Abstract
We describe here a clinical daptomycin treatment failure in a patient with recurrent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in whom daptomycin was administered after a failed empirical treatment course with vancomycin and piperacillin-tazobactam. We had the opportunity to compare the genome sequences of an isogenic pair of daptomycin-susceptible and -resistant MRSA isolates obtained before and after initiation of daptomycin therapy, respectively. The genotype of both isolates was USA800, ST5, SCCmec type IV, agr type II. There was no increase in cell wall thickness in the daptomycin-resistant strain despite having decreased susceptibility to both vancomycin and daptomycin. By comparing the genome sequences by pyrosequencing, we identified a polymorphism (S337L) in the tenth transmembrane segment of the multiple peptide resistance factor, MprF, encoding lysyl phosphatidylglycerol transferase. This enzyme has been shown previously to promote repulsion of daptomycin at the cell surface by addition of positively charged lysine to phosphatidylglycerol. Also, the hlb open reading frame (ORF) encoding the β-toxin was interrupted by a prophage in the daptomycin-susceptible strain; this phage was missing in the daptomycin-resistant isolate and the hlb ORF was restored. Loss of the phage in the resistant isolate also resulted in loss of the virulence factor genes clpP, scn, and sak. This is the first study to use pyrosequencing to compare the genomes of a daptomycin-susceptible/resistant MRSA isolate pair obtained during failed daptomycin therapy in humans.
INTRODUCTION
Staphylococcus aureus is a pathogen that causes a variety of human syndromes ranging in severity from skin and soft tissue infection to endocarditis, osteomyelitis, sepsis, and toxic shock syndrome. Indeed, it is the most common cause of endocarditis (25, 51), bacteremia (64), and skin and soft tissue infection in patients presenting to U.S. emergency departments (50). The increasing prevalence of methicillin-resistant S. aureus (MRSA) infection among both community-associated (50) and healthcare-associated (28, 43) settings has made β-lactam antibiotics alone unreliable for empirical therapy of S. aureus infection (17). Moreover, the emergence of MRSA isolates with resistance to the glycopeptide vancomycin suggests that this agent might also become unreliable for treating MRSA infections (2).
Daptomycin, a bactericidal lipopeptide antimicrobial, is effective against Gram-positive bacteria, including MRSA (5), vancomycin-resistant S. aureus, and vancomycin-resistant Enterococcus faecalis. It was approved in 2003 in the United States for the treatment of complicated skin and soft tissue structure infection and, in 2006, for the treatment of bacteremia and right-sided endocarditis (5). However, failed treatment of S. aureus infection concomitant with the development of daptomycin nonsusceptibility (hereafter called daptomycin resistance) has been increasingly documented (20, 24, 34, 37, 40, 42, 52, 58, 60). Complicating matters is the fact that the development of vancomycin-intermediate resistance resulting from therapy with vancomycin can sometimes confer daptomycin cross-resistance (16, 54, 57). Conversely, stepwise incubation in increasing concentrations of daptomycin can increase the MICs of both daptomycin and vancomycin (7, 48). Since daptomycin is often used as therapy for MRSA infection after treatment failure with vancomycin, a better understanding of the mechanism of cross-resistance between daptomycin and vancomycin is needed.
Several recent studies have provided insight into the basis for development of daptomycin resistance in S. aureus. By performing comparative genomic hybridization, Friedman et al. identified polymorphisms in four genes (mprF, yycG, rpoB, and rpoC) associated with the development of daptomycin resistance following stepwise in vitro incubation of a daptomycin-susceptible (Daps) MRSA isolate in daptomycin (29). These polymorphisms served as the basis for subsequent studies involving DNA sequence comparisons of only these four genes between isogenic Daps and daptomycin-resistant (Dapr) clinical isolate pairs (8, 29, 40, 52). However, since the advent of genome resequencing approaches, there have been no genome-wide DNA sequence comparisons between isogenic Dapr and Daps disease isolates.
We describe here a clinical daptomycin treatment failure in a patient with recurrent MRSA bacteremia in whom daptomycin was administered after failure of initial therapy with vancomycin and piperacillin-tazobactam. A pair of Daps and Dapr isogenic MRSA isolates that were obtained before and after initiation of daptomycin therapy, respectively, provided the opportunity to further explore the mechanism of daptomycin resistance. To this end, we applied state of the art pyrosequencing technology to compare the genome sequences of the two isolates. This allowed us to identify polymorphisms associated with daptomycin resistance obtained in vivo that may be associated with daptomycin treatment failure.
CASE REPORT
A 54-year-old man with end-stage liver disease secondary to alcoholic cirrhosis and morbid obesity (body mass index = 50 kg/m2) was hospitalized after being found on the bathroom floor by his wife with confusion and altered mental status. Three days prior, the patient had been seen by his primary provider for presumed osteoarthritis of his shoulders and given acetaminophen-hydrocodone for pain relief. The patient was initially empirically treated with vancomycin (2 g administered intravenously [i.v.] every 12 h) and piperacillin-tazobactam (3.4 g i.v. every 6 h). The admission blood and urine cultures grew MRSA after 16 h of incubation. Based on automated antimicrobial susceptibility testing (Vitek2; bioMérieux, Inc.), the isolate was determined to be resistant to oxacillin (MIC > 4 μg/ml) and susceptible to clindamycin, erythromycin, gentamicin, levofloxacin, tetracycline, trimethoprim-sulfamethoxazole, linezolid, and vancomycin.
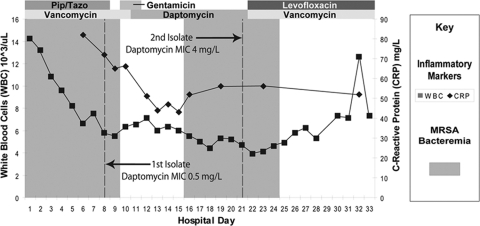
The patient was treated with multiple antibiotics over the subsequent 33 days (Fig. 1). Vancomycin was dosed at 2 g i.v. every 24 h and then at 2 g i.v. every 48 h based on the patient's declining creatinine clearance. No source for the bacteremia was identified despite repeated detailed physical exams, magnetic resonance imaging of his bilateral shoulders, the performance of a trans-esophageal echocardiogram, four limb venous ultrasound studies, and computerized tomography of the chest, abdomen, and pelvis.
