Abstract
Metacavir (PNA) is a novel synthetic nucleoside analogue for the treatment of hepatitis B virus (HBV). Our recent studies showed that PNA, a prodrug of 2′,3′-dideoxyguanosine (ddG), exhibited lower mitochondrial toxicity in long-term cultures of HepG2 cells. In the current study, we examined the long-term effects of PNA on mitochondrial toxicity in Marmota himalayana (Himalayan marmot). Himalayan marmots were treated daily with oral PNA (50 or 100 mg/kg), ziduvidine (AZT) (100 mg/kg), or water (control) for 90 days. PNA treatment did not alter the body weight or plasma lactate acid level. In livers from the animals treated with PNA at 100 mg/kg/day, histopathology showed mild steatosis or small focal liver cell necrosis. Electron microscopy also showed minor proliferation and partial mitochondrial swelling with crista reduction. Measurement of respiratory chain complex enzyme activity and mitochondrial DNA (mtDNA) content revealed no significant differences in skeletal muscle, liver, and kidney tissues between animals treated with PNA and controls. In contrast, in Himalayan marmots treated with AZT we observed delayed toxicity, including lactic acidosis, severe hepatic steatosis, obvious mitochondrial damage, and significant decreases in respiratory chain complex enzyme activity and mtDNA content. This is similar to the delayed toxicity syndrome observed previously in animals and humans. In summary, PNA treatment did not alter mitochondrial enzyme activity or mtDNA content. This suggests that PNA could pose a very low risk for adverse mitochondrion-related effects. However, long-term hepatotoxic effects of PNA were observed, and this indicates a need for continued monitoring of PNA-associated hepatotoxicity in clinical trials.
INTRODUCTION
A variety of nucleoside analogues have been developed for treatment of viral infections, including HIV and hepatitis B virus (HBV), and for a subset of nucleoside analogues mitochondrial injury is associated with long-term therapy (8, 9, 39, 40). Clinical manifestations of mitochondrial toxicity include various hematological disorders, peripheral neuropathy, skeletal and cardiac myopathy, pancreatitis, hepatic failure, and lactic acidosis (1, 9–12, 20, 27, 28, 30, 32, 35). Previous studies demonstrated that these adverse effects of nucleoside analogues are directly associated with mitochondrial injury (16, 27, 28, 35). The mitochondrial injury assessments showed abnormal mitochondrial morphology, depletion of mitochondrially encoded enzymes, and decreased numbers of mitochondrial genes (1, 12, 14). Mitochondrial disruption leads to energy loss, electron leakage from the electron transport system, increased concentrations of reactive oxygen species, oxidative damage, and cellular redox state imbalances (i.e., increased NADH/NAD+ ratio), which reverses the pyruvate/lactate balance in favor of increased lactate production (26, 37, 38).
In vivo and in vitro studies have demonstrated that liver and skeletal muscle tissues are important targets for nucleoside analogue-induced mitochondrial injury (3, 5, 6, 15, 17, 18, 20, 31). Altered liver, kidney, cardiac, and skeletal muscle functions have also been observed in both humans and animals chronically treated with nucleoside analogues (10, 12, 16, 18). In both animals and humans, nucleoside analogue-induced mitochondrial toxicity often has paralleled ultrastructural mitochondrial damage (20, 22, 28, 33). Metacavir (PNA) is a novel synthetic nucleoside analogue designed for the treatment of chronic HBV infection (25). Preclinical studies have demonstrated that it has a potential to be developed as a new anti-HBV drug. We previously examined the effect of metacavir in HepG2 cells maintained in culture and demonstrated that PNA had minimal mitochondrial toxicity at a concentration of 250 μM when given for a duration of 15 days (42).
