Abstract
The pharmacokinetics of orally administered solithromycin (CEM-101), a novel fluoroketolide, were evaluated in healthy subjects in three phase 1 studies. In two randomized, double-blinded, placebo-controlled studies, escalating single oral doses of solithromycin (50 to 1,600 mg) or seven oral daily doses (200 to 600 mg) of solithromycin were administered. A third study evaluated the effects of food on the bioavailability of single oral doses (400 mg) of solithromycin. Following single doses, the median time to peak concentration (Tmax) ranged from 1.5 h to 6 h. The mean maximum measured plasma concentration (Cmax) ranged from 0.0223 μg/ml to 19.647 μg/ml, and the area under the concentration-versus-time curve from time zero to time t (AUC0–t) ranged from 0.0402 μg · h/ml to 28.599 μg · h/ml. There was no effect of high-fat food on the oral bioavailability of solithromycin. In the multiple-dose study, after 7 days, the mean maximum measured plasma solithromycin concentration at steady-state (Cmax,ss) ranged from 0.248 to 1.50 μg/ml, and the area under the concentration-versus-time curve over the final dosing interval (AUCτ) ranged from 2.310 to 18.41 μg · h/ml. These values indicate a greater than proportional increase in exposure at 200 and 400 mg but a proportional exposure at 600 mg. Median Tmax values remained constant between day 1 and day 7. Moderate accumulation ratios of solithromycin were observed after 7 days of dosing. All dose regimens of solithromycin were well tolerated, and no discontinuations due to an adverse event occurred. The human pharmacokinetic profile and tolerability of solithromycin, combined with its in vitro potency and efficacy in animal models against a broad spectrum of pathogens, support further development of solithromycin.
INTRODUCTION
Since the introduction of erythromycin A in the early 1950s, macrolides have proven to be an important class of antibacterial therapeutics for respiratory tract infections (RTIs), including community-acquired pneumonia, acute exacerbations of chronic bronchitis, acute sinusitis, pharyngitis/tonsillitis, and otitis media (23, 25). Clinical usage over the past several decades has been associated with the emergence of microbial resistance to this class, including to clarithromycin and azithromycin, which are currently among the first-line treatments for RTIs (1, 11, 13, 41, 43). Recent surveys document high levels of macrolide resistance among key RTI pathogens, particularly streptococci and staphylococci (4, 10, 11, 12, 15, 17, 22, 24, 43), and emphasize the need to develop new agents with broad activity against resistant pathogens which are also designed to optimize pharmacokinetic and safety properties.
Ketolides are a new macrolide subclass of semisynthetic agents derived from erythromycin A and designed to target macrolide-resistant Gram-positive RTI pathogens. In 2004, telithromycin became the first ketolide approved for marketing and clinical use in the United States (36, 37). Clinical trials demonstrated efficacy of this agent in the treatment of RTIs, including community-acquired pneumonia, but its use has been limited by reports of rare, but significant, hepatic toxicity, reversible visual disturbances, and exacerbation of myasthenia gravis (6, 33). These findings stimulated investigations to develop new and safer ketolides by exploiting new molecular definitions of structure-activity/toxicity relationships (2, 3, 39). Solithromycin, a fluoroketolide, is structurally differentiated from telithromycin by substitution of the hydrogen atom in the 2 position of the macrolide ring with a fluorine atom and a substitution of the 4-pyridin-3-yl-imidazol-1-yl group at the terminus of the butyl side chain of the 11,12-carbamate with a 4-(3-aminophenyl)-1,2,3-triazol-1-yl group. These chemical alterations appear to account for the more potent antimicrobial activity, greater metabolic stability, and greater tolerability of solithromycin than telithromycin (3).
Solithromycin demonstrates potent in vitro activity and a postantibiotic effect against a broad spectrum of pathogens, including macrolide-resistant and multidrug-resistant staphylococci, streptococci, enterococci, and atypical pathogens (12, 15, 19, 22, 24, 30, 31, 34, 43). The overall potency of solithromycin against Gram-positive aerobes is greater than that of clarithromycin, azithromycin, and telithromycin, and it is active against RTI pathogens resistant to these antibiotics (4, 11, 13, 22, 24, 43). Unlike azithromycin and clarithromycin, solithromycin is active against macrolide-resistant pneumococci with the ermB, mefA, ermB-mefA, and/or L4 mutation (5, 11, 18, 24). Solithromycin is also highly active against atypical respiratory pathogens, including macrolide-resistant Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae (9, 11, 12, 20, 21, 31) and was also previously reported to be more active against Gram-negative RTI pathogens (Moraxella catarrhalis and Haemophilus influenzae) than telithromycin (30). Solithromycin's extended antimicrobial spectrum includes biodefense pathogens, such as Bacillus anthracis, Yersinia pestis, and Francisella tularensis (14), and sexually transmitted disease pathogens, including human mycoplasmas and ureaplasmas (40), Chlamydia trachomatis (31), and Neisseria gonorrhoeae (4). Solithromycin has been shown to have excellent activity against intracellular pathogens such as Legionella, Listeria, and Yersinia pestis, when they are tested in macrophages (20). Solithromycin has recently been shown to be curative in a mouse malaria model (7).
This report presents the results of studies of the pharmacokinetics and tolerability of solithromycin after single or multiple oral doses as well as the effects of food on oral bioavailability in healthy adult subjects. These studies support the development of once-daily oral regimens of this novel fluoroketolide in the clinical management of RTI and other infectious disorders.
(These data have been presented in part at recent national and international conferences [35, 38]).
MATERIALS AND METHODS
Study populations and study designs.
Three clinical studies were conducted by Comprehensive Phase One (Miramar, FL) or MDS Pharma (Lincoln, NE, and Tempe, AZ) with 108 healthy adult male and female subjects. Protocols were carried out in accordance with good clinical practices, as described in the Code of Federal Regulations, Title 21, parts 50, 56, and 312, subpart D, as well as the October 2000 version of the Declaration of Helsinki (42). Subjects were apprised of the risks of the study and read, understood, and signed the written informed-consent forms.
