Abstract
The antimicrobial and hemolytic activities of a host defense peptide can be controlled by its modification as a propeptide of reduced net charge, which can then be processed by neutrophil elastase, a serine protease involved in chronic airway inflammation and infections associated with cystic fibrosis.
INTRODUCTION
Host defense peptides (HDPs) are multifunctional molecular effectors of innate immunity in multicellular organisms (3, 8, 26). Deficiencies in their activity or expression levels can be associated with enhanced susceptibility to infections (6) and to chronic infections in conditions such as cystic fibrosis (CF) (19). While there is a sound underlying principle in compensating disease-associated deficiencies in HDPs with exogenous peptides, their potential toxicities contend with their localized administration to sites of infection (10, 26). The activities of endogenous HDPs themselves can be stringently controlled (24). HDPs expressed as inactive prepropeptides are activated by proteolytic cleavage of an anionic profragment masking the net positive charge of the C-terminal mature sequence (9). Human cathelicidin hCAP18/LL-37, for example, is activated by proteinase 3, while other neutrophilic serine proteases, such as neutrophil elastase (NE), can also cleave the profragment in vitro (1, 25). As this enzyme is associated with chronic airway inflammation and infections in CF patients (13), a prodrug approach analogous to the natural control mechanism exerted on HDPs and that exploits the abnormal concentrations of NE in the CF lung is reported here.
Synthetic propeptides were prepared by modification of an HDP with an anionic profragment, using l-P18, an α-helical, salt-resistant, cecropin A-magainin 2 hybrid sequence, identified by K.-S. Hahm's group (20, 21). Peptides were assembled by solid-phase synthesis (14), purified by reversed-phase high-performance liquid chromatography, and characterized by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Six propeptides were synthesized by elongating the parent sequence (KWKLFKKIPKFLHLAKKF-NH2) at its N terminus with a trialanine linker as an NE substrate (2) and from 2 to 7 glutamic acids to mask the net charge determinant (+8) of the mature peptide's antimicrobial activity. When more than 4 glutamic acids were added, a β-alanine spacer was introduced between the fourth and fifth glutamates, to reduce interchain association effects (11).
The antimicrobial activities of these peptides were assessed for two representative CF pathogens, Staphylococcus aureus (SH1000) and Pseudomonas aeruginosa (PAO1). MICs (Table 1) were determined by the broth microdilution method, as recommended by the Clinical and Laboratory Standards Institute. Typical MICs of l-P18 for P. aeruginosa and S. aureus were 32 μM and 64 μM, respectively, while MICs of all propeptides were higher than 128 μM. As the activities of α-helical membrane-active peptides have been suggested to be independent of the stereochemistry of their constitutive amino acids (23), the enantiomeric d-P18 was subsequently used to prevent their proteolytic degradation by bacterial proteases (15) and NE itself. Assembly from l-amino acids was maintained for the trialanine and polyglutamate sequences. d-P18 had lower MICs than its enantiomeric counterpart, with values of 8 and 16 μM for P. aeruginosa and S. aureus, respectively. A propeptide with a MIC of 128 μM against both organisms was generated by extending this d-P18 with a trialanine linker and an N-terminally acetylated tetraglutamate motif (Ac-E-E-E-E-A-A-A-k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2, abbreviated Ac-E4-A3–d-P18 here) (Fig. 1). Reducing the overall charge of the mature sequence from +8 to +3 resulted in activity differentials of 8 and 16 against S. aureus and P. aeruginosa, respectively.
Table 1.
Activities of peptides against representative pathogens associated with CFa
| Peptide sequence | Neutrophil elastase concn (μg/ml) | MIC (μM) |
|
|---|---|---|---|
| P. aeruginosa | S. aureus | ||
| K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | 32 | 64 |
| E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| E-E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| E-E-E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| E-βA-E-E-E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| E-E-βA-E-E-E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| E-E-E-βA-E-E-E-E-A-A-A-K-W-K-L-F-K-K-I-P-K-F-L-H-L-A-K-K-F-NH2 | 0 | >128 | >128 |
| k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0 | 8 | 16 |
| k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0.15 | ND | 16 |
| Ac-E-E-E-E-A-A-A-k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0 | >128 | 128 |
| Ac-E-E-E-E-A-A-A-k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0.075 | 128 | 32 |
| Ac-E-E-E-E-A-A-A-k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0.15 | 16 | 16 |
| Ac-E-E-E-E-A-A-A-OH + k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0 | ND | 16 |
| A-A-k-w-k-l-f-k-k-i-p-k-f-l-h-l-a-k-k-f-NH2 | 0 | ND | 32 |
Lowercase letters denote d-amino acids; uppercase letters denote l-amino acids. βA, β-alanine spacer; Ac, N-terminal acetylation. P. aeruginosa strain PAO1 and S. aureus strain SH1000 were used. ND, not determined.