At present, a variety of mammalian hosts, including mouse, rat, monkey, and woodchuck, have been used for the study of the mitochondrial toxicity of nucleoside analogues (4, 13, 19, 22, 36). Among these animal models, the mouse and rat were not susceptible to mitochondrial toxicity induced by nucleoside analogues, making those systems less than ideal for mitochondrial toxicity studies (4, 29, 33). The woodchuck is a more ideal model system and has been recommended by the FDA for the study of pathogenesis and therapy of chronic HBV infection and disease in humans (4, 36). Likewise, many studies have demonstrated that the results of nucleoside analogue drug toxicity studies using the woodchucks are predictive for responses of patients in clinical treatment (19, 36). In this present study, we used Marmota himalayana (Himalayan marmot) as a new experimental animal model to explore the mitochondrial toxicity potential of metacavir.
MATERIALS AND METHODS
Drugs.
Zidovudine (AZT) (lot no. 0801016) was purchased from the Shanghai Modern Pharmaceutical Co., Ltd. Metacavir (PNA) (lot no. 20080201) was provided by the Nanjing Chang'ao Medicinal and Pharmaceutical Co., Ltd.
Animal treatment.
Prior to initiation of treatment, 24 Himalayan marmots (12 males and 12 females, 3 to 4 years old and 3.0 to 5.0 kg) were identified as candidates based on 1 month of normal physical examinations. The animals were divided into four groups of six Himalayan marmots, comprised of three male and three female animals. All the marmots were housed in indoor pens constructed from concrete blocks and given food and water ad libitum. During the study, animals were individually housed in stainless-steel cages furnished with an arched, galvanized, mailbox-like device with grill doors. Himalayan marmots were dosed orally with a syringe and attached tube with PNA at 50 or 100 mg/kg/day for 90 days. As a positive control, a third group of Himalayan marmots received 100 mg/kg/day of AZT. As a negative control, a fourth group of animals was treated with water on the same schedule as the drug treatment. All animals were maintained in ambient-temperature rooms (10 to 15°C) with a controlled relative humidity of 40 to 60%. Lighting was controlled primarily with incandescent illumination on a cycle corresponding to ambient light and was supplemented with natural daylight that radiated through glass windows. All animals were handled strictly in accordance with institutional guidelines established at Qinghai Experimental Animal Center and regulations issued by the Ministry of Science and Technology of the People's Republic of China for the humane treatment of research animals. Animals were weighed monthly and evaluated for general appearance, mobility, and pharmaco-toxic signs.
Necropsy.
Complete necropsies were performed at the termination of the dosing phase. Himalayan marmots were exsanguinated during anesthesia induced by ketamine. Necropsy included a systematic gross examination of general physical condition, body orifices, internal and external organs, and tissues. The heart, liver, spleen, lung, kidneys, brain, adrenal glands, thymus, thyroid and parathyroid glands, testes, uterus, and ovaries were removed and weighed. Tissue samples from all organs examined were collected at necropsy, preserved in neutral-buffered formalin, and shipped to an experimental pathology laboratory for processing and histologic evaluation. Liver, kidney, skeletal muscle, and heart samples were placed in labeled tubes and frozen at −70°C for subsequent biochemical and molecular analysis.
Electron microscopy studies.
Electron microscopic evaluation of the mitochondrial morphology of liver, kidney, skeletal muscle, and cardiac tissues was performed as described previously (19). Briefly, collected tissue was cut into 1- to 2-mm slices and fixed in 4% paraformaldehyde with 2.0% glutaraldehyde in 100 mM phosphate buffer (pH 7.4) at 4°C for 24 h. The tissues were rinsed in cold phosphate-buffered saline (PBS) and postfixed in 1% OsO4 (Sigma Chemical Co.) in PBS (pH 7.4) for 1 h at room temperature. After OsO4 treatment, the tissue was dehydrated with a series of graded alcohol washes from 50% to 100% followed by 100% propylene oxide. The tissues were then infiltrated in equal volumes of propylene oxide and epoxy resin and embedded in pure epoxy resin. Samples were sectioned (100 nm) with an ultramicrotome and stained with lead citrate and uranyl acetate, and images were randomly captured from different areas using an electron microscope (Tecnai 12; Philips). For each sample, 10 photomicrographs were evaluated for mitochondrial morphology.
