∼36 h postinvasion) were washed and resuspended in bicarbonate-free RPMI 1640 medium (supplemented with 25 mM HEPES, 10 mM glucose, and 0.2 mM hypoxanthine and adjusted to pH 7.4) at a parasitemia of approximately 5% and a hematocrit of approximately 2%. Uptake measurements began with the addition of [3H]CQ (5.2 Ci/mmol; Moravek Biochemicals) in bicarbonate-free RPMI 1640 medium to an equal volume of cell suspension, giving a final [3H]CQ concentration of either 156 nM or 2 nM (as specified). After 1 h of incubation at 37°C, aliquots of the suspension (200 μl) were transferred (in duplicate) to microcentrifuge tubes containing 300 μl of dibutyl phthalate (density of 1.04 g/ml) layered over 30 μl of 30%, wt/vol, perchloric acid. The tubes were centrifuged immediately (17,000 × g, 2 min) to sediment the cells through the oil and into the acid, thereby terminating [3H]CQ uptake, lysing the cells, and precipitating proteins. A 20-μl aliquot of the supernatant solution was then taken from above the oil layer in each tube to permit the calculation of extracellular [3H]CQ concentrations. The remaining supernatant solution was removed by aspiration, and residual radioactivity on the sides of each tube was removed by rinsing the tube four times with water before aspirating the majority of the oil. Trichloroacetic acid (1 ml, 5%, wt/vol) was added to the perchloric acid extracts (as well as to the tubes containing the samples of supernatant solution). The tubes were centrifuged (17,000 × g, 10 min), and the resulting supernatant solutions were transferred to vials for scintillation counting.
The volume of ([3H]CQ-containing) extracellular solution trapped between cells as they were centrifuged through the oil layer was estimated by adding [3H]CQ to uninfected erythrocytes in the presence of 20 μM unlabeled CQ (to minimize uptake of [3H]CQ) and then sampling immediately. Similar results were obtained when using parasitized erythrocytes in place of uninfected erythrocytes. The contribution of extracellular radiolabel to the total radiolabel present in the cell pellets sampled at the end of the 1-h incubation period was calculated by multiplying the volume of trapped extracellular solution by the concentration of radioactivity present in the extracellular solution (as measured at the end of the 1-h incubation). The intracellular radiolabel content was then calculated by subtracting the extracellular radiolabel from the total radiolabel associated with the cell pellet. The small amount of [3H]CQ uptake attributable to the presence of uninfected erythrocytes in the suspension (determined by including an uninfected erythrocyte control in each experiment) was subtracted from the CQ accumulation data for each parasite line. CQ accumulation ratios (i.e., the concentration of radiolabeled CQ within the infected cells relative to the concentration in the extracellular medium) for the infected cells were then calculated using a previous estimate of the water volume of a trophozoite-infected erythrocyte (75 fl) (33).
Parasite proliferation assays.
Parasite proliferation was measured in 96-well plates using the [3H]hypoxanthine incorporation method (10). The cells were suspended in low-hypoxanthine medium (RPMI 1640 medium supplemented with 25 mM HEPES, 10 mM glucose, 2.4 μM hypoxanthine, 25 mg/liter gentamicin sulfate, and 0.5%, wt/vol, Albumax II) at a parasitemia of 0.5% (consisting of early-ring-stage parasites) and a hematocrit of 1%. [3H]hypoxanthine (0.4 μCi, in a volume of 25 μl low-hypoxanthine medium) was added at the 24-h time point in 48-h assays, at the 48-h time point in 72-h assays, and at the 72-h time point in 96-h assays, such that in each case [3H]hypoxanthine incorporation occurred over a 24-h period. IC50s were determined by nonlinear regression using the following equation: y = a/[1 + (x/IC50)c], where y is the percent parasite proliferation, a is the maximal change in the percent parasite proliferation, x is the drug concentration, and c is a fitted constant.
RESULTS
All parasite lines harboring the mutant 7G8 form of PfCRT exhibit a similar chloroquine-associated H+ leak from the digestive vacuole.
One possible explanation for the observation that the D107G8 parasites, expressing mutant (7G8) PfCRT in the D10 background, showed greater CQ sensitivity than the other parasite lines expressing the 7G8 form of PfCRT (3D77G8 and GC037G8) (40) is that mutant PfCRT does not mediate CQ efflux from the DV in the D10 strain. This might be because of inhibitory interactions with other proteins or because the physiological environment is not conducive to the transport activity of the mutant protein. We therefore investigated whether there was a CQ-associated H+ leak (indicative of mutant-PfCRT-mediated CQ efflux [23]) from the DV in each of the pfcrt transfectant lines created by Valderramos et al. (40) using a live-cell method in which the rate of concanamycin A-induced DV alkalinization was monitored over several minutes (23).
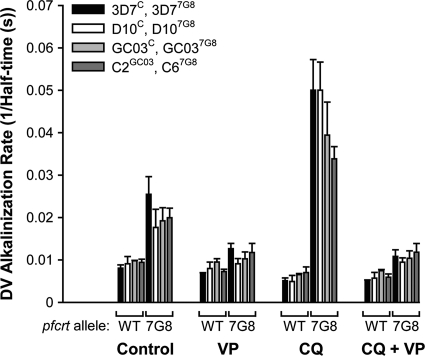
For each of the pfcrt-modified clones studied, the addition of the V-type H+-ATPase inhibitor concanamycin A (100 nM) gave rise to an immediate alkalinization of the DV (Fig. 1A and B), as seen previously with several other strains (17, 22, 23, 31). The rate of alkalinization measured in the absence of CQ (i.e., at a CQ concentration of 0) (Fig. 1C) was significantly higher in the lines bearing the 7G8 form of PfCRT (D107G8, 3D77G8, and GC037G8) than in the lines with wild-type PfCRT (D10C, 3D7C, and GC03C; P ≤ 0.05, unpaired t tests). This is consistent with the previous finding that concanamycin A-induced DV alkalinization is faster in parasites expressing mutant forms of PfCRT than in those expressing the wild-type form of the protein (23, 24). There were no significant differences in the DV alkalinization rates among lines bearing the same form of PfCRT in the absence of CQ (P > 0.3, unpaired t tests).
