Abstract
Studies using patient-level data to determine the attributable cost of invasive fungal diseases (IFDs) are few. Using a case-control study with activity-based costing of patients admitted to a quaternary hospital from 2002 to 2007, we determined attributable hospitalization cost (and 12 weeks thereafter), length of stay (LOS), and costly antifungal treatment (C-AT; liposomal amphotericin B, voriconazole, posaconazole, caspofungin), expressed as defined daily doses (DDDs) per IFD episode, in patients with hematological malignancies and hematopoietic stem cell recipients. Matching criteria and median regression modeling controlled for confounding variables, including LOS prior to IFD onset. Multiple mycoses were identified in 43 matched case-control pairs (n = 86). A separate sensitivity analysis included 22 unmatched patients. IFD status was associated with a median excess cost of AU$30,957 (95% confidence interval [CI] = AU$2,368 to AU$59,546; P = 0.034), approximating at purchasing power parity US$21,203 (95% CI = US$1,622 to US$40,784) and €15,788 (95% CI = €1,208 to €30,368), increasing to AU$80,291 (95% CI = AU$33,636 to AU$126,946; P = 0.001), i.e., US$54,993 (95% CI = US$23,038 to US$86,948) and €40,948 (95% CI = €17,154 to €64,742), with intensive care unit (ICU) requirement. Cost determinants were pharmacy costs (64%; P < 0.001) inclusive of antifungal treatment (27%; P < 0.001) and ward costs (27%; P = 0.091), with proportions persisting through 12 weeks for 25 surviving matched pairs (pharmacy, 60% [P = 0.12]; ward, 31% [P = 0.21]). Median LOS was not significantly increased unless unmatched patients were included (8 days, 95% CI = 1.8 to 14 days; P = 0.012). Excess C-ATs were 17 DDDs (95% CI = 15 to 19 DDDs; P < 0.001) per case patient and 19 DDDs (95% CI = 16 to 22 DDDs; P < 0.001) per ICU patient. The sensitivity analysis was confirmatory (for median cost, AU$29,441, 95% CI = AU$5,571 to AU$53,310, P = 0.016; for C-AT, 17 DDDs, 95% CI = 16 to 18 DDDs, P < 0.001). IFD results in increased hospital and ICU costs, with pharmacy costs, including antifungal treatment, being major determinants. Consumption of costly antifungal drugs may be a novel resource metric with wider generalizability than cost alone.
INTRODUCTION
Improvement in the short-term survival of patients with invasive aspergillosis (IA) (22, 24) is encouraging, but crude mortality rates remain high at >30% in patients with acute myeloid leukemia (AML) (24) and 57% in hematopoietic stem cell (HSCT) recipients (1). As a result, interest in prevention continues, with efficacy demonstrated for posaconazole in patients receiving induction-remission chemotherapy for AML/myelodysplastic syndromes (MDSs) and high-risk allogeneic HSCT (allo-HSCT) recipients (7, 32). However, given incidence rates of invasive fungal diseases (IFDs) of 10 to 15% among patients with AML and HSCT recipients (5, 17, 23), nonselective prophylaxis has raised concerns regarding overtreatment and expenditure (9, 25) because the numbers of eligible patients are high and the duration of prophylaxis is potentially lengthy.
Increasingly, the economic impact of IFDs has been considered in the clinical debate. One center, after determining the attributable mean IA-associated medical cost in AML/MDS patients to be €15,280 in association with a 30% institutional incidence, concluded that antimold prophylaxis was likely cost-beneficial from the patient and hospital perspectives (29). Cost determination methods for IFDs have included gross costs (16, 31), expert opinion (33), and clinical trial data (34, 36); but studies reporting attributable cost, a key component of cost-effectiveness analyses, are few (19–21, 29, 35), and those using patient-level data are even rarer (29). Importantly, sound estimates of attributable cost are dependent on the appropriate selection of case and reference groups in order to disentangle the confounding effect of underlying illness. In addition, measures of resource use alternative to cost which are independent of country and inflation are needed if health economic studies are to have improved generalizability. Thus, our goal was to determine the median hospitalization cost, length of stay (LOS), and consumption of costly antifungal treatment (C-AT: liposomal amphotericin B [L-AMB], voriconazole, posaconazole, caspofungin) attributable to IFD from a hospital perspective in high-risk hematology patients using actual hospital costs, preliminary results of which were used for the listing of posaconazole on Australia's national formulary.
(Preliminary results of this study were presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy-Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 2008, abstr. M-727.)
MATERIALS AND METHODS
Study design and setting.
We undertook a retrospective case-control study of patients with acute leukemia or HSCT from 2002 to 2007 at Alfred Health, a 750-bed adult quaternary university-affiliated hospital network with heart/lung and HSCT units, the latter performing approximately 50 allo-HSCTs per year. Patients were identified from the International Classification of Diseases, 10th revision, Australian Modification, diagnostic codes and underwent manual chart review. Hospitalization costs 12 weeks subsequent to the index admission were also examined. Institutional ethics approval was obtained.
Matching criteria.
Control patients were matched 1:1 with case patients using the following criteria: age within 10 years of a case patient, same underlying hematological disease or year of transplantation, and LOS at least as long as that of case patients prior to IFD, whose date of onset was determined by investigators (M.R.A.-R., M.S.) following manual chart review. The LOS criterion meant that selection of control patients began after chart review of case patients. Exclusion criteria were death at ≤48 h of admission, LOS of <3 days, HIV infection, or key data missing from the chart. If suitable controls were not found, the matching criteria were relaxed sequentially: the age criterion was dropped, alternative hematological conditions were considered, and for HSCT recipients, the year of transplantation within 2 years of the case patient was accepted. The LOS criterion was not relaxed, as this was regarded a key component of cost.