Enzyme assays.
Mitochondrial extracts were obtained using a Genmed animal tissue active mitochondrial extraction kit (Genmed Scientifics Inc.) according to manufacturer's instructions. Briefly, about 500 mg of tissue was homogenized with a glass homogenizer and centrifuged twice at 1,500 × g for 10 min at 4°C. The supernatant was removed and centrifuged at 10,000 × g for 10 min. The pelleted material (mitochondria) was washed three times, resuspended in 10 mM Tris-HCl (pH 7.4) containing 10 mM KCl, 0.25 M sucrose, and 5 mM MgCl2, and stored at −70°C. Protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Genmed Scientifics Inc.) as described by Bradford (7). Mitochondrial respiratory chain complex I-IV enzyme activity was assayed using a Genmed quantitative detection kit according to manufacturer's instructions but modified to allow a 96-well plate format (Genmed Scientifics Inc.) (42).
DNA preparation and quantitation.
Total DNA was extracted from tissues with the QIAamp DNA minikit according to the manufacturer's instructions (Qiagen, Valencia, CA). Briefly, tissues were washed at room temperature with 100 mM PBS (pH 7.4), sliced into small pieces, lysed with Qiagen ATL buffer, and digested with proteinase K and RNase A. The DNA was separated using spin columns provided with the kit, subsequently extracted with elution buffer (10 mM Tris [pH 7.4] containing 1 mM EDTA), and stored at −70°C until further analysis. The quality and quantity of DNA were determined spectrophotometrically by absorbance at 260 nm. Nuclear (actin) and mitochondrial (cytochrome b) genes were amplified in duplicate using a real-time PCR assay with the Light Cycler-Fast Start DNA Master SYBR green I kit (Takara Bio Inc., Dalian, China). DNA was eluted in 100 μl of distilled water and diluted 1:200 with distilled water. Forward and reverse cytochrome b and actin primer sequences were designed using the primer design software Primer 5.0 (primer sequences are available on request). All primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Premix real-time PCR reagent was purchased from Takara Bio Inc. (Dalian, China). Amplification was performed with an iQ5 Bio-Rad Cyclers real-time PCR detector system (Bio-Rad) in a 25-μl reaction mixture containing 1 μ1 DNA, 0.8 μ1 Master SYBR green mix (Takara Bio Inc.), and 200 nM each primer. The thermal profile included denaturation at 95°C for 10 min followed by 45 cycles at 95°C for 10 s, 55°C for 5 s, and 72°C for 10 s. Following amplification, a melting curve analysis was performed to confirm the specificity of the reaction. The product size was confirmed by 2% agarose gel electrophoresis. The threshold cycle (CT) value for each sample was calculated by determining the point at which the fluorescence exceeded the threshold limit. Two recombinant plasmids, containing one copy of the cytochrome b and actin target sequences, were used as a standard for mitochondrial DNA (mtDNA) and nuclear DNA quantification. Results are expressed as the number of copies of mtDNA per cell.
Statistical analysis.
All data were first subjected to tests for normality and equal variance and then analyzed by one-way analysis of variance (ANOVA). The data are presented as the mean ± standard error. When significance was found by ANOVA, the Holm-Sidak test was used to determine which treated groups were significantly different from the unexposed group. Differences were considered significant if the P value was ≤0.05.
RESULTS
Macroscopic examination.