Fig. 1.
Patient's antibiotic treatment course.
Bacteremia persisted. On hospital day 8, blood cultures continued to grow MRSA with the same antimicrobial susceptibilities as the admission isolate. As determined by Etests, this isolate (the Daps isolate, Q2819) had a daptomycin MIC of ≤0.25 μg/ml and a vancomycin MIC of 1.5 μg/ml. The day 8 cultures prompted changing the antimicrobial regimen to daptomycin 750 mg daily (6 mg/kg adjusted, ideal body weight [but a 4.2 mg/kg actual body weight]) with 500 mg of gentamicin given on two consecutive days (44, 61). Subsequent blood cultures from days 9 to 15 were sterile.
On hospital day 14, the patient developed acute renal failure, possibly due to hepatorenal syndrome and/or aminoglycoside toxicity. On hospital day 19, esophageal variceal bleeding prompted transfer to the intensive care unit. On days 16 to 24, blood cultures again grew MRSA in spite of continued daptomycin therapy. The isolate obtained on day 21 (the Dapr isolate, Q2818) had an increased MIC (as determined by Etest) of both daptomycin (2 μg ml) and vancomycin (2 μg ml) (vancomycin susceptible by CLSI guidelines) (11). This finding prompted, on day 22, the changing of the antimicrobial regimen to vancomycin (dosed for a peak serum concentration of 40 μg/ml and trough concentrations ranging from 17 to 26 μg/ml) and levofloxacin (750 mg every 48 h). Due to persisting shoulder pain and growing clinical suspicion of septic arthritis, an arthrocentesis of the shoulder was performed on day 24, which was culture negative. The aspirate had 252 white blood cells and 93% neutrophils. The bacteremia cleared on day 25, without recurrence. The patient thereafter received an allogeneic liver transplant. However, 1 year after transplant, the patient had reactivation of cytomegalovirus and died from septic shock.
MATERIALS AND METHODS
Susceptibility testing.
MIC and growth curve analyses were performed in broth according to CLSI recommendations by incubation at 37°C in 24-well culture plates (Corning). Incubations were performed in an Optima Fluostar microplate reader (BMG-Labtech) programmed to record the optical density at 600 nm (OD600) at 20-min intervals for 24 h. Vancomycin was tested at concentrations of 0.25, 0.75, 1.25, and 2 μg/ml, and daptomycin was tested at 0, 0.25, and 0.5 and at 1, 2, and 4 μg/ml supplemented with 50 mg of Ca2+/ml. The MIC was recorded as the lowest concentration of drug in which no growth was detected by the plate reader after 24 h.
Genotyping.
Pulsed-field gel electrophoresis (PFGE) of SmaI-digested genomic DNA was performed as described previously (47). Pulsotype patterns were assigned using BioNumerics version 5.10 (Applied Maths, Inc., Austin, TX) with a 1% molecular weight position tolerance and >80.0% identity as a similarity cutoff. Multilocus sequence typing was performed as described previously (21) and was confirmed by analysis of the genome sequence. Assignment to SCCmec type IV was made according to recent guidelines by the International Working Group on SCCmec nomenclature (36) and Boyle-Vavra et al. (6). The SCCmec subtype was determined by DNA sequence alignments between SCCmec type IV elements and the matching contigs from the pyrosequencing (46). The agr types were determined by BLAST comparisons of agr locus genes to AF210055.1 (type I), AF001782.1 (type II), AF001783.1 (type III), and AF288215.1 (type IV).
Detection of toxin genes.
As part of routine surveillance by the Minnesota Department of Health, strains were screened for carriage of genes encoding Panton-Valentine leukocidin, enterotoxins (sea, seb, sec, sed, sek, and seq) and toxic shock syndrome toxin 1 (tst1) using previously published methods (9–10, 45, 49), except that tst1 was screened using the following primers: (5′-ATTCCTTAGGATCTATGCGTAT-3′ and 5′-TGGATCCGTCATTCATTGTTAT-3′). Individual PCRs were performed with the HotStarTaq DNA polymerase (Qiagen, Valencia, CA).
Transmission electron microscopy.
An overnight bacterial culture was diluted 1:250 in tryptic soy broth (TSB) and grown to stationary phase (OD600 of 1.0). Cultures were centrifuged and the pellet was fixed in 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer for 2 h, postfixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 60 min, rinsed in maleate buffer (pH 5.0), and poststained in 1% uranyl acetate in maleate buffer for 5 min. The samples were then dehydrated in a graded series of ethanol, followed by infiltration with propylene oxide and Spurr's resin. Thin sections (90 nm) were mounted on coated grids and stained with uranyl acetate and lead citrate. Imaging of cells was performed at 300 kV using an FEI Tecnai F30 transmission electron microscope at the University of Chicago electron microscopy core facility. The images of 20 cells that each contained a cross-wall were photographed at final magnification of ×54,600, and measurements were determined by using ImageJ (version 1.43; http://rsbweb.nih.gov/ij/docs/guide/index.html). The mean and standard deviation of each sample were determined for 40 measurements per strain, and the statistical differences between cell wall thickness were tested by using an independent two-sample t test using Microsoft Excel. P values of ≤0.05 were considered statistically significant.
Genome sequencing.