Fig. 1.
Alkalinization of the DV by the V-type H+-ATPase inhibitor concanamycin A. (A and B) Representative fluorometer traces showing the alkalinization of the DV following the addition of concanamycin A (100 nM, at the point indicated by the black triangle) to isolated mature trophozoite-stage CQS D10C (A) and CQR D107G8 (B) parasites suspended in the presence (gray traces) or absence (solvent control; black traces) of 2.5 μM CQ. CQ was added 4 min before the addition of concanamycin A. (C) Effect of increasing external CQ concentrations on the rate of DV alkalinization (expressed as the inverse of the half-time) following H+-ATPase inhibition in the pfcrt-modified clones. CQ was added to saponin-isolated mature trophozoites containing fluorescein-dextran in their DVs 4 min prior to the addition of concanamycin A (100 nM). Data are averaged from at least three separate experiments for each line and are shown as means ± SEM. For clarity, only positive error bars are shown for the 3D7C, 3D77G8, GC03C, and C67G8 lines, and only negative error bars are shown for the D10C, D107G8, and GC037G8 lines. Where not shown, error bars fall within the symbols.
As shown in the representative traces in Fig. 1B and in the averaged data presented in Fig. 1C and Fig. 2, the inclusion of CQ in the medium increased the rate of DV alkalinization following H+ pump inhibition in all lines expressing the 7G8 form of PfCRT. This is consistent with previous findings obtained with the wild-type 7G8 strain (22) and with the recombinant C67G8 line (23). Figure 1C shows the CQ concentration dependence of the CQ-induced changes in the DV alkalinization rate following H+ pump inhibition. In all lines with the 7G8 form of PfCRT, the rate of DV alkalinization increased with increasing CQ concentration, with a statistically significant effect observed at CQ concentrations of ≥2.5 μM (as well as at lower concentrations in some lines; P < 0.04, paired t tests). With only a single exception (between D107G8 parasites and C67G8 parasites at a CQ concentration of 2.5 μM; P = 0.02, unpaired t test), there were no significant differences in DV alkalinization rates between any of the four lines expressing the 7G8 form of PfCRT at any of the eight CQ concentrations tested (P ≥ 0.05, unpaired t tests). In contrast to the increase in the rate of DV alkalinization seen upon addition of CQ to parasites expressing 7G8 PfCRT, CQ slowed the rate of DV alkalinization in lines with wild-type PfCRT (Fig. 1A and C and 2), as seen previously with all CQS strains tested to date (22, 23).
Fig. 2.
Effect of verapamil (VP) on the CQ-induced changes in the DV alkalinization rate (expressed as the inverse of the half-time) following H+-ATPase inhibition in the transfectant lines expressing wild-type (WT) PfCRT or 7G8 PfCRT. The data are averaged from three independent experiments (shown as means + SEM) for each line. CQ (2.5 μM) and VP (50 μM) were added to suspensions of isolated fluorescein-dextran-loaded mature trophozoites 4 min before the addition of concanamycin A (100 nM). The relevant solvent controls were performed in each case. Parasite lines were generated by Valderramos et al. (40) (3D7C, 3D77G8, D10C, D107G8, GC03C, and GC037G8) and Sidhu et al. (36) (C2GC03 and C67G8).
Figure 2 shows the verapamil sensitivity of the CQ-associated H+ leak. In all lines expressing the 7G8 form of PfCRT, verapamil (at a concentration of 50 μM) inhibited the CQ-associated H+ leak, such that the rate of DV alkalinization in the presence of CQ and verapamil was not significantly different from that in the presence of verapamil alone (P > 0.4, paired t tests). As has been observed with some (but not all) of the parasite lines tested previously (22–24), 50 μM verapamil alone slowed the rate of DV alkalinization in each of the eight lines investigated here, although statistical significance was attained only for the C2GC03, C67G8, and GC037G8 lines (P < 0.04, paired t tests).
The data of Fig. 1C and 2 are consistent with the 7G8 form of PfCRT imparting a similar-sized leak of CQ from the DV in every parasite line expressing this protein, irrespective of the parasite's genetic background and degree of CQ susceptibility.
All lines with the 7G8 form of PfCRT show the same reduced level of chloroquine accumulation relative to lines expressing wild-type PfCRT.
Given the evidence for CQ:H+ efflux from the DV in all lines expressing the 7G8 form of PfCRT, it was postulated that in 3D77G8 and D107G8 parasites, there may be other factors that counter (or partially counter) PfCRT-mediated CQ efflux, such that these parasites accumulate more CQ in their DVs (and are consequently more sensitive to CQ) than the more CQR GC037G8 parasites. One possibility is that the wild-type form of the DV membrane transport protein P-glycoprotein homologue 1 (Pgh1), which is present in the 3D77G8 and D107G8 parasites, might transport CQ into the DV in these parasites, whereas this would not be expected with GC037G8 parasites, which possess a mutant form of Pgh1 that does not transport CQ (35).