Clinical data and definitions.
Collected information included demographics; antifungal drug indications and usage, expressed as defined daily doses (DDDs; WHO Collaborating Centre for Drug Statistics Methodology [http://www.whocc.no]), with prescribed daily doses of 250 mg/day used for L-AMB (11); type/stage of chemotherapy; duration of neutropenia (absolute neutrophil count < 500 cells/mm3); status of underlying disease; HSCT type; graft-versus-host disease (GVHD); Charlson comorbidity index (CCI); intensive care unit (ICU) admission; IFD classification according to accepted criteria (10); and in-hospital mortality and all-cause mortality 12 weeks after IFD diagnosis, as recorded in the medical chart. Date of IFD onset was defined as the first day of suspicious radiological abnormality or positive microbiology result. Although the galactomannan assay became available in 2005, it is not widely used and results of that assay were not used in this study.
Costing data.
Hospitalization costs were obtained from an activity-based costing (ABC) system (Power Business Analytics) in use since 1994. It captures direct (i.e., patient-related) and indirect (e.g., overhead/capital outlay) medical costs reflecting fixed (e.g., salaries) and variable (e.g., investigations and medication costs) costs, ascribing 130 categories per patient which were collated into diagnostics, procedures, operating theater, pharmacy, ICU, and ward costs using mapping tables. Variable costs are patient specific and itemized, with fixed costs apportioned across all inpatients. Collection of detailed resource utilization data was restricted to antifungal treatment after preliminary analysis indicated that antifungal drugs were a major contributor to cost. Antifungal drug acquisition costs are primarily the list price or the Victorian Health Purchasing state contract price (Health Purchasing Victoria tender 2007 to 2009 [http://www.hpv.org.au]). Costs of antifungal drugs available on imprest (only fluconazole) are apportioned across all ward patients. Indirect nonmedical (e.g., loss of productivity) and intangible costs were not evaluated. The short time horizon obviated discounting of future costs or benefits. Costs are reported in Australian dollars inflated to 2009 using the health care component of the Australian consumer price index (Australian Institute of Health and Welfare [http://www.aihw.gov.au/publications/index.cfm/title/10954]), and final costs were converted to 2009 US$/€ using purchasing power parity (PPP) measures (Organisation for Economic Co-Operation and Development Stat Extracts [http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4]).
Statistical analysis.
The highly skewed nature of health outcomes (cost, LOS, antifungal treatment) motivated the choice of median (quantile) regression for data analysis. The median is a more reliable measure of central tendency than the mean or geometric mean, as it is more resistant to outlier influence and median regression models are less sensitive to assumptions that are made in generalized linear models (GLMs), particularly heteroscedasticity and error normality. In this technique, the coefficient represents the incremental median cost associated with a unit change in the explanatory variable; for dummy-coded categorical variables, this was the cost associated with the presence of the factor. We considered dependent variables in a univariable analysis against each outcome variable; all explanatory variables associated with each outcome variable with a P value of <0.1 and eliminated by backwards stepwise selection were selected for three multivariable models. We forced inclusion of case-control status (as the primary dependent variable of interest) and ICU admission (a factor known to be strongly associated with increased cost) into all multivariable models. Model fit was assessed using the link test. Reported P values were two-tailed, and for each analysis a P value of <0.05 was considered significant. All analyses used the Stata (version 11.0) statistical package (Stata Corp., College Station, TX).
RESULTS
Patient characteristics.
A total of 110,744 admissions were screened from coding data, and 43 matched pairs were identified after manual chart review. Study groups were similar with regard to prespecified characteristics (Table 1) and additional clinical features, including prolonged neutropenia (≥10 days; 74% versus 70% for case and control patients, respectively) and baseline neutropenia (35% versus 33% for case and control patients, respectively) and poor-risk hematological disease (86% versus 84% for case and control patients, respectively). More case patients (21%) than control patients (9.3%) required ICU admission (P = 0.23), which occurred after IFD diagnosis in 8 of 9 case patients.
Table 1.