Genomic DNA was extracted from the Daps and Dapr S. aureus clinical isolates by using Epicentre MasterPure gram-positive DNA purification kit (Epicentre Biotechnologies) modified for optimal lysis of S. aureus. Briefly, a 10-ml culture of each strain was grown in brain heart infusion broth to an OD600 of 2.0. Bacterial cultures were centrifuged and then resuspended in a Tris-EDTA-lysozyme-lysostaphin-mutanolysin mixture with incubation at 37°C for 1 h. One volume of MasterPure lysis solution with proteinase K was added to the resuspended cells, followed by incubation at 65°C for 15 min. The remainder of the protocol was performed according to manufacturer's instructions. The DNA concentration was quantified on a NanoDrop 1000 (Thermo Scientific) and visually examined on an ethidium bromide-stained, 1% agarose gel. The genomic DNA was sequenced by using a 454 GS FLX system (Roche) using titanium reagents according to the manufacturer's instructions (Roche). Base-calling was performed using the bundled 454 software. The GS De Novo Assembler (1) was used to assemble the first sequence reads from each strain using the Linux command line using the runAssembly command. The best de novo assembly from each isolate was chosen on the basis of total sequence coverage, total contig length, and fewest numbers of contigs. The sequence reads from each isolate were assembled again by using the GS reference mapper application (1) using the multi-FASTA file resulting from the Daps strain as the reference. Only reads with a maximum of one error in their multiplex identifiers were used. The mapping assemblies were run on the Linux command line using the runMapping command. The sequence contigs will be deposited in the NCBI short-read public archive database.
Polymorphism detection and annotation.
Polymorphisms were determined by accepting all conflicts reported in the GS reference mapper (1), where at least 80% of the sequence reads from the Dapr isolate at the affected location were in conflict with the reference assembly from the Daps isolate and in agreement with one another. To map polymorphisms to genes, we used the annotated genome of strain N315, which has the same genetic background as the Daps/Dapr pair (ST5). A shell script was written (NucmerAlignment.sh) that uses the nucmer application of the MUMmer package (19) to align the assembly reference to the annotation reference and build a mapping file from the resulting alignment. Polymorphisms were then mapped to annotated genes by mapping the coordinates of the polymorphisms from the assembly reference to the coordinate system of the annotation reference.
A Perl script was written (EvaluatePolymorphisms.pl), mapping each polymorphic region onto the annotation reference and determining the effect of the polymorphism on the gene product. The script identifies the gene (if any) affected by the polymorphism and extracts the corresponding sequence data from the assembly reference contig that contains the gene. It then builds a second sequence by applying the polymorphism to the reference sequence. This involves substituting the polymorphic sequence fragment in place of the assembly reference sequence fragment. Both of these gene sequences are then translated in the reading frame of the gene. The script then identifies any early stop codons or amino acid changes that result from the polymorphism.
Polymorphism validation.
Purified genomic DNA from S. aureus strains Q2818 and Q2819 was used as a template for the validation of identified polymorphisms by 3′ quantitative PCR genotyping. To validate the accuracy of single nucleotide polymorphisms (SNP) calls by the CLC software, we utilized 3′ mismatch quantitative PCR. For each potential SNP, we designed one reverse primer, roughly 150-bp downstream of the SNP, and two forward primers, one unique to the SNP and one to the reference sequence.
Transcriptional microarray analysis.
S. aureus RNA was isolated from bacterial cultures at the indicated time points. RNA protect (Qiagen, Valencia, CA) was directly added to the growth medium (2:1 ratio of volume of RNAprotect to bacterial culture), and cells were pelleted and stored at −20°C. Extraction of RNA was performed using an Ambion MirVANA RNA kit (Austin, TX) as recommended, except that cell lysis was carried out in Ambion lysis buffer in matrix B tubes (MP Biomedicals) processed in a Qbiogene FastPrep FP120 at speed 6.0 for 45 s. RNA quantity and quality was assessed by measuring total RNA using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Inc., Rockland, DE) and visualizing RNA on an agarose gel. Purified RNA was stored at −80°C.
DNA probes for microarray experiments and hybridization was performed as described previously (http://intranet.jtc.jcvsf.org/sops/M007.pdf). The microarrays consisted of aminosilane-coated slides printed with a set of 13,767 S. aureus open reading frame sequences (www.jcvi.org). Scanning, image analysis, normalization, and analysis were performed as outlined in (http://intranet.jtc.jcvsf.org/sops/M008.pdf). All wash buffers contained 1 ml of 0.1 M dithiothreitol per liter. Individual TIFF images from each channel were analyzed with TIGR Spotfinder (http://pfgrc.jcvi.org/index.php/bioinformatics.html). Microarray data were normalized by Lowess normalization and with in-slide replicate analysis using TM4 software MIDAS (available at (http://pfgrc.jcvi.org/index.php/bioinformatics.html).
RESULTS
Daptomycin and vancomycin susceptibility.
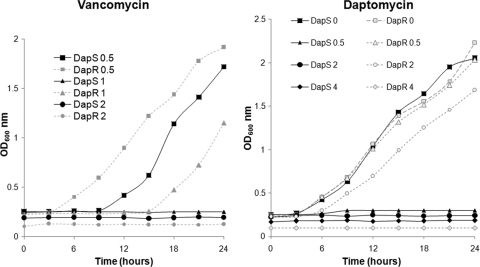
We confirmed the automated susceptibility test results of the MRSA isolates obtained 8 days (Daps isolate, Q2819) and 21 days (Dapr isolate, Q2818) after the initiation of therapy. Based on monitoring growth on a plate reader over a 24-h time period, the MICs of daptomycin were determined to be 0.5 and 4 μg/ml for the Daps and Dapr isolates, respectively (Fig. 2), whereas by Etest, the MICs of daptomycin were determined to be 0.25 and 2 μg/ml for the Daps and Dapr isolates, respectively. Thus, the Etest and broth MICs of daptomycin differed slightly. The MICs of vancomycin in the plate reader, were 1 and 2 μg/ml for the Daps and Dapr isolates, respectively (Fig. 2). Also, the Dapr strain entered early log phase between 3 and 6 h in 1 μg of vancomycin/ml compared to 6 and 9 h for the Daps strain. These analyses confirm the increase in daptomycin and vancomycin resistance phenotypes in strain Q2818.