To investigate this possibility, drug accumulation was measured at two different concentrations of [3H]CQ: 2 nM and 156 nM. The former concentration (2 nM) is well below the IC50 for inhibition of parasite growth in both the D107G8 line and its recombinant control (40). The latter concentration (156 nM) is slightly higher than the IC90 value reported by Valderramos et al. for D107G8 parasites (148 nM) (40) and corresponds to the lowest concentration used in the CQ:H+ leak assays that clearly distinguished between the lines expressing 7G8 PfCRT, on one hand, and those expressing wild-type PfCRT, on the other (Fig. 1C).
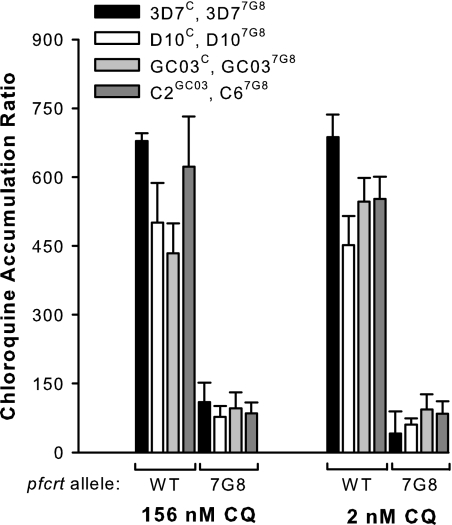
As shown in Fig. 3, at both of the [3H]CQ concentrations tested, the CQ accumulation ratio for every line with the 7G8 form of PfCRT was significantly lower than the ratio for every line with wild-type PfCRT (P ≤ 0.01, unpaired t tests). With one exception (between GC03C parasites and 3D7C parasites with 156 nM CQ; P = 0.02, unpaired t test), there were no significant differences in the CQ accumulation ratios between lines having the same form of PfCRT (P > 0.1, unpaired t tests). Thus, wild-type Pgh1 does not confer an enhanced accumulation of CQ to those lines that express it, and differences in CQ accumulation do not account for the variation in CQ response among lines with the 7G8 form of PfCRT.
Fig. 3.
Accumulation of [3H]CQ by erythrocytes infected with mature trophozoite-stage parasites. [3H]CQ accumulation is expressed in terms of the chloroquine accumulation ratio, i.e., the concentration of radiolabeled CQ within the infected cells relative to the concentration in the extracellular medium. The black, white, and light gray bars show data for the parasite lines generated by Valderramos et al. (40) (with the genetic background and pfcrt allele of each line indicated), and the dark gray bars show data for the parasite lines generated by Sidhu et al. (36). The accumulation assays were performed over 1 h at 37°C with [3H]CQ concentrations of 156 nM and 2 nM. The data represent the means (+SEM) of three to five independent experiments for each line.
D107G8, 3D77G8, and 7G8 parasites display different chloroquine IC50s depending on the duration of the growth assay.
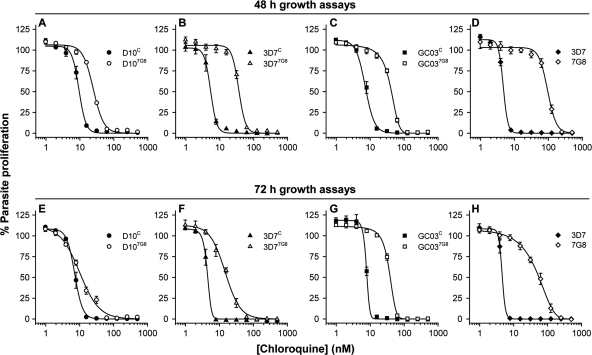
The finding that the D107G8, 3D77G8, and GC037G8 lines all yielded very similar data in both the CQ:H+ leak assays and the CQ accumulation assays prompted us to undertake a series of parasite proliferation assays to investigate in greater detail the differential CQ susceptibilities of the pfcrt transfectant lines. In initial experiments, erythrocytes infected with synchronous early-ring-stage parasites were incubated in the presence of CQ at a range of concentrations for 48 h, with [3H]hypoxanthine added at the 24-h time point. Incorporation of this radiolabeled nucleobase into parasite nucleic acids between 24 h and 48 h after the commencement of the assay provides a measure of parasite proliferation. Figure 4A to D show the results of 48-h CQ growth assays, comparing the D107G8, 3D77G8, and GC037G8 lines with their recombinant control lines (Fig. 4A, B, and C, respectively), as well as nontransfectant 3D7 parasites with nontransfectant 7G8 parasites (Fig. 4D). The CQ IC50s obtained for each strain are presented in Table 1. The results, shown in Fig. 4, reveal a clear separation in the CQ dose-response curves of each 7G8 transfectant line and its recombinant control; in each case there was a significant difference between the CQ IC50s (P < 0.001, unpaired t tests) (Table 1). The fold differences in the mean CQ IC50s between the 7G8 transfectant and recombinant control lines were 2.7 in the D10 background, 6.7 in the 3D7 background, and 5.5 in the GC03 background.
Fig. 4.
The effect of increasing CQ concentrations on parasite proliferation, as assessed using 48-h (A to D) and 72-h (E to H) [3H]hypoxanthine incorporation assays. Data, averaged from 3 to 6 experiments and shown as means ± SEM, are presented for the D10C and D107G8 lines (A and E), the 3D7C and 3D77G8 lines (B and F), the GC03C and GC037G8 lines (C and G), and the nontransfectant 3D7 and 7G8 lines (D and H). Data for lines expressing 7G8 PfCRT and wild-type PfCRT are shown with open symbols and closed symbols, respectively. Where not shown, error bars fall within the symbols.