Characteristics of patients with and without invasive fungal infectionsa
| Variable | IFD group (n = 43) | Control group (n = 43) |
|---|---|---|
| Age (yr) | ||
| Median | 50 | 54 |
| Mean | 44 | 52 |
| range | 26–76 | 20–83 |
| Male sex | 23/43 (53) | 22/43 (51) |
| Length of stay (days) | ||
| Median | 39 | 31 |
| Mean | 44 | 29 |
| Range | 7–193 | 3–54 |
| Hematological malignancy | ||
| Leukemia | 35/43 (81) | 36/43 (84) |
| Leukemia newly diagnosed | 16/43 (37) | 21/43 (49) |
| Relapsed leukemia | 8/43 (19) | 3/43 (7.0) |
| Stem cell transplantation | 13/43 (30) | 12/43 (28) |
| Allogeneic | 11/43 (26) | 11/43 (26) |
| Transformed MDS | 2/43 (4.7) | 2/43 (4.7) |
| Lymphoma | 4/43 (9.3) | 4/43 (9.3) |
| Other | 2/43 (4.7) | 1/43 (2.3) |
| Poor-risk hematological diseaseb | 37/43 (86) | 36/43 (84) |
| Receipt of myelotoxic chemotherapy | 32/43 (74) | 33/43 (77) |
| ICU admission | 9/43 (21) | 4/43 (9.3) |
| Time of neutropenia < 500 cells/μl | ||
| (days) | ||
| Median | 24 | 19 |
| Range | 5–53 | 1–47 |
| Neutropenia for ≥10 days | 32/43 (74) | 30/43 (70) |
| Neutropenia at baseline | 15/43 (35) | 14/43 (33) |
| Date of index admission | ||
| 2003 | 0/43 (0) | 3/43 (7.0) |
| 2004 | 11/43 (26) | 7/43 (16) |
| 2005 | 13/43 (30) | 12/43 (28) |
| 2006 | 15/43 (35) | 15/43 (35) |
| 2007 | 4/43 (9.3) | 6/43 (14) |
| CCI | ||
| <4 | 41/43 (95) | 38/43 (88) |
| ≥4 | 2/43 (4.7) | 5/43 (12) |
| Inpatient death | 6/43 (14) | 5/43 (12) |
| Inpatient death at ≥14 days | 6/43 (14) | 3/43 (7.0) |
| All-cause mortality at 12 wk for | 11/42 (26) | 8/36 (22) |
| evaluable patients | ||
| Infectionc | NAd | |
| Sinopulmonary | 30/43 (70) | |
| Fungemia | 8/43 (19) | |
| Hepatic | 4/43 (9.3) | |
| Othere | 2/43 (4.7) | |
| Disseminated | 10/43 (23) | |
| Localized | 33/43 (77) | |
| Antifungal drug consumption (DDD)f | ||
| Total | ||
| Median | 52 | 32 |
| Mean | 70 | 33 |
| Range | 1.5–287 | 0–113 |
| Costly antifungal treatmentg | ||
| Median | 19 | 0 |
| Mean | 34 | 5.5 |
| Range | 0–122 | 0–32 |
| Receipt of systemic antifungal | 26/43 (60) | 26/43 (60) |
| prophylaxis | ||
| No. of coursesh of antifungal | ||
| prophylaxis | ||
| Fluconazole | 13/31 (42) | 11/37 (30) |
| Itraconazole | 14/31 (45) | 19/37 (51) |
| Voriconazole | 4/31 (13) | 4/37 (11) |
| Posaconazole | 0 | 1/37 (2.7) |
| Caspofungin | 0 | 2/37 (5.4) |
Unless indicated otherwise, data represent number of patients with the characteristic/total number of patients tested (percent).
Poor-risk disease includes relapse, progressive disease, partial remission, induction, and failed induction.
Some patients had one or more sites involved.
NA, not applicable.
One each central nervous system and hepatosplenic.
DDD, defined daily dose.
Denoted by liposomal amphotericin B, voriconazole, posaconazole, and caspofungin.
Course denotes antifungal drug administered for any duration.
IFD complicated chemotherapy-induced aplasia in 24/43 (56%) patients, with induction (n = 21) regimens predominating. Hematological disease progression or leukemic relapse was a factor in 6/43 (14%) patients. Times of IFD onset from allo-HSCT were ≤30 days (n = 4), 30 to 100 days (n = 2), and late (>100 days) in 2 patients (who had GVHD and leukemic relapse, respectively); IFD preceded allo-HSCT in 2 patients.
Antifungal prophylaxis was administered to 60% (n = 26) of the patients in each study group, with fluconazole and itraconazole being the most common and voriconazole used off-label as prophylaxis after 2004 in small numbers. Antifungal prophylaxis was not administered to 11 case patients with IFD complicating postinduction aplasia (n = 2 in 2004, n = 2 in 2005, n = 4 in 2006, n = 3 in 2007).
Characteristics of case patients and clinical outcomes.
The most common infection was sinopulmonary (70%), followed by fungemia (19%). A total of 21 fungal isolates (13 molds, 8 Candida species) were recovered from 20 patients with probable/proven IFDs. Aspergillus species were the most frequently isolated (10/21, 48%), with Aspergillus fumigatus recovered from 9/13 patients. Opportunistic molds (Scedosporium and Rhizopus species) were uncommon (n = 3). Non-Candida albicans Candida species accounted for 6 of 8 Candida isolates. Overall mortality at 12 weeks for 42 evaluable case patients was 26%. The high number of control patients (n = 7) unevaluable at 12 weeks limited outcome comparisons.
Characteristics of unmatched case patients.
Our anticipated goal of 50 matched pairs (on the basis of feasibility considerations) was undermined by insufficient control patients, complicated by the loss of 24 potential candidates due to previous (n = 11) or possible (n = 13) IFD. Thus, our case target was easily met but 13 case patients (10 with probable/proven IFD) lacked suitable controls. Unmatched case patients had a mean age of 44 years (range, 25 to 67 years), median LOS of 36 days (range, 10 days to 133 days), and median hospitalization costs of AU$76,456 (mean, AU$160,854; range, AU$34,548 to AU$820,452). There were 7 HSCT recipients (5 allo-HSCT), and all had poor-risk hematological disease.
Cost, length of stay, and antifungal drug consumption adjusted for additional clinical characteristics.
Differences between groups resulted in a crude median IFD-attributable cost of AU$28,309 (mean, AU$79,129) (Table 2) and LOS of 8 days (mean, 15 days). Median regression analyses adjusted for additional clinical characteristics not accounted for by matching criteria (Table 3). Of several candidate variables (P < 0.1), only receipt of chemotherapy and late (≥14 days) in-hospital mortality (in addition to case status and ICU admission) were included in the final model, on the basis of their consistent association on univariable analyses with all outcome variables.
Table 2.