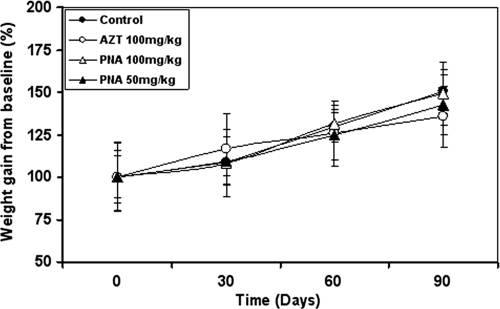
Metacavir (PNA) and AZT were administered orally to Himalayan marmots for 90 days. At termination of the dosing period, there were no differences between body weights or body weight gains of the PNA-treated animals and the control animals (Fig. 1). Remarkably, Himalayan marmots in the AZT-treated group showed more body weight gain than those in the control group after 30 days of treatment. After 60 days of treatment, the AZT group showed less weight gain than the controls. At the termination of the dosing phase (90 days of treatment), the mean body weight of the AZT-treated animals was 15% lower than that of control animals. For all groups, no treatment-related effects on overall body condition, mobility, and behavior were observed at the end of treatment.
Fig. 1.
Relative body weight gain from the baseline in Himalayan marmots after 90 days of treatment with metacavir (PNA), zidovudine (AZT), or water. Data are expressed as means ± standard deviations. No significant weight gains were observed between the treated groups and the control group (n = 6 [3 male and 3 female]).
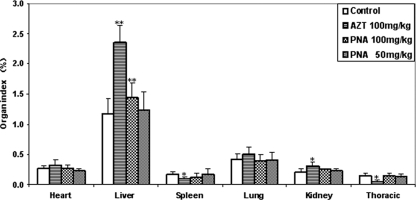
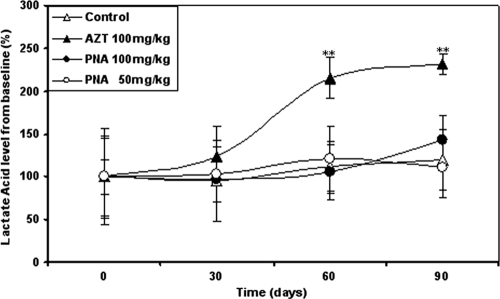
Following 90 days of exposure, treatment-related effects were present in the hearts, spleens, thymuses, kidneys, and livers of Himalayan marmots treated with AZT at 100 mg/kg/day (Fig. 2). Spleen and thymus weights of the AZT-treated group were significantly lower than those of the control group (P < 0.05). Thymic atrophy in the control group was more severe than splenic atrophy in the AZT-treated group. Remarkably, the relative liver weights in the AZT-treated group were about 2-fold higher than those in the control group. Furthermore, mean heart and kidney weight/body weight ratios in the AZT treatment group were significantly higher than those in the control group (P < 0.05). Plasma lactate concentrations in PNA-treated animals were similar to the lactate concentrations in the control animals during and at the end of the dosing phase (Fig. 3). In the AZT group, however, serum lactate concentrations continued to increase during the 90-day treatment and were significantly higher than those of control animals (P < 0.01) after 60 days of treatment, suggesting the development of lactic acidosis.
Fig. 2.
Relative organ weights of Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 Days. Results are expressed as means ± standard deviations. *, P < 0.05; **, P < 0.01 (compared to results for controls, by Dunnett's multiple-comparison test).
Fig. 3.
Mean serum lactate acid concentrations in Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days. Results are expressed as means ± standard deviations. **, P < 0.01 compared to results for controls (by Dunnett's multiple-comparison test).
Histologic findings.