Fig. 2.
MIC determination and growth of Daps (DapS) and Dapr (DapR) strains in various concentrations of vancomycin and daptomycin. Numbers appearing in the legends refer to the amounts (in mg/liter) of antibiotic in which the strains were incubated.
Molecular typing.
Both isolates belonged to ST5 and agr group II. The SmaI PFGE pattern of both the Daps and Dapr isolates was consistent with USA800 (data not shown). The SCCmec elements of both strains were 100% similar to each other and 96.7% similar to the SCCmec subtype IVa carried by strain CA05 (accession number AB063172), which was isolated by us in Chicago from a healthy child in 2001 (35, 46). Both isolates contained seg, sei, sem, and sen enterotoxin genes and five additional enterotoxin genes. Both isolates were negative by PCR for tst-1, sea, seb, sec, sed, sek-l, seq-l, and pvl, findings that were confirmed by inspection of the pyrosequencing data.
Cell wall thickness.
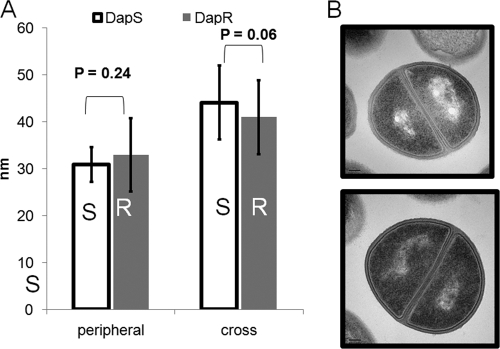
A possible difference in cell wall thickness between the Daps and Dapr isolates was investigated since this parameter has been previously associated with vancomycin-intermediate resistance (18, 33) and daptomycin resistance (16, 40, 66). No significant difference was noted in the thickness of the peripheral walls between the Daps (mean = 30.9 nm ± 7.9) and Dapr isolates (mean = 33 nm ± 7.3) (P = 0.24) (Fig. 3). There was also no difference in cross-wall thickness between these strains (P = 0.06).
Fig. 3.
Cell wall thickness comparisons of Daps (S) and Dapr (R) strains. (A) Cell wall thickness measurements of peripheral and cross walls. (B) Transmission electron microscopy of Daps (top) and Dapr (bottom) strains.
Genome sequence comparison.
We found 2,546 identical open reading frames (ORFs) between the Daps and Dapr strain (see Table S1 in the supplemental material). Relative to the genome sequence of MRSA strain N315 (a prototype ST5 MRSA isolate) the sequence coverage was about ∼94% for the DapR strain and ∼94.9% for the Daps strain N315 (accession number BA000018.3).
The genome sequence comparison of the Daps and Dapr pair revealed two polymorphisms and a large deletion in the latter. The first polymorphism was a C-to-T substitution at nucleotide 1010 in mprF/fmtC. This resulted in a codon change of TCA to TTA, producing a serine-to-leucine substitution in the mprF encoded protein (lysyl phosphatidylglycerol transferase [LPGT]) at amino acid residue 337 (S337L). This was mapped within the tenth putative transmembrane domain of the protein.
In the Daps strain, we found a nucleotide substitution (A184G) that resulted in an early termination codon within the beta-hemolysin ORF (sph or hlb), which was intact in the Dapr isolate. Further inspection revealed the presence of a 41,536-bp prophage (hereafter called phi2819) within hlb in the Daps isolate that was absent in the Dapr isolate. Table 1 lists the ORFs present within this phage (called phi2819). Since isolation of the Daps isolate preceded the Dapr isolate, this difference suggested that the hlb reading frame was restored in the latter isolate upon excision of phi2819 from the chromosome (reverse phage conversion). A BLAST analysis of the GenBank database revealed that phi2819 belongs to a widely distributed phage family among S. aureus, referred to as the beta-toxin converting phages (β-tcp) because they integrate site specifically in hlb (12, 32, 62). Phi2819 had 85.3% identity with a β-tcp inserted in the truncated hlb (SaurJH_2030) in MRSA strain JH1 (coordinates 2128859 to 2171446, dbj accession number CP000736.1). Phage belonging to this family can be found in MRSA strains JH9 (CP000703.1), USA300 (CP000255.1), MRSA252 (BX571856.1), Mu3 (AP009324.1), MW2 (BA000033.2), Mu50 (BA000017.4), TW20 (FN433596.1), and N315 (BA000018.3). MSSA strains that carry a β-tcp include strains 8325 (CP000253.1), Newman (phiNM3, DQ530361), and MSSA476 (BX571857.1). Of note, phi2819 encodes the virulence gene clpP (26, 27), immunomodulatory genes fibrinolytic staphylococcal kinase (sak) and the complement inhibitory protein (skn) that are often carried on S. aureus β-tcp's (3). Thus, although the Dapr strain potentially gained beta-hemolysin activity, it may have lost immunomodulatory function. However, no difference in hot-cold beta-hemolysin activity was distinguished between the strains after incubation on sheep blood agar at 37°C and 4°C.
Table 1.