Hospitalization costs inflated to 2009 AU$ per patient for index hospitalization and hospitalizations 12 weeks after index admission, outpatient care excludeda
| Cost category | Index hospitalization |
Hospitalization up to 12 wk from index hospitalizationb |
||||||
|---|---|---|---|---|---|---|---|---|
| Cost (2009 AU$) |
Pc | Cost (2009 AU$) |
Pc | |||||
| IFD group (n = 43) | Control group (n = 43) | Difference between groups | IFD group (n = 25) | Control group (n = 25) | Difference between groups | |||
| Hospital stay | ||||||||
| Wardd | 49,947 | 33,292 | 16,655 (21)e | 26,512 | 10,927 | 15,585 (30) | ||
| ICU | 7,609 | 2,655 | 4,954 (6.3) | 847 | 196 | 651 (1.2) | ||
| Total | 57,556 | 35,947 | 21,609 (27) | 0.091 | 27,359 | 11,123 | 16,235 (31) | 0.21 |
| Pharmacy (total)f | 72,529 | 22,130 | 50,399 (64) | <0.001 | 40,358 | 8,731 | 31,627 (60) | 0.12 |
| Antifungal drugs | 26,219 | 4,775 | 21,444 (27) | <0.001 | —g | — | — | — |
| Diagnostics | ||||||||
| Pathology | 10,563 | 7,066 | 3,497 (4.4) | 5,307 | 2,918 | 2,389 (4.5) | ||
| Radiology | 4,567 | 2,322 | 2,245 (2.8) | 1,820 | 982 | 838 (1.6) | ||
| Total | 15,130 | 9,388 | 5,742 (7.3) | 0.072 | 7,127 | 3,900 | 3,227 (6.1) | 0.15 |
| Proceduresh | 2,082 | 1,179 | 903 (1.1) | 0.32 | 1,131 | 348 | 783 (1.5) | 0.20 |
| Operating theater | 1,008 | 532 | 476 (0.6) | 0.14 | 1,020 | 201 | 819 (1.6) | 0.043 |
| Total costs | ||||||||
| Mean | 148,305 | 69,176 | 79,129i | 76,995 | 24,303 | 52,692 | ||
| Median | 81,691 | 53,382 | 28,309j | 0.0099 | 37,815 | 11,237 | 26,578 | 0.098 |
| Range | 10,518–687,574 | 3,468–233,020 | 0–425,631 | 0–148,886 | ||||
Values are reported as means (unless otherwise stated).
For surviving matched pairs at 12 weeks.
Test of difference between IFD and control groups used the Mann-Whitney U test.
Ward costs include emergency and inpatient care.
Values in parentheses represent percent difference.
Pharmacy expenditure includes staff salaries as well as medications.
—, data not available.
Therapeutic or diagnostic procedures, e.g., bronchoscopy.
Mean cost at purchasing power parity (2009): US$54,198 and €40,356.
Median cost at purchasing power parity (2009): US$19,390 and €14,438.
Table 3.
Median regression model for hospitalization costs, LOS, and antifungal treatment per patient
| Variable | Result by outcomea |
|||||
|---|---|---|---|---|---|---|
| Hospitalization costb (AU$) |
LOS (days) |
Costly antifungal treatmentc (DDD) |
||||
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | Coefficient (95% CI) | P | |
| Univariable analysis | ||||||
| IFD patient | 28,309 (568 to 56,049) | 0.046 | 8.0 (0.57 to 15) | 0.035 | 19 (18 to 20) | <0.001 |
| LOS | 1,961 (1,518 to 2,405) | <0.001 | —d | — | 0.45 (0.33 to 0.56) | <0.001 |
| ICU admission | 65,470 (19,224 to 111,716) | 0.006 | 13 (0.99 to 25) | 0.034 | 25 (5.6 to 44) | 0.012 |
| HSCT | 53,549 (24,576 to 82,521) | <0.001 | −2.0 (−13 to 9) | 0.71 | 11 (−2.6 to 25) | 0.11 |
| Allo-HSCT | 57,681 (27,602 to 87,760) | <0.001 | 5.0 (−6 to 16) | 0.37 | 12 (−1.4 to 25) | 0.078 |
| Receipt of chemotherapy | 49,268 (13,047 to 85,489) | 0.008 | 25 (15 to 35) | <0.001 | 12 (3.2 to 20) | 0.008 |
| Inpatient death ≥ 14 dayse | 104,164 (51,646 to 156,681) | <0.001 | 45 (31 to 59) | <0.001 | 41 (17 to 65) | 0.001 |
| Inpatient death | 52,502 (5,555 to 99,449) | 0.029 | 10 (−3.24 to 23) | 0.14 | 25 (5.6 to 44) | 0.012 |
| Age (yr) | −1,392 (−2,377 to −407) | 0.006 | −0.29 (−0.60 to 0.02) | 0.066 | −0.34 (−0.63 to −0.06) | 0.018 |
| Receipt of costly antifungal treatmentc | 426 (−162 to 1,014) | 0.15 | 0.39 (0.23 to 0.54) | <0.001 | — | — |
| Neutropenia ≥ 10 days | 19,529 (−22,524 to 61,581) | 0.36 | 19 (11 to 27) | <0.001 | 11 (2.5 to 20) | 0.012 |
| Neutropenia at baseline | −12,252 (−52,628 to 28,124) | 0.55 | −1.0 (−11 to 9.3) | 0.85 | −1.05 (−17 to 14) | 0.89 |
| Poor-risk hematological diseasef | −40,267 (−85,936 to 5,402) | 0.083 | 1.0 (−11 to 13) | 0.87 | 1.4 (−20 to 23) | 0.90 |
| Newly diagnosed leukemia | −10,048 (−44,615 to 24,520) | 0.57 | 7.0 (−1.81 to 16) | 0.12 | −5.3 (−20 to 9.2) | 0.47 |
| Multivariable analysisg | ||||||
| Base caseh | 23,964 (−8,819 to 56,747) | 0.15 | 12 (2.8 to 21) | 0.011 | 0 (−1.5 to 1.5) | 1.0 |
| IFD patienti | 30,957 (2,368 to 59,546) | 0.034 | 7.0 (−0.95 to 15) | 0.083 | 17 (15 to 19) | <0.001 |
| ICU admissioni | 80,291 (33,636 to 126,946) | 0.001 | 6.0 (−7.23 to 19.23) | 0.37 | 19 (16 to 22) | <0.001 |
| Receipt of chemotherapy | 29,418 (−4,695 to 63,531) | 0.09 | 20 (11 to 29) | <0.001 | 0 (−1.9 to 1.9) | 1.0 |
| Inpatient death ≥ 14 dayse | 24,824 (−32,713 to 82,360) | 0.39 | 33 (17 to 49) | <0.001 | 13 (9.0 to 17) | <0.001 |
Values are reported as medians.