Table 1.
CQ IC50 values for the pfcrt transfectant lines and the (nontransfectant) 3D7 and 7G8 strains, as determined using 48-h and 72-h [3H]hypoxanthine incorporation assaysa
| Parasite line | Mean CQ IC50 ± SEM (nM) |
P value (no. of experiments)b | |
|---|---|---|---|
| 48 h | 72 h | ||
| D10C | 9.1 ± 0.7 | 7.2 ± 0.6 | 0.07 (6) |
| D107G8 | 24.9 ± 0.7 | 10.2 ± 0.8 | <0.001 (5) |
| 3D7C | 5.3 ± 0.4 | 4.3 ± 0.2 | 0.05 (3) |
| 3D77G8 | 35.7 ± 1.8 | 14.2 ± 2.0 | <0.001 (5) |
| GC03C | 7.2 ± 0.6 | 7.0 ± 0.6 | 0.9 (4) |
| GC037G8 | 39.4 ± 1.6 | 33.6 ± 2.0 | 0.05 (6) |
| 3D7 | 4.8 ± 0.2 | 4.6 ± 0.2 | 0.6 (6) |
| 7G8 | 95 ± 4 | 48 ± 5 | 0.002 (3) |
The pfcrt transfectant lines were made by Valderramos et al. (40). In each assay, [3H]hypoxanthine was added 24 h before the termination of the assay. The IC50s are averaged from those obtained in 3 to 6 separate experiments. The 48-h and 72-h assays were set up concurrently.
P values as determined for 48-h versus 72-h IC50s, unpaired t tests.
These results contrast with those presented by Valderramos et al. (40), who reported that expression of the 7G8 form of PfCRT caused a substantial increase in the CQ IC50 in the GC03 background, a smaller shift in the CQ IC50 in the 3D7 background, and no significant shift in the CQ IC50 in the D10 background. In the study by Valderramos et al. (40), parasite growth assays were performed over 72 h, with [3H]hypoxanthine added at the 48-h time point. To investigate whether the different incubation times might account for the apparent discrepancies in the CQ sensitivity data, we performed 72-h growth assays. Erythrocytes infected with synchronous early-ring-stage parasites were incubated in the presence of CQ at a range of concentrations for 72 h, with [3H]hypoxanthine added at the 48-h time point. The results of the 72-h assays are presented in Fig. 4E to H and Table 1. For the D107G8, 3D77G8, and 7G8 strains, the CQ IC50s were significantly lower when measured in the 72-h assays than when measured in the 48-h assays (P ≤ 0.002, unpaired t tests). In contrast, the CQ IC50s of the D10C, 3D7C, GC03C, GC037G8, and 3D7 strains measured in the 72-h assays were similar to those measured in the 48-h assays (P ≥ 0.05, unpaired t tests).
As shown in Fig. 4E, the separation that was observed between the CQ dose-response curves of D107G8 and D10C parasites in the 48-h assays (Fig. 4A) virtually disappeared in the 72-h assays (although a statistically significant difference in the CQ IC50s between the lines remained in the 72-h assays; P = 0.02, unpaired t test). There was also a narrowing of the separation between the 3D77G8 and 3D7C parasites (Fig. 4F and B) and between the 3D7 and 7G8 parasites (Fig. 4H and D) in the 72-h assays, though in both cases there remained a significant difference in the CQ IC50s (P ≤ 0.006, unpaired t tests). The separation in the CQ dose-response curves between GC037G8 and GC03C parasites after 72 h (Fig. 4G) did not differ significantly from that observed after 48 h (Fig. 4C).
It should be noted that the CQ IC50s for all the strains (both CQS and CQR) were lower than those reported by Valderramos et al. (40), for reasons that are unknown. Nonetheless, the fold differences in the mean CQ IC50s between each 7G8 transfectant line and its recombinant control line after 72 h (as shown in Table 1) were consistent with those reported previously by Valderramos et al. for the relevant clones (40): 1.4- and 4.8-fold for the D10 and GC03 backgrounds, respectively, both here and previously; and 3.3-fold for the 3D7 background here versus 2.9-fold previously (40).
In the 48-h assays, the [3H]hypoxanthine incorporation window (24 to 48 h) encompasses the trophozoite and schizont stages of the first parasite cycle and the invasion of new erythrocytes by second-generation merozoites. In the 72-h assays the [3H]hypoxanthine incorporation window encompasses the transition of second-generation ring-stage parasites into trophozoites (48 to 72 h) (see micrographs of untreated parasites in Fig. 5C). To investigate whether the decrease in the CQ IC50 observed with several of the 7G8-PfCRT-expressing strains upon increasing the length of the assay from 48 h to 72 h was due to an increased sensitivity of parasites to CQ in the second generation after exposure (rather than being a consequence of comparing [3H]hypoxanthine incorporation by parasites at different developmental stages), we compared the levels of CQ sensitivity of the D10C and D107G8 lines in concurrent 48-h, 72-h, and 96-h assays. In the 96-h assays, the [3H]hypoxanthine incorporation window (72 to 96 h) encompasses the trophozoite and schizont stages of the second parasite cycle and the invasion of new erythrocytes by third-generation merozoites; the parasite developmental stages over which [3H]hypoxanthine incorporation is measured are therefore the same as those in the 48-h assays. These results are presented in Fig. 5.