ORFs within phi2819
| ORF | ORF length (bp) | Gene | Common name |
|---|---|---|---|
| ORF00412 | 1,038 | Null | Phage integrase family protein |
| ORF00413 | 717 | Null | 77ORF017 |
| ORF00415 | 186 | Null | Conserved hypothetical protein |
| ORF00416 | 147 | Null | Conserved hypothetical protein |
| ORF00417 | 528 | Null | Hiran |
| ORF00418 | 684 | Null | Peptidase S24 S26A and S26B |
| ORF00419 | 261 | Null | Transcriptional regulator |
| ORF00420 | 330 | Null | Conserved hypothetical protein |
| ORF00421 | 162 | Null | Conserved domain protein |
| ORF00422 | 270 | Null | Conserved hypothetical protein |
| ORF00423 | 1,956 | Null | Hypothetical protein |
| ORF00424 | 921 | Null | Recombination protein RecT |
| ORF00425 | 486 | Null | gp51 |
| ORF00426 | 471 | ssb | Single-stranded DNA-binding protein |
| ORF00427 | 567 | Null | DNA replication protein DnaD |
| ORF00428 | 219 | Null | Conserved hypothetical protein |
| ORF00429 | 405 | Null | Endodeoxyribonuclease RusA |
| ORF00430 | 378 | Null | PVL ORF-50 family protein |
| ORF00431 | 243 | Null | Phi PVL ORF 51 analogue |
| ORF00432 | 261 | Null | Phi PVL ORF 52 analogue |
| ORF00433 | 153 | Null | Conserved hypothetical protein |
| ORF00434 | 162 | Null | Conserved hypothetical protein |
| ORF00435 | 534 | Null | dUTPase |
| ORF00436 | 207 | Null | Conserved domain protein |
| ORF00437 | 213 | Null | Conserved hypothetical protein |
| ORF00438 | 219 | Null | Conserved hypothetical protein |
| ORF00439 | 387 | Null | Conserved hypothetical protein |
| ORF00440 | 150 | Null | Conserved domain protein |
| ORF00441 | 651 | Null | 77ORF019 |
| ORF00442 | 204 | Null | Conserved domain protein |
| ORF00443 | 417 | Null | 77ORF026 |
| ORF00444 | 300 | Null | gp65 |
| ORF00445 | 345 | Null | Conserved hypothetical protein |
| ORF00446 | 1,662 | Null | Putative phage terminase, large subunit |
| ORF00447 | 1,140 | Null | Phage portal protein, HK97 family |
| ORF00448 | 738 | clpP | Endopeptidase ClpP |
| ORF00449 | 1,146 | Null | 77ORF006 |
| ORF00450 | 285 | Null | 77ORF045 |
| ORF00451 | 285 | Null | gp7 |
| ORF00452 | 354 | Null | Putative phage head-tail adaptor |
| ORF00453 | 405 | Null | 77ORF029 |
| ORF00454 | 378 | Null | Conserved hypothetical protein |
| ORF00455 | 645 | Null | 77ORF020 |
| ORF00456 | 141 | Null | Conserved hypothetical protein |
| ORF00457 | 351 | Null | Hypothetical protein |
| ORF00458 | 162 | Null | Conserved domain protein |
| ORF00459 | 4,530 | Null | TMP repeat family |
| ORF00460 | 1,485 | Null | 77ORF004 |
| ORF00461 | 3,786 | Null | Phage minor structural protein, N region |
| ORF00462 | 153 | Null | Conserved hypothetical protein |
| ORF00463 | 288 | Null | Conserved hypothetical protein |
| ORF00464 | 297 | Null | Conserved hypothetical protein |
| ORF00465 | 135 | Null | Conserved hypothetical protein |
| ORF00466 | 234 | Null | Holin, phage phi LC3 family |
| ORF00467 | 756 | Null | N-Acetylmuramoyl-l-alanine amidase |
| ORF00468 | 492 | sak | Staphylokinase |
| ORF00469 | 300 | Null | Truncated amidase |
| ORF00470 | 351 | skn | Staphylococcal complement inhibitor |
| ORF00471 | 192 | Null | Conserved hypothetical protein |
| ORF00472 | 156 | Null | Conserved hypothetical protein |
| ORF00473 | 180 | Null | Conserved hypothetical protein |
Transcriptional profiling.
The global transcriptional profiles of the Daps and Dapr pair were compared at four time points during growth. Only five genes with a >2.0-fold difference in expression that mapped outside phi2819 are shown in Table 2. These were all downregulated in the Dapr strain compared to the Daps strain. No genes were upregulated in the Dapr strain. Three of the five downregulated ORFs encode small peptides of ca. 20 to 45 amino acids in length that are homologous to antimicrobial peptides called phenol soluble modulins (PSMs) (63). One of these, delta-hemolysin, is encoded within a key regulator of virulence, RNAIII (53).
Table 2.
Microarray results for ORFs found to be underexpressed in the DapR strain Q2818a
| Annotation |
Gene type | Fold difference in expression at: |
||||
|---|---|---|---|---|---|---|
| Strain | Locus tag | 2.5 h | 3 h | 3.5 h | 4 h | |
| COL | SACOL1186 | Antibacterial protein (psmβ) | 1.4 | 2.1 | 10.1 | 5.0 |
| COL | SACOL1187 | Antibacterial protein (psmβ) | 11.0 | 5.3 | ||
| MSSA476 | SAS1862 | Pseudo | 27.0 | 3.8 | 16.2 | 11.3 |
| MSSA476 | SAS1940a | Delta-hemolysin precursor | 3.4 | 5.6 | 9.4 | 4.4 |
| Mu50 | SAV2568 | Hypothetical protein | 13.5 | 19.7 | 17.7 | 16.4 |
Only ORFs that did not map to the beta-toxin converting phage, phi2819, are shown. RNA was harvested at the indicated time points after inoculation.
DISCUSSION
The availability of a Daps/Dapr isogenic pair of isolates from the same patient, isolated before and after exposure to daptomycin, respectively, afforded the opportunity to investigate the genetic correlates of conversion to daptomycin resistance during therapy in humans. To this end, we used pyrosequencing and found only two genetic differences between the isolates. One change was a point mutation in the multiple peptide resistance factor, mprF, encoding LPGT. This enzyme alters the cell membrane charge by the addition of lysine to phosphatidylglycerol (55). The other genetic difference we found was the restoration of the beta-toxin encoding ORF upon deletion of the β-tcp and the disappearance of phage-encoded genes. Transcriptional profiling revealed few changes other than those associated with phage excision.