Healthy male and female subjects aged 19 to 55 years with body mass indexes between 18 and 32 kg/m2, inclusive, and a total body weight of >60 kg were eligible for enrollment. Females of childbearing potential were excluded if they were found to be pregnant by screening assays. Those enrolled were required to use acceptable birth control methods starting 14 days prior to dosing, throughout the study, and for 30 days after the last dose of study drug. Enrolled subjects had negative urine drug screens and were willing to adhere to lifestyle guideline restrictions in the protocol; drug or alcohol abusers and tobacco users were excluded from the study. Subjects with positive tests for human immunodeficiency virus, hepatitis B virus surface antigen, or anti-hepatitis C virus antibody were excluded. Subjects with electrocardiographic (ECG) QTc values of >450 ms for males and >470 ms for females were also excluded.
(i) Single-oral-dose study.
An initial first-in-humans, single-center, randomized, double-blinded, placebo-controlled, dose escalation study was conducted to determine the tolerability and pharmacokinetics of single oral doses of solithromycin compared to placebo. A total of 49 subjects completed this study, and all were in good health, as determined by medical history, physical examination, clinical laboratory tests, and 12-lead ECGs. Subjects were sequentially enrolled into one of seven cohorts consisting of 7 subjects each; 5 subjects in each cohort were randomized to receive a single oral dose of 50, 100, 200, 400, 800, 1,200, or 1,600 mg solithromycin and 2 subjects received placebo. This sample size was considered adequate to provide the clinical information required to satisfy the objectives of the study.
Escalating single doses were administered to fasting subjects using 50-, 100-, or 200-mg capsules of solithromycin or matching placebo capsules. Nominal escalations were selected on the basis of an approximation of a modified Fibonacci scheme. Dose escalation proceeded only after the safety of the previous dose was determined. Pharmacokinetics and safety were assessed throughout the study. The study duration from initial dosing of the first cohort until the follow-up of the last cohort was 16 weeks. Screening for eligibility occurred up to 3 weeks prior to dosing, and enrolled subjects were admitted to the clinical research unit (CRU) on the day before dosing (day −1) and remained there for 72 h after the dose of study drug for pharmacokinetic sampling and safety monitoring.
(ii) Food effects on bioavailability study.
The food effects on bioavailability study was a single-center, randomized, two-period, fed/fasted crossover bioequivalence study conducted to assess the effects of food on the tolerability and pharmacokinetics of a single oral dose of solithromycin 400 mg (two 200-mg capsules) in healthy adult subjects. The study was conducted in two periods. Twenty-four healthy adult subjects were enrolled and randomized equally to one of two oral dosing sequences as follows. In period 1, group A (n = 12) subjects received a single 400-mg dose (two 200-mg capsules) of solithromycin in the fasted state (treatment A); these subjects were fasted overnight for at least 10 h prior to dosing and remained fasted for 4 h after dosing. Group B (n = 12) subjects received a single 400-mg dose (two 200-mg capsules) of solithromycin in the fed state (treatment B); these subjects were fasted overnight for at least 10 h prior to the start of a high-fat caloric meal containing approximately 900 cal (50% of the caloric content from fat) that was entirely consumed in 30 min or less. The dose of solithromycin was administered 30 min after the start of the meal. In period 2, group A (n = 12) subjects received a single 400-mg dose (two 200-mg capsules) of solithromycin in the fed state (as described for group B in period 1), and group B (n = 12) subjects received a single 400-mg dose (two 200-mg capsules) of solithromycin in the fasted state (as described for group A in period 1).
In each period, subjects were admitted to the CRU on day −1, were dosed on day 1, and remained in the CRU for 72 h after the dose of study drug for pharmacokinetic sampling and safety monitoring prior to discharge. There was a ≥7-day washout period between discharge from the CRU in period 1 and dosing in period 2.
(iii) Multiple-oral-dose study.
The multiple-oral-dose study was a single-center, randomized, double-blinded, placebo-controlled study to determine the tolerability and pharmacokinetics of multiple oral doses of solithromycin compared to placebo in healthy subjects. A total of 35 subjects participated in this study. The subjects were enrolled in five cohorts of 7 subjects each and randomized in a 5:2 ratio to receive oral doses of solithromycin or placebo administered once daily for 7 days. Escalating multiple doses were administered to fasting subjects using 200-mg capsules of solithromycin. The rationale for a starting dose of 200 mg was based on the estimated area under the concentration-versus-time curve (AUC) from 0 to 24 h (AUC0–24) of approximately 2 μg · h/ml and the results of pharmacokinetic and safety assessments in the single-dose study. The escalation schedule was planned in increments of 200 mg of solithromycin, with the actual escalation being determined on the basis of completed follow-up assessments and careful review of blinded clinical and pharmacokinetic data confirming the safety of each preceding dose. The study duration from initial dosing of the first dosage cohort until the follow-up of the last dosage cohort was 16 weeks.
Study drug.
Study drug was supplied by Cempra Pharmaceuticals, Inc., in gelatin capsules containing 50, 100, or 200 mg of solithromycin. Gelatin capsules containing placebo (microcrystalline cellulose, USP) were identical to those containing active solithromycin in order to maintain the study blind. All study drugs were kept in a secure, limited-access storage area under recommended storage conditions until they were needed or until they were returned to the sponsor.
Blood sample collection.
In the single-dose study, blood samples for assay of solithromycin concentrations were collected predosing (0 h) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 16, 24, 36, 48, and 72 h postdosing. In the food effect study, samples were collected predosing (0 h) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 16, 24, 36, 48, and 72 h postdosing in both periods. In the multiple-dose study, samples were collected predosing (0 h) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, and 16 h postdosing on day 1 and day 7, prior to dosing (trough determinations) in the morning of days 2 to 6, and 24, 36, 48, and 72 h following the last dose of study drug on day 7 (days 8 to 10). Plasma was separated from blood collection tubes by refrigerated centrifugation, transferred to labeled storage tubes, and stored at −20°C until analysis.