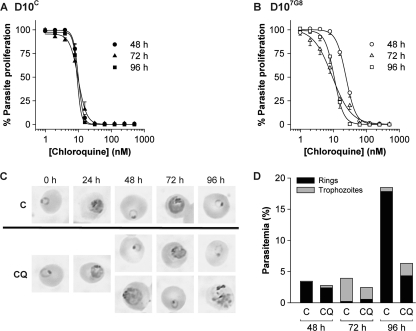
Fig. 5.
The effect of increasing CQ concentrations on parasite proliferation in D10C (A) and D107G8 (B) parasites, as assessed using 48-h (circles), 72-h (triangles), and 96-h (squares) [3H]hypoxanthine incorporation assays. Data are averaged from four independent experiments and are shown as means ± SEM. For clarity, only positive error bars are shown for D10C, and only negative error bars are shown for D107G8. Where not shown, error bars fall within the symbols. (C) Giemsa-stained smears of untreated (control [“C”]) D107G8 parasites (top) and of D107G8 parasites treated with 15 nM CQ (bottom) at various time points following the start of CQ exposure. Where two photos are shown for one time point and condition, they represent parasite stages that were both present in significant numbers. (D) Parasitemias of untreated (“C”) and CQ (15 nM)-treated cells and proportions of ring-stage parasites and trophozoite-stage parasites at the 48-h, 72-h, and 96-h time points in the experiment from which the micrographs in panel C were obtained.
For D10C parasites (Fig. 5A), there was no significant difference between the CQ IC50s obtained in the 48-h, 72-h, or 96-h assays (10.0 ± 0.7, 10.3 ± 1.7, and 9.2 ± 0.9 nM [mean ± standard error of the mean {SEM}], respectively; P > 0.5, unpaired t tests), nor was there a discernible difference in the slope of the dose-response curves. However, for D107G8 parasites, the CQ IC50 obtained in the 48-h assay (24.0 ± 1.7 nM) was significantly higher than those obtained in both the 72-h (8.6 ± 1.4 nM; P = 0.0005, unpaired t test) and the 96-h (10.7 ± 1.1 nM; P = 0.0007, unpaired t test) assays. The CQ IC50s obtained in the 72-h and 96-h assays were not significantly different from one another (P = 0.3, unpaired t test). However, the dose-response curves obtained in the 72-h assays were less steep than those observed in the 96-h (and 48-h) assays (Fig. 5B).
The CQ response of D107G8 parasites was investigated further using Giemsa-stained smears of parasites exposed to 15 nM CQ, with samples taken every 24 h from the commencement of CQ exposure (starting with early-ring-stage parasites at a parasitemia of 0.5%). The experiment was done concurrently with one of the experiments giving rise to the [3H]hypoxanthine incorporation data presented in Fig. 5B. Representative micrographs obtained at each time point are presented in Fig. 5C. Untreated control parasites attained parasitemias of 3.5% after 48 h and 18% after 96 h and remained highly synchronous throughout the experiment (Fig. 5D). In D107G8 parasites exposed to 15 nM CQ, the second- and third-generation parasitemias were lower: 2.8% at 48 h and 6.3% at 96 h. At the 48-h time point, 13% of the parasites were morphologically abnormal trophozoites (see representative micrograph in Fig. 5C) that had presumably succumbed to death in the first cycle. In the concurrent 48-h [3H]hypoxanthine incorporation assay, the percent parasite proliferation with 15.6 nM CQ was 81 ± 1 (mean ± SEM of triplicate measurements) of control levels, consistent with a portion of the parasites undergoing death in their first cycle at this CQ concentration.
In the 72-h and 96-h [3H]hypoxanthine incorporation assays, the values for the percent parasite proliferation with 15.6 nM CQ were 30 ± 1 and 35 ± 1, respectively, of control levels. The 96-h value agrees well with the finding of a 3-fold-lower parasitemia in parasites treated with 15 nM CQ relative to untreated parasites. The finding that at 72 h the percent parasite proliferation was 30 ± 1 of control levels, yet that the parasitemia was at 62% of control levels, suggests that some of the CQ-treated parasites at the 72-h time point were dead or slowed in their development. At the 72-h time point, the CQ-treated parasite population consisted of 23% rings and 77% trophozoites, and at 96 h, it consisted of 69% rings and 31% (morphologically abnormal) trophozoites (Fig. 5C and D).
Thus, it appears that the exposure of D107G8 parasites to 15 nM CQ resulted in the death of some parasites in the first generation, as well as of some of the progeny of the parasites that survived the first cycle. Furthermore, the presence of rings at the 72-h time point is indicative of a delay or halt in the development of some second-generation parasites.
DISCUSSION
Genetically distinct parasites expressing the 7G8 form of PfCRT show a similar chloroquine-associated H+ leak and a similarly low level of chloroquine accumulation.
P. falciparum parasite lines harboring mutant forms of PfCRT vary considerably in their sensitivity to CQ (8, 12, 27, 30). A recent transfection study in which the same mutant form of PfCRT was introduced into three different parasite strains revealed that the genetic background influences the extent to which mutant PfCRT gives rise to CQ resistance (40). Here, we performed biochemical studies on these recombinant lines to investigate the mechanistic basis for this observation.
Neither the CQ:H+ leak studies nor the CQ accumulation assays performed on the pfcrt transfectant lines of Valderramos et al. (40) uncovered any differences among lines bearing the same form of PfCRT. The verapamil-sensitive CQ-associated H+ leak from the DV, described previously (22, 23), was seen with all lines with the 7G8 form of PfCRT and in none of the lines with wild-type PfCRT (Fig. 1 and 2). Furthermore, all parasites with the 7G8 form of PfCRT accumulated CQ to a level that was approximately the same in each case and that was much lower than that seen for parasites with wild-type PfCRT (Fig. 3).