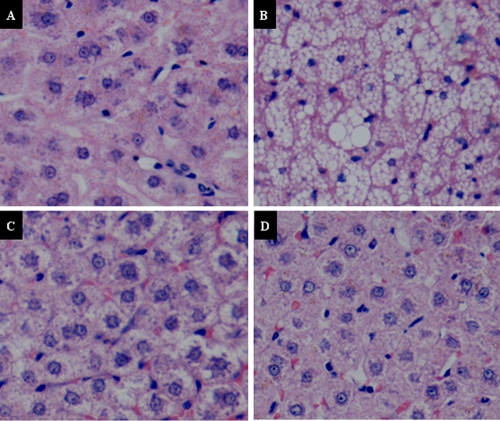
Histologic examination of the liver revealed that more than 80% of the hepatocytes from the AZT-treated group had severe macrovesicular steatosis (Fig. 4), as observed in patients treated with nucleoside analogue reverse transcriptase inhibitors (NRTIs) (20). Additional small lobular necrosis and centrolobular cholestasis were present in four of six animals from this group. Mild steatosis (occasional vacuoles of steatosis) was also observed in the animals treated with PNA at 100 mg/kg/day. In two of the six Himalayan marmots treated with PNA at 100 mg/kg/day, small focal liver cell necrosis and unclear hepatic lobule structures were also observed at the termination of treatment. However, with the exception of liver, there were no additional histologic differences observed in other tissues between the control and treated groups. No obvious histopathologic alterations were observed in tissues from control and 50 mg/kg/day PNA-treated animals.
Fig. 4.
Histopathologic examination of liver steatosis in Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days (hematoxylin and eosin [H&E] staining; magnification, ×400). (A) Control group (normal). (B) Group treated with AZT at 100 mg/kg/day, showing severe microvesicular steatosis of more than 90% hepatocytes. (C) Group treated with PNA at 100 mg/kg/day, showing mild steatosis of fewer than 20% of hepatocytes. (D) Group treated with PNA at 50 mg/kg/day (normal).
Electron microscopic examination.
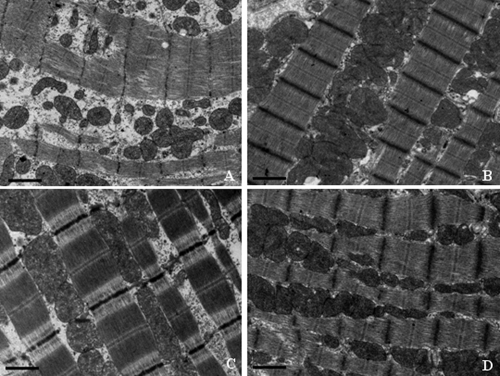
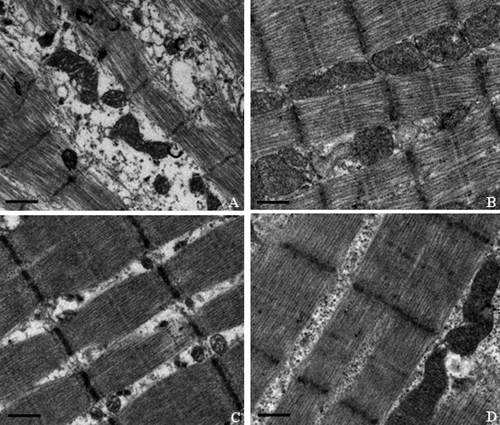
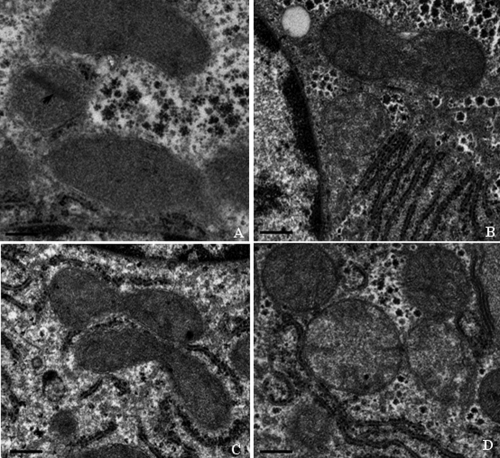
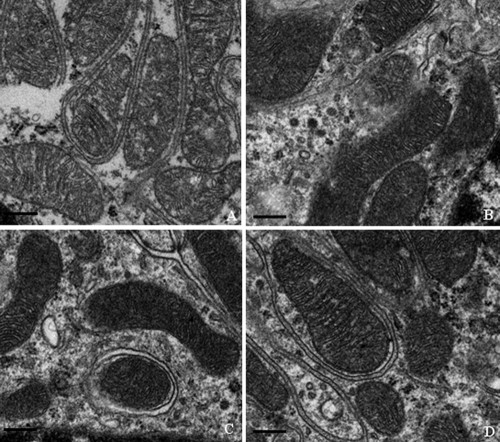
Electron microscopic evaluation of cardiac and skeletal muscle, liver, and kidney mitochondrial morphology was performed at the termination of the dosing phase. Ultrastructural findings are illustrated in Fig. 5, 6, 7, and 8. Marked ultrastructural differences between the AZT-treated and control Himalayan marmot cardiac muscle, skeletal muscle, kidney, and liver mitochondria were observed. Cardiac and skeletal muscle samples from AZT-treated Himalayan marmots revealed mitochondrial proliferation, mitochondrial swelling with loss of cristae, sarcomere disruption (Z-line and M-line irregularities), partial or complete erosion of mitochondrial membranes, and replacement of cristae with clear space. Significant mitochondrial damage observed in liver from AZT-treated Himalayan marmots was typified by severe mitochondrial enlargement, fragmented and missing cristae, presence of crystalline inclusions, lower matrix electron density, and swollen mitochondrial membranes compared with that of control animals. In kidney mitochondria of AZT-treated animals, conspicuous