Pharmacokinetics and statistical analyses. (i) Analytical procedures.
Plasma concentrations of solithromycin were measured at MicroConstants, Inc. (San Diego, CA), using a validated liquid chromatography coupled to tandem mass spectrometry (LC-MS-MS) method (MicroConstants' analytical method MN08060) with a lower limit of quantitation of 0.010 μg/ml and a validation range of 0.010 to 20.000 μg/ml. For quality control (QC) samples containing 0.030, 0.300, or 16 μg/ml prepared in human plasma, values for interday precision were 2.33, −3.33, and −5.00% deviations of the means from theoretical values, respectively, and the coefficients of variation (CVs) for intraday precision values ranged from 1.41 to 6.45%. The intraday accuracy of the method was determined by comparing the mean concentrations from each validation run with the theoretical concentrations of solithromycin for each of three QC sample concentrations; mean values ranged from −8.13 to 6.33% DEV.
(ii) Pharmacokinetic parameter analysis.
Pharmacokinetic analyses were performed for solithromycin in plasma using noncompartmental methods with WinNonlin Professional software, version 5.2 (Pharsight Corp., Mountain View, CA). Pharmacokinetic parameter estimates were derived from the plasma concentration-versus-time curves for single- or multiple-dose regimens of solithromycin. For multiple doses, figures were created to display mean and individual solithromycin concentration-versus-time curves on days 1 and 7 for each dose level. For single-dose and multiple-dose day 1 assessments, parameters calculated for plasma solithromycin included maximum measured plasma concentration (Cmax), time to peak concentration (Tmax), AUC from time zero to time t (AUC0–t), AUC from time zero to infinity (AUC0–∞), apparent terminal elimination half-life (t1/2), and apparent first-order terminal elimination rate constant (kel); and these were summarized for each treatment group using descriptive statistics. For day 7 (end of therapy) assessments in the multiple-dose study, calculated pharmacokinetic parameters for plasma solithromycin included maximum measured plasma concentration at steady state (Cmax,ss), measured plasma concentration at the end of the dosing interval (Cmin), AUC over the final dosing interval (AUCτ), and time to peak concentration at steady state (Tmax,ss). The accumulation index (AI) parameter was estimated by comparing the plasma exposure of solithromycin on day 7 (AUCτ) to the exposure on day 1 (AUC0–t). Steady-state evaluation was performed using the predose Cmins collected on days 3, 4, 5, 6, 7 and 8 (−96, −72, −48, −24, 0, and 24 h relative to day 7 dosing) and descriptive statistics. Pharmacokinetic linearity was evaluated by comparing AUCτ values (day 7) against AUC0–∞ values (day 1) using analysis of variance on the ln-transformed values for solithromycin. Dose proportionality was evaluated for solithromycin using a regression approach. A statistical linear relationship between the ln-transformed pharmacokinetic parameters (AUC0–t, AUC0–∞, and Cmax on day 1 and AUCτ and Cmax on day 7) and the ln-transformed dose was fitted by using a regression model with ln-transformed dose as a covariate. Dose proportionality was confirmed if a statistical linear relationship was demonstrated and if the 95% confidence interval (CI) for these parameters included the value of 1 for dose-dependent parameters (AUC0–t, AUC0–∞, AUCτ, and Cmax).
(iii) Food effects on bioavailability.
Assessments of the bioequivalence of drug exposure under fasted and fed conditions were based on the 90% confidence interval of the ratio of least-squares means for the ln-transformed exposure measurements (AUC0–t, AUC0–∞, and Cmax) of the fed to fasted drug exposures. The sample size for this study, i.e., 24 subjects, was not determined by formal statistical methods, due to a lack of intrasubject variability data from crossover studies. However, 24 subjects were considered sufficient to satisfy the objectives of the study.
Safety assessment.
Evaluations of safety and tolerability included physical examinations, vital signs, clinical laboratory parameters (blood chemical analyses, hematology and coagulation tests, and urinalysis) and standard 12-lead ECGs prior to dosing, during dosing, and/or following administration of study drug per protocols. Adverse events (AEs) were monitored throughout each study, and subjects were contacted by telephone 7 days after the final dosing (multiple-dose study) for follow-up safety assessments. The number and percentage of subjects who experienced at least 1 AE were assessed in terms of severity (based on the Division of Microbiology and Infectious Diseases Adult Toxicity Table, November 2007), relationship to drug, system organ class, preferred term, and dose level; and the AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA). Clinically significant laboratory abnormalities were considered AEs only if they required medical or surgical intervention or resulted in study drug interruption or discontinuation.
RESULTS
Subjects.
In the single-dose study, 49 subjects (30 males, 19 females) were randomized to receive solithromycin (mean age, 37.3 years; age range, 20 to 55 years; 97% Hispanic; mean weight, 74.4 kg) or placebo (mean age, 36.6 years; age range, 20 to 54 years; 100% Hispanic; mean weight, 73.5 kg) and completed the study. In the multiple-dose study, 35 subjects (22 males, 13 females) were randomized to receive solithromycin (mean age, 34.1 years; 76% Hispanic; mean weight, 75.5 kg) or placebo (mean age, 29.7 years; 60% Hispanic; mean weight, 72.1 kg). This group included additional 400-mg and 600-mg cohorts that were added to reaffirm the tolerability of these doses. In the food effects-bioavailability study, 24 subjects (mean age, 34.1 years; age range, 19 to 50 years; 100% Hispanic; mean weight, 74.6 kg) were enrolled, and all completed the study. For each study, demographic characteristics (age, race, and weight) of subjects receiving solithromycin were comparable to those receiving placebo. The only notable difference across dose cohorts for any demographic or baseline characteristic was gender (solithromycin groups, 75% male; placebo groups, 54% male [pooled data]).
Pharmacokinetics.
All the subjects who were dosed in the three studies had evaluable data and were included in the pharmacokinetic analyses.
(i) Single-dose pharmacokinetics.