The CQ accumulation data reflect the total uptake of CQ by parasitized erythrocytes and do not exclude the possibility that there may be differences in intravacuolar CQ concentrations. Measuring the concentration of CQ in the DV is inherently difficult. Using an approach that entailed loading infected erythrocytes with [3H]CQ and then rapidly performing parasite isolation and DV isolation procedures, Bray et al. estimated that under the conditions of their experiment ∼85% of the CQ contained in CQS C2GC03-infected erythrocytes was present within the parasite DV, whereas only about half of the CQ present in CQR C3Dd2-infected erythrocytes was intravacuolar (5). Small differences in the levels of intravacuolar CQ accumulation between the lines expressing 7G8 PfCRT are likely to be difficult to observe (if the cytoplasmic CQ concentrations are similar between the strains) and/or may be obscured by differences in the uptake of CQ into other subcellular compartments. Nevertheless, it is clear that the D107G8 line, which on the basis of its 72-h CQ IC50 would be considered CQS, clusters with the CQR lines in terms of its CQ accumulation profile.
Valderramos et al. (40) reported that in 72-h assays, the D107G8 and 3D77G8 lines, both of which express the wild-type form of Pgh1 (shown previously to transport CQ when expressed in Xenopus oocytes [35]), are more susceptible to CQ than GC037G8 parasites (which express a mutant form of Pgh1 that does not transport CQ [35]). However, under the conditions of our CQ accumulation experiments, the presence of the wild-type form of Pgh1 did not confer a detectable increase in CQ accumulation to D107G8 and 3D77G8 parasites relative to GC037G8 and C67G8 parasites, nor to D10C and 3D7C parasites relative to GC03C and C2GC03 parasites (Fig. 3). This suggests that the relative contribution of Pgh1 to the flux of CQ across the DV membrane in these strains is negligible (34), that its influence on CQ accumulation in the DV is too small to be detected in CQ accumulation assays performed on intact erythrocytes, or that other genetic differences between the strains counteract any effect that Pgh1 might have on CQ accumulation. Thus, if wild-type Pgh1 does contribute to the lower 72-h CQ IC50s of D107G8 and 3D77G8 parasites relative to GC037G8 parasites, it is likely to be via an accumulation-independent mechanism. One possibility is that if wild-type Pgh1 is actively transporting CQ into the DV in D107G8 and 3D77G8 parasites, then the associated metabolic cost might hamper the ability of the parasites to counter CQ-mediated cellular damage. Another is that CQ may competitively inhibit the transport of endogenous substrates via Pgh1 and that this may be a facet of the mechanism of action of CQ against strains with wild-type Pgh1.
It should be noted that Reed et al. (29) reported an approximately 2-fold increase in CQ accumulation in their pfmdr1 transfectant 7G8-mdrD10 lines relative to the 7G8-mdr7G8 recombinant control line. However, only the three 3′ mutations were altered in 7G8-mdrD10 parasites relative to the 7G8 allele, and both of the forms examined (NFCDY and NFSND at positions 86, 184, 1034, 1042, and 1246) were shown not to transport CQ when expressed in Xenopus oocytes (35). Thus, the basis for the difference in CQ accumulation between these pfmdr1 transfectant lines (29) is not clear.
For some strains expressing 7G8 PfCRT, the chloroquine IC50 varies with the duration of the growth assay.
The CQ dose-response curves obtained for D107G8, 3D77G8, and 7G8 parasites differed depending on the time period at which parasite proliferation was assessed (Fig. 4 and Table 1). In assays in which parasites were exposed to CQ for 72 h, with [3H]hypoxanthine incorporation measured over the last 24 h, Valderramos et al. (40) observed a minimal difference in the CQ IC50s between the D107G8 line and its recombinant control line D10C. A similar result was obtained here (Table 1). However, when the parasites were exposed to CQ for just 48 h (i.e., the approximate duration of one asexual blood-stage cycle), with [3H]hypoxanthine incorporation again measured over the last 24 h, a different picture emerged; for each of the D107G8, 3D77G8, and 7G8 parasite strains, the IC50s determined in 48-h assays were significantly higher than those determined in 72-h assays, whereas for each of the other strains the IC50s measured at 48 h were similar to those measured at 72 h. In the 48-h assays there was a significant separation in the CQ dose-response curves of D107G8 and D10C parasites (Fig. 4A). These data are consistent with the earlier designation of D107G8 parasites as CQ tolerant and not CQS (40).
There was no change in the CQ IC50s of D107G8 parasites (or D10C parasites) when growth assays were lengthened from 72 h to 96 h. Thus, the different CQ responses observed with 72-h assays compared to 48-h assays indicate a genuine “second-cycle effect” and are not a result of comparing [3H]hypoxanthine incorporation by different developmental stages. There was, however, a marked difference in the slopes of the 72-h and 96-h dose-response curves in D107G8 parasites (but not D10C parasites), with the 96-h dose-response curves reverting to the steeper slope observed in 48-h assays. The reason for the reduced slope in the 72-h assays is not clear but may indicate the contributions of multiple effects of CQ on the parasite during the 48-h-to-72-h [3H]hypoxanthine incorporation window.