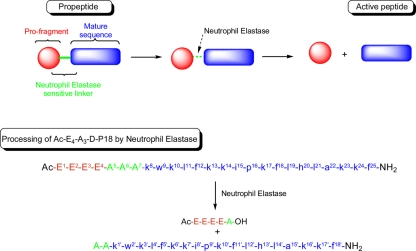
Fig. 1.
Proposed approach for the generation of neutrophil elastase-dependent host defense peptide prodrugs and its application to Ac-E4-A3–d-P18. Residue numbering used in the NMR studies is included as superscript numbers in the sequences of d-P18 and its propeptide; Ac indicates N-terminal acetylation, OH is a C-terminal carboxyl group, and NH2 is a C-terminal amide group.
Reactivation studies of Ac-E4-A3–d-P18 were conducted by susceptibility testing in the presence of purified human NE at concentrations of 0.075 and 0.15 μg/ml. These concentrations, while significantly lower than physiological concentrations in sputum or bronchoalveolar lavage fluid (26 to 100 μg/ml) of CF patients (7, 17), were sufficient to reactivate partly or fully the propeptide. Its MICs against S. aureus were 32 and 16 μM at enzyme concentrations of 0.075 and 0.15 μg/ml, respectively. Propeptide reactivation was also observed against P. aeruginosa, with a MIC of 16 μM at an enzyme concentration of 0.15 μg/ml, although NE can have direct antibacterial activity against Gram-negative organisms (16). For S. aureus, control tests performed with NE alone or with d-P18 showed that NE had neither antimicrobial activity alone nor additive activity in the presence of d-P18. Also, the activity of d-P18 remained unaffected by the equimolar addition of the profragment linker (Ac-E-E-E-E-A-A-A-OH; synthesized separately), indicating that this promoiety (18) does not hinder the released sequence by electrostatic interactions. The results reported in Table 1 indicate that the reactivation of Ac-E4-A3–d-P18 can be performed in a concentration-dependent manner with NE and could be exploited to confine the activity of d-P18 to the neutrophil-dominated airway secretions of CF patients.
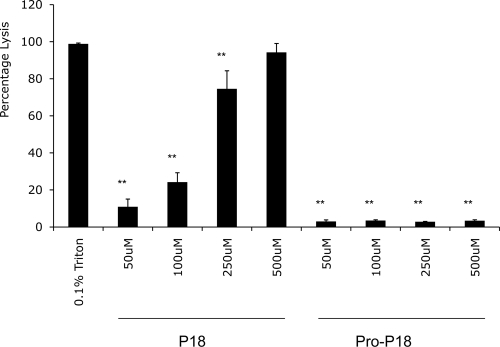
To determine if toxicity differentials also exist between the mature and proforms of d-P18, a hemolytic assay (4, 5, 22) was performed with these peptides. While the active peptide induced dose-dependent lysis of erythrocytes at concentrations between 50 and 500 μM, its proform was almost devoid of an erythrolytic effect (less than 3.5%) in this concentration range (Fig. 2).
Fig. 2.
Hemolytic activity of the active HDP d-P18 and its proform, Ac-E4-A3–d-P18. The results are normalized to the 0.1% Triton positive control. Statistics were carried out using a repeated-measures analysis of variance followed by Dunnett's post test. **, P < 0.01.
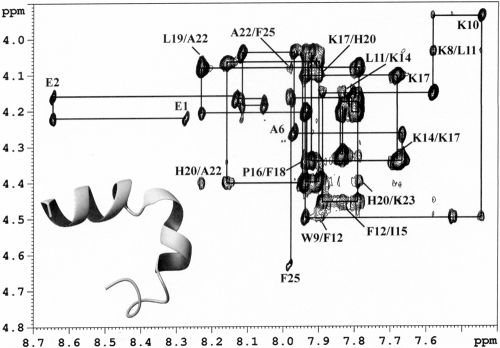
Finally, 1H nuclear magnetic resonance (NMR) studies were performed to assess if the activity differentials between d-P18 and Ac-E4-A3–d-P18 could be attributed to conformational changes. In aqueous solutions, both peptides showed a lack of secondary structure. In a 50% 2,2,2-trifluoroethanol (TFE-d3)–water mixed solvent system, d-P18 was characterized by a left-handed short α-helical conformation between residues d-Ile8′-d-Leu14′, while Ac-E4-A3–d-P18 adopted an extendedα-helical conformation between residues d-Lys8-d-Ile15 and d-Lys17-d-Lys24 (Fig. 3). Therefore, the lower activities of the propeptide most likely originate from the diminution of its net charge and/or hydrophobicity (5, 12), rather than from a lower capacity to adopt a functional conformation.
Fig. 3.
Fingerprint region of the 200-ms two-dimensional nuclear Overhauser effect spectra of propeptide Ac-E4-A3–d-P18 in a 50% TFE–water mixed solvent system. The backbone assignment is indicated, along with important short- and medium-range residue connectivities. A structural model of Ac-E4-A3–d-P18 is shown on the bottom left, with a ribbon representation of the helical motif for the average conformation. The N-terminal random coil segment is shown with a wire for clarity.