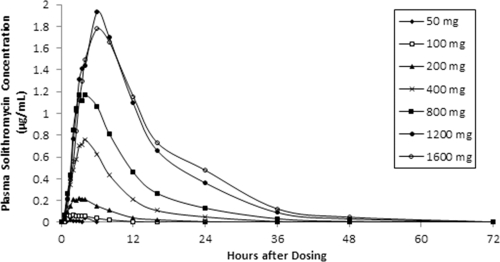
Table 1 shows the mean values for the pharmacokinetic parameters in plasma after single doses of solithromycin, and mean plasma concentrations of solithromycin for each dose level are depicted in a concentration-time plot in Fig. 1. Across the dose range studied, mean Cmaxs ranged from 0.0223 μg/ml to 1.964 μg/ml and increased in a greater than dose-proportional manner to 1,200 mg. The mean Cmax of solithromycin for the 1,600-mg dose group (1.782 μg/ml) was approximately 10% less than that observed for the 1,200-mg cohort, because one subject in the 1,600-mg cohort had a much lower Cmax (0.298 μg/ml) than the other four subjects in this cohort. This deviant value contributed to the higher variability and overall lower mean Cmax for this cohort. The median Tmax occurred 1.5 to 6.0 h following drug administration, and there was a trend for Tmax values to increase as the dose of solithromycin increased.
Table 1.
Pharmacokinetic parameters for solithromycin in plasma after single oral doses in fasted subjectsa
| Pharmacokinetic parameter | Result by dose cohortb |
||||||
|---|---|---|---|---|---|---|---|
| 50 mg (n = 3c) | 100 mg (n = 5) | 200 mg (n = 5) | 400 mg (n = 5) | 800 mg (n = 5) | 1,200 mg (n = 5) | 1,600 mg (n = 5) | |
| Cmax (μg/ml) | 0.0223 (20.99) | 0.0683 (56.01) | 0.255 (24.30) | 0.784 (33.15) | 1.324 (69.38) | 1.964 (14.19) | 1.782 (67.51) |
| Tmax (h) | 1.50 (1.0, 1.5) | 2.00 (1.5, 2.5) | 3.00 (2.0, 4.0) | 4.00 (3.0, 4.0) | 3.50 (2.5, 6.0) | 6.00 (3.5, 6.0) | 6.00 (4.0, 6.0) |
| AUC0–last (μg · h/ml) | 0.0402 (44.99) | 0.394 (84.95) | 1.730 (30.30) | 6.941 (41.14) | 13.665 (69.98) | 26.758 (24.09) | 28.599 (79.31) |
| AUC0–∞ (μg · h/ml) | NDd | 0.624e (55.79) | 1.859 (29.69) | 7.128 (40.36) | 13.836 (69.44) | 26.957 (23.90) | 28.861 (78.53) |
| t1/2 (h) | ND | 3.16e (20.74) | 4.00 (21.74) | 5.14 (23.66) | 6.65 (15.21) | 6.87 (10.76) | 7.42 (9.43) |
For any subject whose extrapolated portion of AUC0–∞ (AUClast–∞) was greater than 20% of AUC0–∞, the parameters associated with kel were set to missing due to the high level of extrapolation. This affected the 3 subjects with quantifiable concentrations in the 50-mg dose cohort and 2 subjects in the 100-mg dose cohort.
Cmax, AUC0–last, AUC0–∞, and t1/2 are expressed as means (% CVs). Tmax values are expressed as medians (minimum, maximum).
Only 3 of the 5 subjects had quantifiable solithromycin plasma concentrations.
ND, not determined.
n = 3.
Fig. 1.
Mean plasma concentrations of solithromycin following escalating single-dose regimens.
Exposure to solithromycin, determined by measurement of AUC0–t and AUC0–∞, increased in a greater than dose-proportional manner up to the 1,200-mg dose. The variability in AUC0–∞ and AUC0–t was moderate to high and ranged from 24 to 85% across cohorts. In the 1,600-mg dose cohort, one subject exhibited a much lower exposure (AUC0–∞ = 5.186 μg · h/ml) to solithromycin than the four other subjects within the cohort. The mean t1/2 increased from 3.16 to 7.42 h over the 50- to 1,600-mg dose range.
(ii) Food effect on bioavailability and pharmacokinetics.
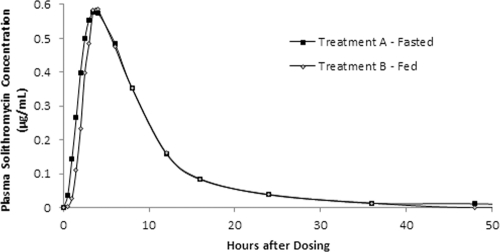
The solithromycin concentration-time profiles characterized over the 36-hour postdosing period appeared to be comparable under fasted and fed conditions, with mean peak concentrations of approximately 0.6 μg/ml at 3.5 h postdosing (median Tmax range, 2.5 to 6 h) for both treatments (Fig. 2). Except for one subject in the treatment A group, all plasma solithromycin concentrations for both treatments were below the limits of quantitation (0.01 μg/ml) at 48 h postdosing. The values of the pharmacokinetic parameters for solithromycin in plasma are shown in Table 2. The harmonic mean t1/2s for the fasted and fed treatments were 5.37 and 5.02 h, respectively. The ratios of least-squares means for the ln-transformed Cmax, AUC0–t, and AUC0-∞ of the fed to fasted drug exposures were 107%, 97.1%, and 97.8%, respectively, with the 90% CIs falling within the 80 to 125% range. These results indicate that the pharmacokinetics of solithromycin in the fed state were bioequivalent to those in the fasted state and that food had no effect on the bioavailability of solithromycin following a single oral dose of 400 mg (two 200-mg capsules).
Fig. 2.
Mean plasma concentrations of solithromycin under fasted and fed conditions.
Table 2.