Cost inflated to 2009 AU$.
For liposomal amphotericin B, 250 mg intravenously was regarded to be equivalent to 1 DDD (11), denoted by liposomal amphotericin B, voriconazole, posaconazole, and caspofungin.
—, variables not included in the model.
The reference group comprised surviving patients and 2 control patients who died early in their admission, i.e., within the first 7 days.
Poor-risk disease includes relapse, progressive disease, partial remission, induction, and failed induction.
Multivariable analysis included forced inclusion of case status and ICU admission and variables significant on univariable analysis (P < 0.1).
The baseline patient who did not develop an IFD received chemotherapy and survived a minimum of 14 days in hospital. All values for each variable in the multivariable analysis refer to the excess cost, LOS, or antifungal drug consumption attributable to an IFD and added to the base case.
Median excess cost for an IFD patient at purchasing power parity (2009): US$21,203 (95% CI = US$1,622 to US$40,784)/€15,788 (95% CI = €1,208 to €30,368) increasing to US$54,993 (95% CI = US$23,038 toUS$86,948)/€40,948 (95% CI = €17,154 to €64,742) with intensive care unit admission.
On multivariable analysis, IFD status was associated with an excess median cost over that for the baseline patient of AU$30,957 (95% confidence interval [CI] = AU$2,368 to AU$59,546; P = 0.034) and 17 DDDs of C-AT. If ICU admission was also required, then the excess median cost increased to AU$80,291 (95% CI = AU$33,636 to AU$126,946; P = 0.001) and an additional 19 DDDs of C-AT were required (P < 0.001). Late in-hospital mortality was strongly associated with prolonged excess median LOS (33 days; P < 0.001) and 13 DDDs of C-AT (P < 0.001) but not cost (P = 0.39), unless ICU admission was omitted from the multivariable model (AU$105,115; 95% CI = AU$60,437 to AU$149,792; P < 0.001). Case status was not associated with increased median LOS (P = 0.83), but following inclusion of 22 unmatched patients (13 case patients, 9 control patients) in a sensitivity analysis akin to that of Dubberke et al. (12), a significant association (LOS, 8 days; 95% CI = 1.8 to 14 days; P = 0.012) emerged, while median cost (AU$29,441; 95% CI = AU$5,571 to AU$53,310; P = 0.016) and C-AT (17 DDDs; 95% CI = 16 to 18; P < 0.001) remained largely unchanged.
Distribution of costs is shown in Table 2. Main determinants of the difference in mean cost were pharmacy costs (64%; P < 0.001), of which antifungal drugs comprised 27% (P < 0.001), followed by ward costs (27%; P = 0.091). Proportionate differences in mean hospitalization cost were maintained 12 weeks from the index hospitalization for the 25 surviving matched pairs (pharmacy costs, 60%; ward costs, 31%) but were not statistically significant.
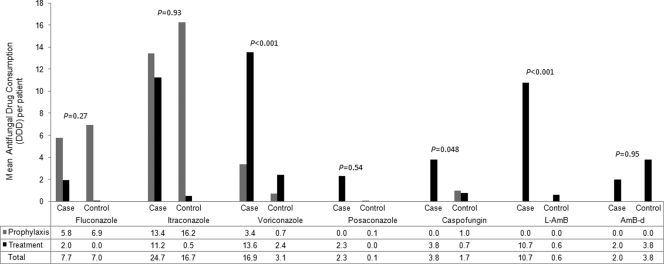
Antifungal drug consumption according to treatment indication is presented in Fig. 1. Mean drug consumption was higher among case patients, with the exception of amphotericin B deoxycholate (AMB-d), but significant differences were observed for voriconazole (P < 0.001), L-AMB (P < 0.001), and caspofungin (P = 0.048). The small numbers (n = 3) using posaconazole (prior to its licensure) limited its interpretation. In case patients, median drug administrations were as follows: L-AMB 240 mg/day (mean, 266 mg/day) for 7.5 days (mean, 11 days); voriconazole, 400 mg/day (mean, 464 mg/day) for 6 days (mean, 10 days); and caspofungin, 50 mg/day (mean, 52 mg/day) for 6 days (mean, 10 days).
Fig. 1.