Microscopic examination of D107G8 parasites treated with 15 nM CQ (a concentration at which, according to [3H]hypoxanthine incorporation assays, there was little reduction in parasite proliferation after 48 h but a marked reduction after 72 h and 96 h) revealed a complex picture. A proportion of first-generation and second-generation parasites appeared to form parasites with the appearance of abnormal trophozoites, suggesting parasite death at this developmental stage. Additionally, some second-generation parasites appeared to be halted or slowed in their development at the ring stage.
In a recent study in which parasites were exposed to CQ either continuously or for short time periods at specific stages of development, Gligorijevic et al. (16) reported four different effects of CQ: (i) an inhibition of hemozoin formation, (ii) a decrease in the number of nuclei observed in segmented schizonts, (iii) a decrease in the infectivity of the progeny of CQ-treated parasites, and (iv) a decrease in the number of second-generation parasites that progress from the ring to the trophozoite stage. The first three of these would be expected to contribute to the CQ IC50 in 48-h assays, whereas the fourth would impact on the IC50 only in longer (72-h and 96-h) assays. Gligorijevic et al. reported that mutant PfCRT conferred resistance to all four facets of CQ toxicity (16). The strains compared by Gligorijevic et al. were Dd2 (CQR), HB3 (CQS), GC03 (a CQS progeny of the HB3 × Dd2 cross), C2GC03 (CQS), and C4Dd2 (CQR, expressing the Dd2 form of PfCRT in the GC03 background). Our results are consistent with that study, because with the GC03 background a similar difference between the CQ IC50s of the 7G8 PfCRT-expressing and wild-type PfCRT-expressing parasites was seen in 48-h and 72-h assays. However, our results might suggest that mutant PfCRT does not confer resistance to the delayed effect of CQ in the D10 background and confers only partial resistance in the 3D7 background. Perhaps the highly CQR Dd2 strain acquired an adaptation subsequent to mutations in PfCRT that allowed it to avoid the delayed component of CQ toxicity in addition to the earlier effects of the drug, a trait that could have been passed on to GC03.
The molecular mechanism(s) by which CQ exerts its toxicity against P. falciparum strains with a mutant form of PfCRT are not clear; thus, it is difficult to predict the basis for the enhanced potency of CQ against some such strains in the second cycle following CQ exposure. The apparent lack of a correlation between CQ accumulation and (first- or second-cycle) CQ sensitivity among strains with mutant PfCRT might indicate that the primary mechanism of CQ action in (some or all) parasites with mutant PfCRT is not the inhibition of heme detoxification in the DV (the proposed mechanism of action of the drug in CQS parasites [6, 11, 18, 38, 39]).
The pfcrt transfectant lines generated to date (36, 40) have reduced levels of PfCRT protein and in most cases lower CQ IC50s (measured at 72 h in references 36 and 40), compared to the strains from which the mutant pfcrt alleles were obtained, yet accumulate CQ to a similarly low level compared to that seen with highly resistant nontransfectant strains (36; this study). This raises the possibility that mutant PfCRT might itself be a target of CQ. PfCRT is likely to have an essential role in the parasite (42). CQ is a transport substrate for mutant (but not wild-type) PfCRT (25) and, as such, may act as a competitive inhibitor of the transport of the (unknown) endogenous substrates via the mutant protein. This would provide one possible explanation for why parasites having lower levels of expression of mutant PfCRT (i.e., lower levels of the putative target) are more sensitive to growth inhibition by CQ than those with higher levels of expression, despite showing similar levels of CQ accumulation.
Another possibility is that the inhibition of heme detoxification is the mechanism of action of CQ in strains with mutant PfCRT but that strains vary in their management of the downstream consequences of a toxic buildup of heme and CQ-heme complexes. For example, different strains could differ in the power of their antioxidant defense mechanisms, which would be relevant if heme and CQ-heme complexes exert their toxic effects on the parasite through the oxidation of biomolecules (a scenario for which there is some support; for a review, see reference 3). Further work on the precise mechanism of CQ action is likely to aid in the elucidation of factors that determine the degree of mutant PfCRT-mediated CQ resistance in the first and subsequent cycles following CQ exposure.
ACKNOWLEDGMENTS
This work was supported by Australian National Health and Medical Research Council (NHMRC) grant 418055 (to K.K.), the U.S. National Institutes of Health grant R01 A150234 (to D.A.F.), and an Investigator in Pathogenesis of Infectious Diseases Award from the Burroughs Wellcome Fund (to D.A.F.). A.M.L. is supported by an NHMRC Overseas Biomedical Fellowship (585519).