Pharmacokinetic parameters for solithromycin in plasma after single doses in fed or fasted subjects
| Pharmacokinetic parameter | Valuea |
% MRb (90% CI) for treatment B/treatment A (fed vs fasted) | |
|---|---|---|---|
| Treatment A (fasted) | Treatment B (fed) | ||
| Cmax (μg/ml) | 0.609 (38.6) | 0.633 (31.8) | 106.81 (97.56, 116.93) |
| AUC0–t (μg · h/ml) | 5.470 (41.9) | 5.083 (34.6) | 97.08 (86.86, 108.50) |
| AUC0–∞ (μg · h/ml) | 5.614 (40.7) | 5.267 (33.6) | 97.81 (87.89, 108.85) |
| Tmax (h)b | 3.50 (2.50, 6) | 3.50 (2.50, 6) | NDc |
| t1/2 (h)c | 5.37 (14.0) | 5.02 (13.1) | ND |
Cmax, AUC0–t, and AUC0–∞ are expressed as means (% CVs). Tmax is expressed as median (minimum, maximum). t1/2 is expressed as harmonic mean (% CV from pseudo-SD).
MR, mean ratio, based on the ratio of least-squares means.
ND, not determined.
(iii) Multiple-dose pharmacokinetics.
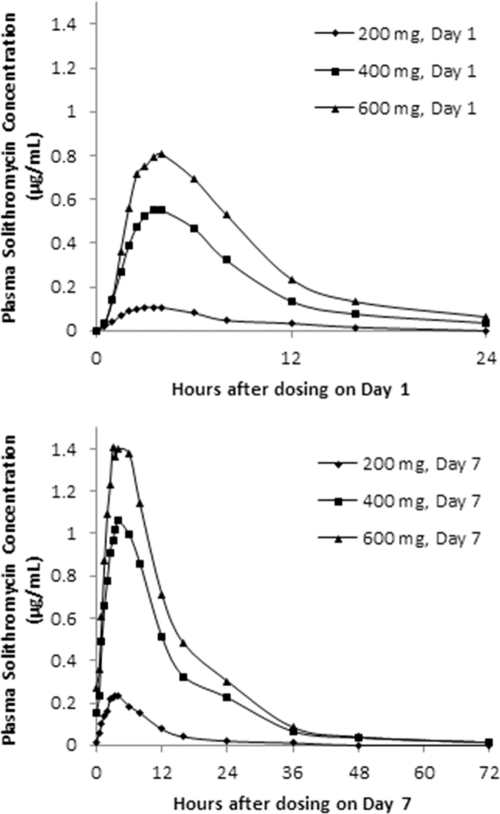
Table 3 shows the mean values of the pharmacokinetic parameters for solithromycin in plasma estimated on day 1 (after single doses) and on day 7 of multiple-dose protocols. The 400-mg and 600-mg dose groups were tested in a second set of cohorts (D and E), in order to verify the tolerability of these doses, and the results presented in this report are combined. Mean plasma concentrations of solithromycin are depicted in a linear plot for each dose level and are compared for day 1 and day 7 in Fig. 3. In five consecutive cohorts studied, dose regimens were 200 mg (cohort A), 400 mg (cohort B), 600 mg (cohort C), 400 mg (cohort D), and 600 mg (cohort E) once daily for 7 days.
Table 3.
Pharmacokinetic parameters for solithromycin in plasma on days 1 and 7 of multiple-dose protocolsa
| Day and pharmacokinetic parameter | Value |
||
|---|---|---|---|
| Cohort A, 200 mg/day (n = 5) | Cohorts B and D, 400 mg/day (n = 10) | Cohorts C and E, 600 mg/day (n = 10) | |
| Day 1 | |||
| Cmax (μg/ml) | 0.113 (42.6) | 0.579 (64.5) | 0.862 (61.2) |
| Tmax (h) | 3.00 (2, 4) | 3.50 (3, 4) | 3.75 (2, 6) |
| t1/2 (h) | 3.56 (8.5)b | 4.82 (18.7) | 5.53 (32.9) |
| AUC0-24 (μg · h/ml) | 0.841 (49.3) | 4.835 (64.3) | 7.656 (60.0) |
| AUC0–∞ (μg · h/ml) | 0.895 (56.0)b | 5.062 (63.3) | 9.049 (47.9)c |
| kel (1/h) | 0.195 (8.4)b | 0.144 (18.6) | 0.125 (26)c |
| Day 7 | |||
| Cmax,ss (μg/ml) | 0.248 (33.8) | 1.09 (47.7) | 1.50 (26.9) |
| Tmax,ss (h) | 3.50 (2.5, 3.5) | 4.00 (2.5, 6) | 3.50 (2.5, 6) |
| t1/2 (h) | 5.84 (23.9) | 7.47 (21.4) | 8.72 (13.8) |
| AUCτ (μg · h/ml) | 2.310 (33.4) | 13.27 (55.4) | 18.41 (30.2) |
| Accumulation indexd | 3.02 (2.77) | 5.22 (2.12) | 7.76 (2.19) |
For any subject whose extrapolated portion of AUC0–∞ (AUClast–∞) was greater than 20% of AUC0–∞, the parameters associated with kel were set to missing due to the high level of extrapolation. This affected one subject in the 200-mg/day cohort and one subject in the 600-mg/day cohort. Cmax, AUC0-24, AUC0–∞, and kel are expressed as means (% CVs). Tmax is expressed as median (minimum, maximum). t1/2 is expressed as harmonic mean (% CV from pseudo-SD).
n = 4; see footnote a.
n = 9; see footnote a.
AUCτ on day 7/AUC0-24 on day 1 expressed as mean (median value).
Fig. 3.
Mean plasma concentrations of solithromycin (CEM-101) after 200-, 400-, or 600-mg multiple-dose regimens on day 1 (top) and day 7 (bottom).
Solithromycin plasma exposures on day 1 increased in a greater than dose-proportional manner across the 200- to 600-mg dose range, with mean Cmax and AUC0–∞ values increasing from 0.113 to 0.862 μg/ml and 0.895 to 9.049 μg · h/ml, respectively. Median Tmax values ranged from 3 to 3.75 h, and the harmonic mean t1/2 increased (from 3.6 to 5.5 h) with increasing dose.