Mean antifungal drug consumption (in DDDs) for patients with and without invasive fungal diseases. For liposomal amphotericin B, 250 mg intravenously was regarded equivalent to 1 DDD (11). Differences in total drug consumption between case and control patients were determined using the Mann-Whitney U test.
DISCUSSION
Determining disease attribution and broadening the generalizability of economic analyses are challenges we attempted to overcome in this study. A comparative attribution approach using a case-control method, followed by regression modeling to adjust for clinical characteristics not accounted for by matching criteria, was used to separate the confounding effect of underlying illness from IFD-related outcomes. Informed by Graves et al.'s caution against overestimating the cost of hospital-acquired infections (14), we therefore reported median outcomes to describe the typical value for most patients rather than the arithmetic mean, which, while relevant to payers, e.g., the hospital, is highly sensitive to outliers, thus limiting its generalizability while potentially overstating cost. Commonly used approaches for adjustment of highly skewed data include linear regression models for log-transformed dependent variables and GLMs with a logarithmic link function (26); however, normalization of data is not always successful (13), and for linear regression models, retransformation of predicted results may be misleading (26). Quantile regression models, in contrast, make no assumptions about distribution of errors or outcomes and can accommodate different quantiles, depending on the focus of interest, i.e., the median to describe the general population or higher quantiles for outliers (2). Predictably, the crude hospitalization cost in our matched-pairs analysis was right skewed, with median and mean IFD-attributable costs being AU$28,309 and AU$79,129 per patient, respectively. By median regression analysis adjusted for ICU requirement, receipt of chemotherapy and late (≥14 days) in-hospital mortality, the IFD cost was AU$30,957 (95% CI = AU$2,368 to AU$59,546; P = 0.034; approximating at a PPP of US$21,203/€15,788) over that for the baseline patient and consistent with the crude estimate, differing by <10%. Median excess cost increased to AU$80,291 (95% CI = AU$33,636 to AU$126,946; P = 0.001) if intensive care was also required, which occurred in 21% of case patients.
Antifungal drugs being a substantial component of our IFD-attributable cost is contrary to the findings of previous studies (8, 27), which have found LOS to be the main determinant of hospitalization cost. This result was not unexpected due to the high acquisition costs of drugs we commonly use for treatment, namely, L-AMB, voriconazole, caspofungin, and, to a lesser extent, posaconazole.
Driving the difference in mean cost per patient was overwhelmingly pharmacy (64%; P < 0.001), of which antifungal drugs accounted for 43% of pharmacy expenditure or 27% of the overall difference (P < 0.001), with no significant difference in ward costs seen (27%; P = 0.091). The robustness of these results was confirmed in the 12-week analysis of subsequent inpatient care (pharmacy costs, 60%; ward costs, 31%), which was not significant, probably due to fewer surviving matched pairs (n = 25). Historically, antifungal drugs have accounted for 7 to 15% of total treatment costs (4, 27, 35), a finding supported by a recent U.S. study where intravenous antifungal drugs accounted for 7.2% of IA-associated hospitalization costs (16), but differences in case mix and clinical care are likely responsible.
Slobbe et al. (29), in a cohort similar to ours (2002 to 2007), used fluconazole prophylaxis and AMB-d (pre-2003) or voriconazole (typically, the less costly oral form) for treatment of IA. In contrast, our practice is characterized by antimold prophylaxis (58% of case patients, 70% of control patients) and C-AT (i.e., voriconazole, posaconazole, caspofungin, L-AMB), with voriconazole (means, 16.9 DDDs/case patient and 3.1 DDDs/control patient; P < 0.001) and L-AMB (means, 10.7 DDDs/case patient and 0.6 DDDs/control patient; P < 0.001) predominating principally for empiric or definitive treatment of IFD with AMB-d rarely used due to its recognized toxicities. The high contribution of antifungal treatment to hospitalization costs is also recognized in the ICU, where it is regarded a costly intervention, along with hemodialysis and blood product administration (18).
Alternatives to cost as a descriptor of resource utilization were sought in order to enhance generalizability. IFD status was associated with an excess crude median LOS of 8 days (mean, 15 days; P = 0.083) which reached significance after inclusion of 22 unmatched patients into the model (median, 8 days; 95% CI = 1.7 days to 14 days; P = 0.012), suggesting a sample size effect. Thus, a conservative estimate of the opportunity cost per IFD episode includes the loss of 8 ward-bed days at AU$700/day and a crude mean difference in antifungal treatment of AU$21,444, approximating AU$27,044 in total, notwithstanding other marginal costs, e.g., diagnostics and potential loss of ICU-bed days at AU$3,200/day.
C-AT represented another measure not previously described in the economic literature but proved useful in comparing subgroups, including case patients (17 DDDs; 95% CI = 15 to 19 DDDs; P < 0.001), ICU patients (19 DDDs; 95% CI = 16 to 22 DDDs; P < 0.001), and patients with late in-hospital mortality (13 DDDs; 95% CI = 9.0 to 17 DDDs; P < 0.001). In case patients, 17 DDDs approximates L-AMB at 250 mg/day for 7 to 10 days, followed by voriconazole at 400 mg/days for 7 days, and is consistent with documented prescribing, thus validating the model. Late in-hospital mortality, i.e., nonsurvivor care, was not more costly (compared to the reference group, comprising survivors and 2 control patients who died early in hospital), despite a strong association with C-AT and prolonged LOS (33 days; P < 0.001), perhaps due to the competing effect of intensive care, which was required by some nonsurvivors (6 of 10; data not shown) but by more patients overall (n = 13). Indeed, with omission of ICU admission from the final model, nonsurvivor care was substantial (AU$105,115; P < 0.001), suggesting that IFD could prolong hospitalization and increase cost before death supervenes.