Only one other investigation has used a whole-genome approach to identify daptomycin resistance-associated mutations in S. aureus. Friedman et al. compared three Dapr isolates obtained by in vitro stepwise passage of three Daps clinical isolates in daptomycin (29). In that study, hybridization of genomic DNA to tiled microarrays that represented the entire genome sequence of S. aureus (called comparative genomic sequencing [CGS]) was used. In contrast to CGS, the pyrosequencing approach we used offered the potential to identify novel genes not previously recognized in S. aureus. Another difference in our study is that our Dapr isolate developed resistance in a patient while undergoing daptomycin therapy, whereas Friedman et al. studied in vitro-derived resistance, which might not select for mutations that are relevant to resistance that develops in human tissues and body fluids. Despite the different approaches, both studies identified point mutations in mprF. It is noteworthy that we did not find polymorphisms in rpoB, rpoC, and yycG as Friedman et al. did. We also did not find a polymorphism in the dlt operon, which has also been implicated in resistance to daptomycin and cationic peptides (48, 56, 65). However, the deletion of a phage in our isolate prevents us from concluding that the mprF polymorphism was the sole explanation for the daptomycin resistance phenotype. Also, since coverage relative to strain N315 was ca. 95%, there may be sequence polymorphisms we did not detect.
Despite the association of other mutations with daptomycin resistance, mprF has the strongest association with daptomycin resistance in S. aureus. This is supported by our data and the facts that (i) the earliest polymorphism detected by Friedman et al. in all three passage isolates during stepwise passage was in mprF and (ii) mprF polymorphisms were present in all daptomycin-selected, Dapr isolates studied to date (8, 29, 38, 40, 48, 52). Conversely, deletion or mutation of mprF in S. aureus increases the susceptibility to daptomycin as well as leukocyte-derived cationic peptides (22, 55). However, this has only been investigated in a strain already susceptible to daptomycin.
What role could polymorphisms in mprF play in daptomycin resistance? Daptomycin binds to the bacterial cell membrane, which leads to depolarization and disruption of macromolecular synthesis and cell death (without lysis) (14, 59). Accordingly, in Dapr isolates, a decreased amount of daptomycin binding to the cell membrane is associated with decreased daptomycin-mediated dissipation of membrane potential (41, 52). Daptomycin resistance-associated polymorphisms in the mprF gene product, LPGT, have been associated with a gain in its lysinylation activity, which has been proposed to explain the resistance phenotype (40, 48, 52). Thus, Dapr strains with polymorphisms in LPGT display an increased amount of lysinylated phosphatidylglycerol, [LPG]) on the outer leaflet of the cell membrane that might decrease the interaction of daptomycin with the cell surface by charge repulsion (38). This may explain the resistance phenotype; however, at least one Dapr strain with increased LPGT function exhibited decreased net positive surface charge (48).
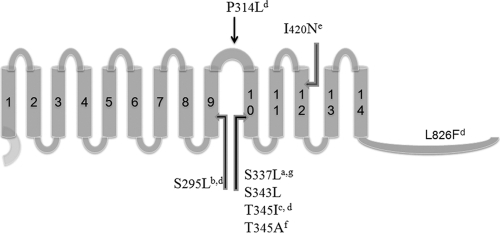
LPGT has at least two functional domains, one that that adds lysine to phosphatidylglycerol and one that translocates lysinylated phosphatidylglycerol across to the exterior face of the cytoplasmic membrane (“flippase” activity) where the positive charge can best affect repulsion (22). Mutations in mprF abolishing either of these activities increase susceptibility to daptomycin and cationic peptides (22). Of the reported daptomycin resistance-associated polymorphisms in LPGT, most are in close proximity to our polymorphism, within the ninth or tenth transmembrane segments or the intermembrane loop connecting them (Fig. 4). Ernst et al. demonstrated that deletion of the first eight segments of LPGT are dispensable to lysinylation activity in E. coli (22). However, further deletion into the ninth and tenth transmembrane segments abolish lysinylation. Since most of the daptomycin resistance-associated mutations map to the ninth and tenth transmembrane segments, these data suggest that the mutations might be involved in lysinylation. However, it is also possible that these mutations are involved in translocation of LPG to the outer leaflet of the cytoplasmic membrane since the ninth and tenth transmembrane segments are also contained in the N-terminal translocase (or “flippase”) domain identified by Ernst et al. (22). Interestingly, no daptomycin resistance polymorphisms have been reported in the first eight transmembrane segments of LPGT which, although dispensable for lysinylation, are required for “flippase” activity and resistance to daptomycin (22).
Fig. 4.
Dap resistance-associated polymorphisms in the translated mprF gene product, lysyl phosphatidylglycerol transferase (LPGT). Bars 1 to 14 refer to transmembrane domains in which mutations were found. Superscript letters indicate reference sources as follows: a, the present study; b, Yang et al. (65); c, Mishra et al. (48); d, Friedman et al. (29); e, Julian et al. (40); f, Murthy et al. (52); and g, Pillai et al. (56a).
It has been suggested that decreased susceptibility to both daptomycin and vancomycin can sometimes be selected for by either agent (4, 16, 48), suggesting a common pathway to development of resistance for both agents. Despite the complex treatment course of our patient, the increased MICs of vancomycin and daptomycin in the MRSA isolate developed many days after vancomycin had been discontinued and the patient had been receiving prolonged daptomycin therapy. Increased cell wall thickness, a common feature of vancomycin-intermediate resistant strains (15, 18, 33), has also been found in some Dapr strains (16). However, consistent with Yang et al. (66), the cell wall thickness was not different between our Daps and Dapr isolates. However, since an isolate prior to daptomycin exposure was not available, we do not know whether vancomycin therapy led to a thickened cell wall prior to daptomycin exposure. This is a possibility since the cell walls of both the Daps and Dapr isolates were significantly thicker than an unrelated Daps USA300 isolate which had an MIC of vancomycin of 0.5 μg/ml (data not shown). Nevertheless, these data show that decreased susceptibility to both vancomycin and daptomycin occurred without increasing cell wall thickness.