Following repeated once-daily oral administration for 7 days, plasma solithromycin exposure again increased in a greater than dose-proportional manner across the 200- to 600-mg dose range, with mean Cmax,ss and AUCτ values increasing from 0.25 to 1.50 μg/ml and 2.3 to 18.4 μg · h/ml, respectively. Solithromycin median Tmax values were 3.5 to 4.0 h, and the harmonic mean t1/2 increased (from 5.8 to 8.7 h) for the 200-, 400-, and 600-mg doses (Table 3).
With repeated once-daily oral solithromycin administration, the median Tmax values remained constant between day 1 and day 7 over the dose range studied (median ranges, 3 to 3.75 h and 3.50 to 4 h, respectively). At all doses, solithromycin exposures were higher on day 7 than on day 1, indicating accumulation over this dosing period. AUC and Cmaxs were 1.7 to 2.2 times greater on day 7 than on day 1. The median AIs were approximately 3, 2, and 2 for the 200-, 400-, and 600-mg doses, respectively (Table 3). Mean plasma Cmins appeared to be comparable during days 4 to 7, suggesting that a steady state was probably attained (Table 4). The ratio of AUCτ on day 7 versus AUC0–t on day 1 was 254%, suggesting nonlinear kinetics of solithromycin over time for the dose range studied. Statistical results (Table 5) demonstrated that slope estimates for AUC and Cmax ranged from 1.60 to 2.04 on days 1 and 7, indicating that the pharmacokinetic parameters increased in a more than dose-proportional manner over the dose range studied. In addition, the 95% CIs for the slope were very large on days 1 and 7 and did not include the value of 1, except for AUC0–t and Cmax on day 1.
Table 4.
Plasma solithromycin trough concentrations in multiple-dose protocols
| Dose cohort | Mean solithromycin Cmina (% CV) |
|||||
|---|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 7 (h 24) | |
| 200 mg (n = 5) | 0.0164 (27.6) | 0.0153 (25.6) | 0.0159 (41.3) | 0.0148 (29.4) | 0.0159 (22.2) | 0.0195 (31.9) |
| 400 mg (n = 10) | 0.0671 (71.7) | 0.0946 (81.8) | 0.118 (83.6) | 0.14 (74.1) | 0.153 (80.1) | 0.225 (67.4) |
| 600 mg (n = 10) | 0.180 (60.6) | 0.210 (57.0) | 0.263 (54.9) | 0.261 (73.6) | 0.273 (59.7) | 0.303 (44.6) |
Plasma Cmins are reported in μg/ml. Values that were below the limit of quantitation (BLQ) of 0.01 μg/ml were treated as missing.
Table 5.
Dose proportionality results for solithromycin in plasmaa
| Dosing day | Pharmacokinetic parameter | Slope (b) | SE | 95% CI for slope |
|---|---|---|---|---|
| 1 | Cmax (μg/ml) (n = 25) | 1.6046 | 0.4823 | (0.7780, 2.4312) |
| AUC0–t (μg · h/ml) (n = 25) | 1.7765 | 0.5042 | (0.9123, 2.6406) | |
| AUC0–∞ (μg · h/ml) (n = 25) | 2.0375 | 0.4686 | (1.2312, 2.8438) | |
| 7 | Cmax (μg/ml) (n = 25) | 1.6567 | 0.3285 | (1.0937, 2.2198) |
| AUCτ (μg · h/ml) (n = 25) | 1.9043 | 0.3713 | (1.2679, 2.5407) |
The statistical analyses were performed using the SAS mixed procedure. A linear regression model with ln-transformed parameter and ln-transformed dose was used. Dose proportionality was not rejected if the 95% CI for the slope included the value of 1.
Safety.
Single oral doses of solithromycin were well tolerated at all dose levels. No subjects died, and none experienced treatment-emergent serious adverse events (SAEs) or were withdrawn from the study after dosing due to an AE. No clinically significant laboratory results or trends were reported for any dose at any time point. No clinically meaningful changes in physical examinations, vital signs, or ECG studies were apparent during the study. There were no clinical chemistry abnormalities reported as AEs by the investigator. No alterations of clinical laboratory values were considered of clinical significance, including a mild (<2 times the upper limit of normal [ULN]) and transient elevation of serum alanine aminotransferase (ALT) levels in a single subject (800-mg dose group) that was unassociated with other chemical abnormalities of hepatic function.
Single oral doses of 400 mg (two 200-mg capsules) of solithromycin administered in the fasting and fed states were generally well tolerated in the healthy adult subjects in the food effects-bioavailability study. No subjects died, experienced treatment-emergent SAEs, or were withdrawn from the study after dosing due to an AE. One subject in period 1 (group A, fasted) had an increase in ALT level to 84 IU/liter on day 4 from a baseline value of 56 IU/liter at screening (normal range, 10 to 60 IU/liter). Seven days later, the predosing value for this subject in period 2 (group A, fed) was normal at 54 IU/liter and thereafter was just above the ULN at 61 IU/liter. These increases were not considered clinically significant, and there were no treatment-emergent abnormal values for aspartate aminotransferase (AST), alkaline phosphatase, or bilirubin for any subject, nor were there any clinically significant changes in physical exams, vital signs, ECGs, or other laboratory parameters.
Multiple-oral-dose regimens of solithromycin administered to healthy adult subjects for 7 days were well tolerated at all dose levels. No subjects died, experienced treatment-emergent SAEs, or were withdrawn from the study after dosing due to an AE. No clinically significant changes in physical exams, vital signs, or ECGs were observed at any dose level. Clinical laboratory studies showed normal predosing and postdosing values in subjects at the 200- and 400-mg dose levels. Four of 10 subjects administered 600 mg of solithromycin had low-level hepatic transaminase increases (for ALT, 3 determinations at ≤2 times the ULN and 1 determination at <3 times the ULN; for AST, 2 determinations at <2 times the ULN) during dosing that rapidly returned to normal values within 5 to 8 days after dosing was completed. All other laboratory studies showed normal values, including serum bilirubin determinations, and no associated clinical symptoms were observed in these subjects.