While NE preferentially cleaves peptides at residues with medium-sized aliphatic side chains (e.g., valine) (2, 16), the trialanine sequence was originally selected on the basis of synthetic efficiencies, to facilitate the assembly of the tetraglutamate sequence. Mass spectrometry analysis of samples from reactivation assays with purified NE showed that this enzyme hydrolyzes the linker between the first and second alanines (Ala5-Ala6 bond) (Fig. 1), yielding a reactivated peptide with two N-terminal alanines. A synthetic peptide with the corresponding sequence had a MIC of 32 μM against S. aureus. Therefore, the control exerted on the activity of an HDP through the conjugation of an oligo-glutamyl promoiety can be further optimized by the sequence of the linker and its selection among the cleavage motifs of enzymes with narrow substrate specificities, the sequence selected here being only suitable for topical application. This approach is currently being developed to generate HDP prodrugs for targeted delivery.
Acknowledgments
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (05/RFP/CHE0063 and 06/RFP/CHO024/EC07 to M.D. and 06/RFP/CHO006 to C.M.H.).
We also acknowledge Kevin McGuigan (Physiology and Medical Physics Department, Royal College of Surgeons in Ireland) for hosting and facilitating some of the microbiological work in his laboratory and Maria Boyle for her assistance during this work.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Bergsson G., et al. 2009. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 183:543–551 [DOI] [PubMed] [Google Scholar]
- 2. Bode W., Meyer E., Jr., Powers J. C. 1989. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry 28:1951–1963 [DOI] [PubMed] [Google Scholar]
- 3. Bowdish D. M., Davidson D. J., Hancock R. E. W. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6:35–51 [DOI] [PubMed] [Google Scholar]
- 4. Chen H.-C., Brown J. H., Morell J. L., Huang C. M. 1988. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 2:462–466 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y. X., et al. 2006. Comparison of biophysical and biologic properties of alpha-helical enantiomeric antimicrobial peptides. Chem. Biol. Drug Des. 67:162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finlay B. B., Hancock R. E. W. 2004. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497–504 [DOI] [PubMed] [Google Scholar]
- 7. Greene C. M., et al. 2005. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 174:1638–1646 [DOI] [PubMed] [Google Scholar]
- 8. Hancock R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156–164 [DOI] [PubMed] [Google Scholar]
- 9. Hancock R. E. W., Diamond G. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402–410 [DOI] [PubMed] [Google Scholar]
- 10. Hancock R. E. W., Sahl H.-G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 11. Hyde C., Johnson T., Owen D., Quibell M., Sheppard R. C. 1994. Some ‘difficult sequences’ made easy. A study of interchain association in solid-phase peptide synthesis. Int. J. Pept. Protein Res. 43:431–440 [PubMed] [Google Scholar]
- 12. Jiang Z. Q., et al. 2008. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers 90:369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelly E., Greene C. M., McElvaney N. G. 2008. Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets 12:145–157 [DOI] [PubMed] [Google Scholar]
- 14. Merrifield R. B. 1986. Solid phase synthesis. Science 232:341–347 [DOI] [PubMed] [Google Scholar]
- 15. Peschel A., Sahl H.-G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536 [DOI] [PubMed] [Google Scholar]
- 16. Pham C. T. N. 2006. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 6:541–550 [DOI] [PubMed] [Google Scholar]
- 17. Poncz L., Jentoft N., Ho M.-C. D., Dearborn D. G. 1988. Kinetics of proteolysis of hog gastric mucin by human neutrophil elastase and by Pseudomonas aeruginosa elastase. Infect. Immun. 56:703–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rautio J., et al. 2008. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 7:255–270 [DOI] [PubMed] [Google Scholar]
- 19. Rowe S. M., Miller S., Sorscher E. J. 2005. Cystic fibrosis. N. Engl. J. Med. 352:1992–2001 [DOI] [PubMed] [Google Scholar]
- 20. Shin S. Y., Kang J. H., Hahm K.-S. 1999. Structure-antibacterial, antitumor and hemolytic activity relationships of cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 53:82–90 [DOI] [PubMed] [Google Scholar]
- 21. Shin S. Y., et al. 2002. Salt resistance and synergistic effect with vancomycin of α-helical antimicrobial peptide P18. Biochem. Biophys. Res. Commun. 290:558–562 [DOI] [PubMed] [Google Scholar]
- 22. Tossi A., Sandri L., Giangaspero A. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30 [DOI] [PubMed] [Google Scholar]
- 23. Wade D., et al. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. U. S. A. 87:4761–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeaman M. R., Yount N. Y. 2007. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 5:727–740 [DOI] [PubMed] [Google Scholar]
- 25. Zanetti M. 2005. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 7:179–196 [PubMed] [Google Scholar]
- 26. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]