The phage that was missing from the Dapr isolate belongs to a family of beta-toxin-converting phages commonly found in S. aureus (Sa3-int family or F serogroup) (13, 32, 62), which variably carries virulence genes encoding enterotoxins, clpP, and staphylococcal kinase (sak), as well as the immunomodulatory genes scn and chp (30) (although phi2819 did not carry an enterotoxin gene or chp). Whereas excision of the phage restored the beta-toxin ORF, which might increase virulence potential (3), the resistant strain had also lost some virulence factors that were present in the Daps parent strain. Considering the fact that the infection cleared while the patient was receiving vancomycin, despite an increased vancomycin MIC, it is tempting to speculate that clearance was facilitated by the loss of the virulence factors clpP, sak, and/or scn. The loss of skn could have decreased the bacterium's resistance to complement thereby depriving the bacteria of an important defense. The loss of clpP has been shown to result in decreased expression of the agr global regulator and the agr-regulated extracellular proteins (26). Accordingly, we found decreased agr expression in the DapR strain. However, we cannot discount the possible role of the fluoroquinolone that was administered with vancomycin in contributing to clearance. It is also interesting to consider the possibility that the loss of these virulence factors might have been promoted by prolonged antibiotic therapy, which induced excision of the prophage (31).
Our comparison of the transcriptomes by microarray analysis demonstrated very few differences between the Daps and Dapr isolates, other than those associated with loss of the phi2819 phage. Also, we found no upregulated genes in the Dapr isolate. Of the five downregulated genes in the Dapr isolate located outside phi2819, three encoded PSM antimicrobial peptides (PSMβ1, PSMβ2, and delta-toxin). Unlike the PSMα peptides, the PSMβ peptides are not involved in the virulence of CA-MRSA or response to protein synthesis inhibitors, such as gentamicin (39, 63). Although all PSMs in S. aureus are regulated by agr, a major virulence-associated quorum-sensing global regulatory locus (53), PSMβ1 and PSMβ2 were the only agr-regulated genes dysregulated in the Dapr isolate.
Since S. aureus bacteremia is becoming increasingly common, microbiology laboratories and healthcare providers should be aware of possible development of daptomycin resistance during therapy. Due to fluctuating renal function and the patient's morbid obesity, the dose of daptomycin was in this case determined by using an adjusted rather than the actual body weight. Consequently, the patient was treated with 4 mg/kg of actual body weight instead of the recommended 6 mg/kg. Consistent with this, in a retrospective review of daptomycin therapy for bacteremia and endocarditis, treatment failure occurred more often when doses of 2 to 4 mg/kg compared to 6 mg/kg were used (23). Taking these findings into consideration, pharmacists and clinicians should be mindful to adjust daptomycin to actual body weight.
Phenotypic studies are needed to shed light on whether the polymorphisms in mprF are sufficient for increasing daptomycin resistance and whether antibiotic therapy with daptomycin promotes reverse lysogenization and alters virulence.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the Grant Healthcare Foundation to S.B.-V. and R.S.D. S.B.-V. and R.S.D. are also supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH/NIAID) R56 AI040481. The genome sequencing was supported by the Pathogen Functional Genomics Resource Center (PFGRC) (contract N01-AI15447) and funding by the NIH/NIAID. D.R.B. is supported by NIH grants L30AI066779 and K23AI073192.
We acknowledge the assistance of the University of Minnesota microbiology staff and Patricia Ferrieri.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Anonymous. 2008. Genome sequencer data analysis software manual, version 2.0.00. Roche Diagnostics GmbH, Berlin, Germany [Google Scholar]
- 2. Appelbaum P. C. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398–408 [DOI] [PubMed] [Google Scholar]
- 3. Bae T., Baba T., Hiramatsu K., Schneewind O. 2006. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62:1035–1047 [DOI] [PubMed] [Google Scholar]
- 4. Bamberger D. M. 2007. Bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: the potential role of daptomycin. Ther. Clin. Risk Manag. 3:675–684 [PMC free article] [PubMed] [Google Scholar]
- 5. Boucher H. W., Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601–608 [DOI] [PubMed] [Google Scholar]
- 6. Boyle-Vavra S., Ereshefsky B., Wang C. C., Daum R. S. 2005. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J. Clin. Microbiol. 43:4719–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camargo I. L., Neoh H. M., Cui L., Hiramatsu K. 2008. Serial daptomycin selection generates daptomycin-nonsusceptible Staphylococcus aureus strains with a heterogeneous vancomycin-intermediate phenotype. Antimicrob. Agents Chemother. 52:4289–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers H. F., et al. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob. Agents Chemother. 53:1463–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiang Y. C., Chang L. T., Lin C. W., Yang C. Y., Tsen H. Y. 2006. PCR primers for the detection of staphylococcal enterotoxins K, L, and M and survey of staphylococcal enterotoxin types in Staphylococcus aureus isolates from food poisoning cases in Taiwan. J. Food Prot. 69:1072–1079 [DOI] [PubMed] [Google Scholar]
- 10. Chiang Y. C., et al. 2008. PCR detection of staphylococcal enterotoxins (SEs) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poisoning cases in Taiwan. Int. J. Food Microbiol. 121:66–73 [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100–S19 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12. Coleman D. C., Arbuthnott J. P., Pomeroy H. M., Birkbeck T. H. 1986. Cloning and expression in Escherichia coli and Staphylococcus aureus of the beta-lysin determinant from Staphylococcus aureus: evidence that bacteriophage conversion of beta-lysin activity is caused by insertional inactivation of the beta-lysin determinant. Microb. Pathog. 1:549–564 [DOI] [PubMed] [Google Scholar]
- 13. Coleman D. C., et al. 1989. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: molecular mechanism of triple conversion. J. Gen. Microbiol. 135:1679–1697 [DOI] [PubMed] [Google Scholar]
- 14. Cotroneo N., Harris R., Perlmutter N., Beveridge T., Silverman J. A. 2008. Daptomycin exerts bactericidal activity without lysis of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2223–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui L., Murakami H., Kuwahara-Arai K., Hanaki H., Hiramatsu K. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui L., Tominaga E., Neoh H. M., Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daum R. S. 2007. Clinical practice: skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 357:380–390 [DOI] [PubMed] [Google Scholar]