DISCUSSION
Three studies, reported here, investigated the pharmacokinetics and tolerability of escalating single- and multiple-oral-dose regimens of solithromycin and evaluated the effects of food on the bioavailability of single oral doses in healthy adult subjects. Results of evaluations of 84 subjects demonstrated the overall tolerability of oral (capsule) dosage regimens of this fluoroketolide, currently under development and in phase 2 clinical trials for treatment of community-acquired bacterial pneumonia. Previous studies have demonstrated efficacy in various animal models of respiratory pathogen infections, as well as other infections (7, 26, 27, 28). The pharmacokinetic characteristics of solithromycin described here support the development of once-daily oral regimens capable of achieving tissue exposure to ensure efficacy for RTIs.
Since the pharmacodynamics of solithromycin are driven by AUC/MIC ratios, as for other macrolides (8), the high systemic exposure achieved with once-a-day dosing of solithromycin should allow therapeutic efficacy and broad utility in RTIs and other infectious disorders. Mean Cmax and AUCτ values for the 600-mg oral dose of solithromycin on day 7 were 1.50 μg/ml and 18.4 μg · h/ml, respectively (Table 3), values which should prove to be therapeutically effective against a variety of respiratory pathogens, on the basis of MICs reported elsewhere (9, 11, 12, 22, 30, 31). Moreover, excellent distribution in the lung (the targeted site for RTIs) and penetration into epithelial lining fluid (ELF) and alveolar macrophages (AM) have been observed in a study with healthy subjects (32). Following five daily oral doses of 400 mg of solithromycin, mean AUC0–24 values in ELF and AM were 63.2 and 1,282 μg · h/ml, respectively, and the ratios of AUC0–24 values in ELF and AM to AUC0–24 in plasma were 8.88 and 180, respectively (32).
While oral doses of solithromycin are efficiently absorbed irrespective of food intake, a front-loaded dosing strategy may allow more rapid achievement of steady-state plasma concentrations and optimized tissue exposure targets for specific clinical indications. Monte Carlo simulations based on human population pharmacokinetic models and animal model pharmacokinetic-pharmacodynamic targets should support dose regimens for future studies of solithromycin. The relatively short terminal half-life of solithromycin should obviate prolonged subinhibitory tissue exposures and the selection/emergence of microbial resistance (29).
The pharmacokinetic profile of solithromycin derived from these studies indicates systemic accumulation following 7 once-daily doses of 200 mg to 600 mg (Fig. 2; Table 3). Observed Cmax and AUC0–∞ increases were more than dose proportional for administered single doses of 50 to 400 mg (Table 1), as well as for multiple doses of 200 mg to 400 mg (Table 3). The moderate plasma accumulation ratios observed after 7 daily doses of solithromycin may likely reflect its metabolic characteristics. Like all macrolides, in vitro studies have demonstrated metabolism of solithromycin in human hepatocytes, primarily as a result of cytochrome CYP3A4 interactions (Cempra Pharmaceuticals, data on file).
In conclusion, on the basis of its tolerability in human subjects and its pharmacokinetic profile, in addition to its potent in vitro activities against diverse respiratory and other intracellular pathogens, solithromycin represents a potentially unique and important drug candidate for clinical development in the treatment of bacterial RTIs and other infections.
ACKNOWLEDGMENTS
We thank the study participants, clinical investigators, and study coordinators who made these studies possible. We thank C. James Kissling and Scott Sharples of MDS Pharma (now Celerion) for their assistance with these studies. We also thank Cynthia Gomez and Ahmed Kousba at MicroConstants for their help in analysis and presentation of pharmacokinetic data.
This study was supported by Cempra Pharmaceuticals, Inc. We thank Brian Jamieson and Donald Anderson for their help in drafting the manuscript.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1. Bartlett J. G., et al. 1998. Community-acquired pneumonia in adults; guidelines for management. Clin. Infect. Dis. 26:811–838 [DOI] [PubMed] [Google Scholar]
- 2. Berisio R., et al. 2003. Structural insight into the antibiotic action of telithromycin against resistant mutants. J. Bacteriol. 185:4276–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertrand D., Bertrand S., Neveu E., Fernandes P. 2010. Molecular characterization of off-target activities of telithromycin: a potential role of nicotinic acetylcholine receptors. Antimicrob. Agents Chemother. 54:5399–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biedenbach D. J., Deshpande L. M., Fritsche T. R., Sader H. S., Jones R. N. 2008. Antimicrobial characterization of CEM-101: activity against enterococci, uncommon Gram-positive pathogens, N. gonorrhoeae and anaerobes, abstr. F1-3976. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 5. Clark C., Kowasaka-Shick K., McGhee P., Nagai K., Appelbaum P. 2009. Capability of CEM-101 to select for resistant pneumococcal and group A streptococcal clones by multistep resistance selection, abstr. F1-2039. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 6. Clay K. D., et al. 2006. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann. Intern. Med. 144:415–420 [DOI] [PubMed] [Google Scholar]
- 7. Craft J. C., Wittlin S., Lotharius J., Bathurst I., Fernandes P. 2010. Solithromycin (CEM-101)—a new fluoroketolide with antimalarial activity, abstr. 269. Abstr. 59th Annu. Meet. Am. Soc. Trop. Med. Hyg. American Society of Tropical Medicine and Hygiene, Deerfield, IL [Google Scholar]
- 8. Craig W. A., Kiem S., Andes D. R. 2002. PK/PD target correlates with in vivo efficacy of macrolides, azilides, ketolides and clindamycin, abstr. A-1264. Abstr. 42nd Intersci. Conf. Antmicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 9. Dubois J., Fernandes P. 2009. In vitro activity of CEM-101 against Legionella spp., abstr. F1-2036. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 10. Dubois J., Fernandes P. 2009. In vitro activity of CEM-101 against resistant strains of Staphylococcus aureus, abstr. F1-2037. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 11. Farrell D. J., et al. 2010. Antimicrobial characterization of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 45:537–543 [DOI] [PubMed] [Google Scholar]
- 12. Farrell D. J., Castanheira M., Sader H. S., Jones R. N. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J. Infect. 61:476–483 [DOI] [PubMed] [Google Scholar]
- 13. Felmingham D. 2002. Evolving resistance patterns in community-acquired respiratory tract pathogens: first results from the PROTEKT global surveillance study. Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin. J. Infect. 44(Suppl. A):3–10 [PubMed] [Google Scholar]
- 14. Heine H. S., Miller L., Bassett J., Holman K. 2008. Antimicrobial activity of CEM-101 a new macrolide, tested against diverse collections of bacterial biowarfare/bioterrorism agents, abstr. F1-3980. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 15. Jones R. N., Sader H. S., Biedenbach D. J. 2009. Antimicrobial characterization of CEM-101: activity against staphylococci, β-haemolytic and viridans group streptococci, abstr. P-1100. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 16. Reference deleted.