We thank the Canberra Branch of the Australian Red Cross Blood Service for the provision of blood.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Abu Bakar N., Klonis N., Hanssen E., Chan C., Tilley L. 2010. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci. 123:441–450 [DOI] [PubMed] [Google Scholar]
- 2. Allen R. J., Kirk K. 2010. Plasmodium falciparum culture: the benefits of shaking. Mol. Biochem. Parasitol. 169:63–65 [DOI] [PubMed] [Google Scholar]
- 3. Becker K., et al. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol. 34:163–189 [DOI] [PubMed] [Google Scholar]
- 4. Bray P. G., et al. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 56:323–333 [DOI] [PubMed] [Google Scholar]
- 5. Bray P. G., et al. 2006. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol. Microbiol. 62:238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bray P. G., Mungthin M., Ridley R. G., Ward S. A. 1998. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 54:170–179 [DOI] [PubMed] [Google Scholar]
- 7. Cabrera M., Paguio M. F., Xie C., Roepe P. D. 2009. Reduced digestive vacuolar accumulation of chloroquine is not linked to resistance to chloroquine toxicity. Biochemistry 48:11152–11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen N., Russell B., Fowler E., Peters J., Cheng Q. 2002. Levels of chloroquine resistance in Plasmodium falciparum are determined by loci other than pfcrt and pfmdr1. J. Infect. Dis. 185:405–407 [DOI] [PubMed] [Google Scholar]
- 9. Cooper R. A., et al. 2002. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 61:35–42 [DOI] [PubMed] [Google Scholar]
- 10. Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egan T. J. 2008. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J. Inorg. Biochem. 102:1288–1299 [DOI] [PubMed] [Google Scholar]
- 12. Ferdig M. T., et al. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985–997 [DOI] [PubMed] [Google Scholar]
- 13. Fidock D. A., et al. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitch C. D. 2004. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 74:1957–1972 [DOI] [PubMed] [Google Scholar]
- 15. Fitch C. D. 1970. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science 169:289–290 [DOI] [PubMed] [Google Scholar]
- 16. Gligorijevic B., Purdy K., Elliott D. A., Cooper R. A., Roepe P. D. 2008. Stage independent chloroquine resistance and chloroquine toxicity revealed via spinning disk confocal microscopy. Mol. Biochem. Parasitol. 159:7–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayward R., Saliba K. J., Kirk K. 2006. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J. Cell Sci. 119:1016–1025 [DOI] [PubMed] [Google Scholar]
- 18. Klonis N., et al. 2010. Hematin-hematin self-association states involved in the formation and reactivity of the malaria parasite pigment, hemozoin. Biochemistry 49:6804–6811 [DOI] [PubMed] [Google Scholar]
- 19. Krogstad D. J., et al. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283–1285 [DOI] [PubMed] [Google Scholar]
- 20. Krogstad D. J., Schlesinger P. H., Gluzman I. Y. 1985. Antimalarials increase vesicle pH in Plasmodium falciparum. J. Cell Biol. 101:2302–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambros C., Vanderberg J. P. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420 [PubMed] [Google Scholar]
- 22. Lehane A. M., Hayward R., Saliba K. J., Kirk K. 2008. A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J. Cell Sci. 121:1624–1632 [DOI] [PubMed] [Google Scholar]
- 23. Lehane A. M., Kirk K. 2008. Chloroquine resistance-conferring mutations in pfcrt give rise to a chloroquine-associated H+ leak from the malaria parasite's digestive vacuole. Antimicrob. Agents Chemother. 52:4374–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lehane A. M., Kirk K. 2010. Efflux of a range of antimalarial drugs and ‘chloroquine resistance reversers’ from the digestive vacuole in malaria parasites with mutant PfCRT. Mol. Microbiol. 77:1039–1051 [DOI] [PubMed] [Google Scholar]
- 25. Martin R. E., et al. 2009. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science 325:1680–1682 [DOI] [PubMed] [Google Scholar]
- 26. Martin S. K., Oduola A. M., Milhous W. K. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899–901 [DOI] [PubMed] [Google Scholar]
- 27. Mu J., et al. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 49:977–989 [DOI] [PubMed] [Google Scholar]
- 28. Paul F., Roath S., Melville D., Warhurst D. C., Osisanya J. O. 1981. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet ii:70–71 [DOI] [PubMed] [Google Scholar]
- 29. Reed M. B., Saliba K. J., Caruana S. R., Kirk K., Cowman A. F. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909 [DOI] [PubMed] [Google Scholar]
- 30. Sá J. M., et al. 2009. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U. S. A. 106:18883–18889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saliba K. J., et al. 2003. Acidification of the malaria parasite's digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J. Biol. Chem. 278:5605–5612 [DOI] [PubMed] [Google Scholar]
- 32. Saliba K. J., Folb P. I., Smith P. J. 1998. Role for the Plasmodium falciparum digestive vacuole in chloroquine resistance. Biochem. Pharmacol. 56:313–320 [DOI] [PubMed] [Google Scholar]
- 33. Saliba K. J., Horner H. A., Kirk K. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273:10190–10195 [DOI] [PubMed] [Google Scholar]
- 34. Saliba K. J., Lehane A. M., Kirk K. 2008. A polymorphic drug pump in the malaria parasite. Mol. Microbiol. 70:775–779 [DOI] [PubMed] [Google Scholar]
- 35. Sanchez C. P., Rotmann A., Stein W. D., Lanzer M. 2008. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum. Mol. Microbiol. 70:786–798 [DOI] [PubMed] [Google Scholar]
- 36. Sidhu A. B., Verdier-Pinard D., Fidock D. A. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Staalsoe T., Giha H. A., Dodoo D., Theander T. G., Hviid L. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329–336 [DOI] [PubMed] [Google Scholar]
- 38. Sullivan D. J. 2002. Theories on malarial pigment formation and quinoline action. Int. J. Parasitol. 32:1645–1653 [DOI] [PubMed] [Google Scholar]
- 39. Sullivan D. J., Gluzman I. Y., Russell D. G., Goldberg D. E. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U. S. A. 93:11865–11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valderramos S. G., et al. 2010. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PloS Pathog. 6:e1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Schalkwyk D. A., Egan T. J. 2006. Quinoline-resistance reversing agents for the malaria parasite Plasmodium falciparum. Drug Resist. Updat. 9:211–226 [DOI] [PubMed] [Google Scholar]
- 42. Waller K. L., et al. 2003. Chloroquine resistance modulated in vitro by expression levels of the Plasmodium falciparum chloroquine resistance transporter. J. Biol. Chem. 278:33593–33601 [DOI] [PubMed] [Google Scholar]
- 43. Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. 1990. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature 345:253–255 [DOI] [PubMed] [Google Scholar]