- 18. Daum R. S., Gupta S., Sabbagh R., Milewski W. M. 1992. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J. Infect. Dis. 166:1066–1072 [DOI] [PubMed] [Google Scholar]
- 19. Delcher A. L., et al. 1999. Alignment of whole genomes. Nucleic Acids Res. 27:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Enoch D. A., Bygott J. M., Daly M. L., Karas J. A. 2007. Daptomycin. J. Infect. 55:205–213 [DOI] [PubMed] [Google Scholar]
- 21. Enright M. C., Day N. P., Davies C. E., Peacock S. J., Spratt B. G. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ernst C. M., et al. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falagas M. E., Giannopoulou K. P., Ntziora F., Vardakas K. Z. 2007. Daptomycin for endocarditis and/or bacteraemia: a systematic review of the experimental and clinical evidence. J. Antimicrob. Chemother. 60:7–19 [DOI] [PubMed] [Google Scholar]
- 24. Fowler V. G., Jr., et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 25. Fowler V. G., Jr., et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021 [DOI] [PubMed] [Google Scholar]
- 26. Frees D., Savijoki K., Varmanen P., Ingmer H. 2007. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol. Microbiol. 63:1285–1295 [DOI] [PubMed] [Google Scholar]
- 27. Frees D., Sorensen K., Ingmer H. 2005. Global virulence regulation in Staphylococcus aureus: pinpointing the roles of ClpP and ClpX in the sar/agr regulatory network. Infect. Immun. 73:8100–8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fridkin S. K., et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 29. Friedman L., Alder J. D., Silverman J. A. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goerke C., et al. 2007. High phenotypic diversity in infecting but not in colonizing Staphylococcus aureus populations. Environ. Microbiol. 9:3134–3142 [DOI] [PubMed] [Google Scholar]
- 31. Goerke C., Koller J., Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goerke C., et al. 2009. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 191:3462–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanaki H., et al. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199–209 [DOI] [PubMed] [Google Scholar]
- 34. Hayden M. K., et al. 2005. Development of Daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussain F. M., Boyle-Vavra S., Daum R. S. 2001. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr. Infect. Dis. J. 20:763–767 [DOI] [PubMed] [Google Scholar]
- 36. Ito T. 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): a guideline for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacobson L. M., Milstone A. M., Zenilman J., Carroll K. C., Arav-Boger R. 2009. Daptomycin therapy failure in an adolescent with methicillin-resistant Staphylococcus aureus bacteremia. Pediatr. Infect. Dis. J. 28:445–447 [DOI] [PubMed] [Google Scholar]
- 38. Jones T., et al. 2008. Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joo H. S., Chan J. L., Cheung G. Y., Otto M. 2010. Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated MRSA. Antimicrob. Agents Chemother. 54:4942–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Julian K., et al. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaatz G. W., Lundstrom T. S., Seo S. M. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int. J. Antimicrob. Agents 28:280–287 [DOI] [PubMed] [Google Scholar]
- 42. Kirby A., et al. 2009. In vivo development of heterogeneous glycopeptide-intermediate Staphylococcus aureus (hGISA), GISA and daptomycin resistance in a patient with meticillin-resistant S. aureus endocarditis. J. Med. Microbiol. 58:376–380 [DOI] [PubMed] [Google Scholar]
- 43. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 44. LaPlante K. L., Rybak M. J. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lina G., et al. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 46. Ma X. X., et al. 2002. A novel type of staphylococcal cassette chromosome mec (SCCmec) identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46:1147–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDougal L. K., et al. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mishra N. N., et al. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monday S. R., Bohach G. A. 1999. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J. Clin. Microbiol. 37:3411–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moran G. J., et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 51. Murdoch D. R., et al. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 169:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murthy M. H., Olson M. E., Wickert R. W., Fey P. D., Jalali Z. 2008. Daptomycin non-susceptible methicillin-resistant Staphylococcus aureus USA300 isolate. J. Med. Microbiol. 57:1036–1038 [DOI] [PubMed] [Google Scholar]
- 53. Novick R. P., et al. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO 12:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel J. B., Jevitt L. A., Hageman J., McDonald L. C., Tenover F. C. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 42:1652–1653 [DOI] [PubMed] [Google Scholar]
- 55. Peschel A., et al. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peschel A., et al. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405–8410 [DOI] [PubMed] [Google Scholar]
- 56a. Pillai S. K., et al. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in mprF. Antimicrob. Agents Chemother. 51:2223–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sakoulas G., Alder J., Thauvin-Eliopoulos C., Moellering R. C., Jr., Eliopoulos G. M. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 50:1581–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakoulas G., Brown J., Lamp K. C., Friedrich L. V., Lindfield K. C. 2009. Clinical outcomes of patients receiving daptomycin for the treatment of Staphylococcus aureus infections and assessment of clinical factors for daptomycin failure: a retrospective cohort study utilizing the Cubicin Outcomes Registry and Experience. Clin. Ther. 31:1936–1945 [DOI] [PubMed] [Google Scholar]
- 59. Silverman J. A., Perlmutter N. G., Shapiro H. M. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skiest D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsuji B. T., Rybak M. J. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Wamel W. J., Rooijakkers S. H., Ruyken M., van Kessel K. P., van Strijp J. A. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 188:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang R., et al. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]
- 64. Wisplinghoff H., et al. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 65. Yang S. J., et al. 2009. Enhanced expression of dltABCD is associated with the development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 200:1916–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang S. J., et al. 2010. Cell wall thickening is not a universal accompaniment of the daptomycin nonsusceptibility phenotype in Staphylococcus aureus: evidence for multiple resistance mechanisms. Antimicrob. Agents Chemother. 54:3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.