The economic burden of IFDs on hospitals is recognized (16, 20), but methodological differences between our study and others limit comparisons. Kim et al. (16), using actual costs reported median gross hospitalization costs of US$72,029 for a subset of hematology patients with IA (2000 to 2006), while Tong et al. (31), using cost-to-charge ratios (2003), reported a median gross cost of US$47,949 for non-HSCT hematology patients. Menzin et al. (20) estimated the mean IFD-attributable cost in patients with hematological malignancies and HSCT recipients to be US$37,046 (P < 0.001) and US$60,190 (P < 0.001), respectively, a range which includes our crude mean estimate approximating at a PPP of US$54,198. These studies (16, 20, 31), like others now ≥10 years old (8, 27, 28, 35), used administrative data sets, which have poor case detection (6); in our case, an administrative IFD diagnosis was absent for 13 possible case patients.
Study limitations include the small sample size, reflecting the epidemiology of a disease with a low institutional incidence (17), compounded by difficulties in finding suitable controls. Inpatient care underestimates the true burden of IFDs, which have outpatient and societal costs, but previous studies have suggested that >50% of costs are incurred in hospital (35). Generalizability, a concern of single-center studies, was mitigated by median regression modeling and C-AT as a resource metric. Use of retrospective data in combination with ABC is a valid approach (15), with ABC being a highly regarded cost-capturing tool (3); however, studies utilizing bottom-up methods are few (29), with proxies such as cost-to-charge ratios popular in the United States, despite their recognized shortcomings as billing parameters rather than actual expenses (15).
Gross hospitalization costs are of interest, but attributable estimates are preferred for pharmacoeconomic analyses. To this extent, our case and reference groups were well-defined, and chart review ensured that only patients truly with or without IFD were included. Time-dependent bias was addressed by controlling for LOS prior to IFD onset, thus separating preinfection from postinfection costs (14). Inclusion of parameters (e.g., receipt of chemotherapy) predictive of treatment-related complications not controlled for (e.g., mucositis) minimized residual confounding. A sensitivity analysis addressed omitted variables and selection biases inherent in matched-cohort studies (14) by including all unmatched patients and showed similar results. The CCI was poorly discriminatory in our cohort, as in HSCT recipients (30), because many comorbidities are exclusion criteria for chemotherapy, and therefore, no adjustment for comorbidities was made. Strategies such as prophylaxis as part of a stewardship program may reduce costs, as highlighted by the few patients (2 to 3/year, 2004 to 2007) who failed to receive antifungal prophylaxis and developed IFD during postinduction aplasia.
Ameliorating the economic burden of IFDs while optimizing the return from finite health care resources is possible with better diagnostics, improved antifungal stewardship, and individualized prophylaxis. In our setting, the attributable cost of an IFD is driven by pharmacy expenditure, of which antifungal drugs are a major contributor, with supportive care, i.e., ICU admission, also being substantial. Our methods are applicable to other settings, and the results provided can inform future studies assessing the cost-effectiveness of IFD interventions.
ACKNOWLEDGMENTS
This study was partially funded by Schering-Plough Pty. Ltd. using an unrestricted educational grant. Michelle R. Ananda-Rajah is the recipient of a National Health and Medical Research Council postgraduate medical scholarship.
We thank Michelle Frost from Schering-Plough Pty. Ltd. for facilitating data collection and Karin Thursky for reviewing the manuscript.
Study conception and design were by Michelle R. Ananda-Rajah, C. Orla Morrissey, and Monica Slavin; data collection was by Michelle R. Ananda-Rajah, A. Munro Neville, Michael Dooley, C. Orla Morrissey, and Monica Slavin; data analysis and interpretation were by Michelle R. Ananda-Rajah, Allen Cheng, Tim Spelman, and Monica Slavin; drafting of the manuscript was by Michelle R. Ananda-Rajah; and all of us participated in critical revision of the manuscript.