- 17. Jones R. N., Woosley L. N., Moet G. J., Rhomberg P. R. 2009. Activity of CEM-101 tested against emerging telithromycin-resistant β-haemolytic streptococci, abstr. P-1101. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 18. Jones R. N., Stilwell M. G., Sader H. S., Fernandes P. 2009. CEM-101, a novel fluoroketolide: activity against recent (2008) isolates of multidrug-resistant S. pneumoniae, abstr. 208. Abstr. 47th Annu. Meet. Infect. Dis. Soc. Am [Google Scholar]
- 19. Jones R. N., Rhomberg P. R., Sader H. S. 2008. Antimicrobial characterization of CEM-101: single step, selection by passaging and inducible resistances, abstr. F1-3982. Abstr. 48th Intersci. Conf. Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 20. Lemaire S., Van Bambeke F., Tulkens P. M. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X., et al. 2009. Emerging macrolides resistance in Mycoplasma pneumoniae in children: detection and characterization of resistant isolates. Pediatr. Infect. Dis. J. 28:693–696 [DOI] [PubMed] [Google Scholar]
- 22. Lismond A., Van Bambeke F., Tulkens P. M. 2009. Comparative activities of the novel ketolide CEM-101 and telithromycin (TEL) towards Streptococcus pneumoniae (SP) resistant to macrolides (ML) from patients with confirmed community-acquired pneumonia (CAP), abstr. P-1099. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 23. Mandell L. A., et al. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGhee P., et al. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGuire J. M., Bunch P. L., Anderson R. C. 1952. Ilotycin, a new antibiotic. Antibiot. Chemother. 2:281–283 [PubMed] [Google Scholar]
- 26. Murphy T. M., et al. 2009. Efficacy and pharmacodynamic evaluation of CEM-101, a novel macrolide, in murine infection models, abstr. P-1098. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 27. Murphy T. M., Little S., Slee A. M., Fernandes P. 2010. Evaluation of solithromycin (CEM-101), a novel fluoroketolide, in murine infection models, abstr. F1-2152. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 28. Murphy T. M., Little S., Wu R., Slee A. M., Fernandes P. 2009. Evaluation of CEM-101, a novel fluoroketolide, in a rat H. influenzae pulmonary infection model, abstr. F1-2040. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 29. Neiderman M. S. 2005. Principles of appropriate antibiotic use. Int. J. Antimicrob. Agents 26(Suppl. 3):S170–S175 [DOI] [PubMed] [Google Scholar]
- 30. Putnam S. D., Castanheira M., Moet G. J., Farrell D. J., Jones R. N. 2010. CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 66:393–401 [DOI] [PubMed] [Google Scholar]
- 31. Robin P. M., Kohlhoff S. A., Parker C., Hammerschlag M. R. 2010. In vitro activity of CEM-101, a new ketolide antibiotic against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob. Agents Chemother. 54:1358–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodvold K. A., et al. 2010. Intrapulmonary penetration of CEM-101 in healthy adult subjects, abstr. A1-690. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 33. Ross D. B. 2007. The FDA and the case of Ketek. N. Engl. J. Med. 356:1601–1604 [DOI] [PubMed] [Google Scholar]
- 34. Sader H. S., Biedenbach D. L., Rhomberg P. R., Jones R. N. 2008. Antimicrobial characterization of CEM-101: PAE, bactericidal activity and combinations, abstr. F1-3981. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 35. Schranz J., et al. 2010. Phase 1 pharmacokinetic and safety of multiple doses and effects of food on the bioavailability of oral solithromycin (CEM-101) in healthy adult subjects, abstr. A1-689. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 36. Shain C. S., Amsden G. W. 2002. Telithromycin: the first of the ketolides. Ann. Pharm. 36:452–464 [DOI] [PubMed] [Google Scholar]
- 37. Shi J., Montay G., Bhargava V. O. 2006. Clinical pharmacokinetics of telithromycin, the first ketolide antibacterial. Clin. Pharmacokinet. 44:915–934 [DOI] [PubMed] [Google Scholar]
- 38. Still J. G., Clark K., Degenhardt T., Scott D., Fernandes P. 2010. Multiple dose pharmacokinetics and safety of CEM-101, a new fluoroketolide, in healthy subjects, abstr. 902. Abstr. 20th Eur. Congr. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 39. Tu D., Blaha G., Moore P. B., Steitz T. A. 2005. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121:257–270 [DOI] [PubMed] [Google Scholar]
- 40. Waites K. B., Crabb D. M., Duffy L. B. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wierzbowski A. K. K., et al. 2007. Macrolide resistance mechanisms among Streptococcus pneumoniae isolated over 6 years of Canadian Respiratory Organism Susceptibility Study (CROSS) (1998-2004). J. Antimicrob. Chemother. 60:733–740 [DOI] [PubMed] [Google Scholar]
- 42. WMADH. 2000. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 20:3043–3045 [PubMed] [Google Scholar]
- 43. Woosley L. N., Castanheira M., Jones R. N. 2010. Characterization of CEM-101 activity against gram-positive organisms. Antimicrob. Agents Chemother. 54:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]