M.R.A.-R. has received speaker's fees from Schering Plough Pty. Ltd. to formally present the results of this study; C.O.M. serves or has served on advisory boards for, has received investigator-initiated grants from, and has given lectures for Gilead Sciences, Pfizer, Merck, and Schering Plough; A.C., T.S., and M.D. have no conflicts; A.M.N. has received contractual funding from Schering Plough Pty. Ltd.; and M.S. serves or has served on advisory boards for, has received investigator-initiated grants from, and has given lectures for Gilead Sciences, Pfizer, Merck, and Schering Plough.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Baddley J. W., et al. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50:1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bang H., Tsiatis A. A. 2002. Median regression with censored cost data. Biometrics 58:643–649 [DOI] [PubMed] [Google Scholar]
- 3. Barnett P. G. 2009. An improved set of standards for finding cost for cost-effectiveness analysis. Med. Care 47:S82–S88 [DOI] [PubMed] [Google Scholar]
- 4. Cagnoni P. J., et al. 2000. Pharmacoeconomic analysis of liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. J. Clin. Oncol. 18:2476–2483 [DOI] [PubMed] [Google Scholar]
- 5. Caira M., et al. 2008. Invasive fungal infections in patients with acute myeloid leukemia and in those submitted to allogeneic hemopoietic stem cell transplant: who is at highest risk? Eur. J. Haematol. 81:242–243 [DOI] [PubMed] [Google Scholar]
- 6. Chang D. C., et al. 2008. Comparison of the use of administrative data and an active system for surveillance of invasive aspergillosis. Infect. Control Hosp. Epidemiol. 29:25–30 [DOI] [PubMed] [Google Scholar]
- 7. Cornely O. A., et al. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359 [DOI] [PubMed] [Google Scholar]
- 8. Dasbach E. J., Davies G. M., Teutsch S. M. 2000. Burden of aspergillosis-related hospitalizations in the United States. Clin. Infect. Dis. 31:1524–1528 [DOI] [PubMed] [Google Scholar]
- 9. De Pauw B., Donnelly J. P. 2007. Prophylaxis and aspergillosis—has the principle been proven? N. Engl. J. Med. 356:409–411 [DOI] [PubMed] [Google Scholar]
- 10. De Pauw B., et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de With K., et al. 2005. Hospital use of systemic antifungal drugs. BMC Clin. Pharmacol. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubberke E. R., Reske K. A., Olsen M. A., McDonald L. C., Fraser V. J. 2008. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin. Infect. Dis. 46:497–504 [DOI] [PubMed] [Google Scholar]
- 13. Graves N., Birrell F., Whitby M. 2005. Effect of pressure ulcers on length of hospital stay. Infect. Control Hosp. Epidemiol. 26:293–297 [DOI] [PubMed] [Google Scholar]
- 14. Graves N., et al. 2010. Estimating the cost of health care-associated infections: mind your p's and q's. Clin. Infect. Dis. 50:1017–1021 [DOI] [PubMed] [Google Scholar]
- 15. Jegers M., Edbrooke D. L., Hibbert C. L., Chalfin D. B., Burchardi H. 2002. Definitions and methods of cost assessment: an intensivist's guide. ESICM Section on Health Research and Outcome Working Group on Cost Effectiveness. Intensive Care Med. 28:680–685 [DOI] [PubMed] [Google Scholar]
- 16. Kim A., Nicolau D. P., Kuti J. L. 14 June 2010. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 17. Kontoyiannis D. P., et al. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50:1091–1100 [DOI] [PubMed] [Google Scholar]
- 18. McLaughlin A. M., Hardt J., Canavan J. B., Donnelly M. B. 2009. Determining the economic cost of ICU treatment: a prospective “micro-costing” study. Intensive Care Med. 35:2135–2140 [DOI] [PubMed] [Google Scholar]
- 19. Menzin J., et al. 2005. Excess mortality, length of stay, and costs associated with serious fungal infections among elderly cancer patients: findings from linked SEER-Medicare data. Value Health 8:140–148 [DOI] [PubMed] [Google Scholar]
- 20. Menzin J., et al. 2009. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am. J. Health Syst. Pharm. 66:1711–1717 [DOI] [PubMed] [Google Scholar]
- 21. Morgan J., et al. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 26:540–547 [DOI] [PubMed] [Google Scholar]
- 22. Neofytos D., et al. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin. Infect. Dis. 48:265–273 [DOI] [PubMed] [Google Scholar]
- 23. Pagano L., et al. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068–1075 [PubMed] [Google Scholar]
- 24. Pagano L., et al. 2010. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 95:644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pagano L., Fianchi L., Caira M. 2008. Pulmonary aspergillosis in hematologic malignancies: lights and shadows. Haematologica 93:1611–1616 [DOI] [PubMed] [Google Scholar]
- 26. Polsky D., Glick H. 2009. Costing and cost analysis in randomized controlled trials: caveat emptor. Pharmacoeconomics 27:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rentz A. M., Halpern M. T., Bowden R. 1998. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin. Infect. Dis. 27:781–788 [DOI] [PubMed] [Google Scholar]
- 28. Slavin M., et al. 2004. Burden of hospitalization of patients with Candida and Aspergillus infections in Australia. Int. J. Infect. Dis. 8:111–120 [DOI] [PubMed] [Google Scholar]
- 29. Slobbe L., et al. 2008. Outcome and medical costs of patients with invasive aspergillosis and acute myelogenous leukemia-myelodysplastic syndrome treated with intensive chemotherapy: an observational study. Clin. Infect. Dis. 47:1507–1512 [DOI] [PubMed] [Google Scholar]
- 30. Sorror M. L., et al. 2005. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong K. B., Lau C. J., Murtagh K., Layton A. J., Seifeldin R. 2009. The economic impact of aspergillosis: analysis of hospital expenditures across patient subgroups. Int. J. Infect. Dis. 13:24–36 [DOI] [PubMed] [Google Scholar]
- 32. Ullmann A. J., et al. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347 [DOI] [PubMed] [Google Scholar]
- 33. Van Campenhout H., Marbaix S., Derde M. P., Annemans L. 2008. Voriconazole treatment of invasive aspergillosis: real-world versus health-economic model results. Clin. Drug Invest. 28:509–521 [DOI] [PubMed] [Google Scholar]
- 34. Wenzel R., et al. 2005. Economic evaluation of voriconazole compared with conventional amphotericin B for the primary treatment of aspergillosis in immunocompromised patients. J. Antimicrob. Chemother. 55:352–361 [DOI] [PubMed] [Google Scholar]
- 35. Wilson L. S., et al. 2002. The direct cost and incidence of systemic fungal infections. Value Health 5:26–34 [DOI] [PubMed] [Google Scholar]
- 36. Wingard J. R., et al. 2007. Resource use and cost of treatment with voriconazole or conventional amphotericin B for invasive aspergillosis. Transpl. Infect. Dis. 9:182–188 [DOI] [PubMed] [Google Scholar]