swelling, dissolution of cristae, and homogenization and decreased density of the matrix also were observed. In all of these tissues from the AZT-treated animals, fat droplet accumulation was present. Conversely, there were no obvious mitochondrial morphological changes in cardiac muscle, skeletal muscle, kidney, and liver from control and 50 mg/kg/day PNA-treated animals. Mitochondrial cristae from these tissues were dense and clear, and mitochondrial membranes were intact. It is noteworthy that minor proliferation and partial mitochondrial swelling with reduction of cristae were observed in skeletal muscle and liver from the group treated with PNA at 100 mg/kg/day.
Fig. 5.
Representative electron photomicrographs of cardiac muscle from Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days. (A) AZT; (B) PNA at 100 mg/kg/day; (C) PNA at 50 mg/kg/day; (D) control. Bars show 1 μm. Ultrastructural features include registered sarcomeres with thick and thin filaments and mitochondria. Severe distortion of mitochondrial internal architecture with swelling, disruption of mitochondrial membranes and lysis of mitochondrial cristae, and sarcomere disruption (Z-line and M-line irregularities) are observed in the AZT-treated group. Conversely, no obvious mitochondrial morphological changes are observed in the PNA-treated and control groups. Original magnification, ×18,500.
Fig. 6.
Representative electron photomicrographs of skeletal muscle from Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days. (A) AZT; (B) PNA at 100 mg/kg/day; (C) PNA at 50 mg/kg/day; (D) control. Bars show 1 μm. Ultrastructural features include registered sarcomeres with thick and thin filaments and mitochondria. Swelling of mitochondria, giant mitochondria, disruption of mitochondrial membranes, and sarcomere disruptions with Z-line misalignment are observed in the AZT-treated group. Conversely, no obvious mitochondrial morphological changes are observed in the PNA-treated and control groups. Original magnification, ×18,500. In each sample, more than 10 fields were examined under low or high magnification.
Fig. 7.
Representative electron photomicrographs of liver biopsy specimens from Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water at the termination of the 90-day dosing phase. (A) AZT; (B) PNA at 100 mg/kg/day; (C) PNA at 50 mg/kg/day; (D) control. Bars show 1 μm. Mitochondrial inclusions and alterations, such as loss of the cristae and a crystalline inclusion, were observed in the AZT-treated group. Conversely, no obvious mitochondrial cytopathy is observed in the PNA-treated and control group. Original magnification, ×18,500. In each sample, more than 10 fields were examined under low or high magnification.
Fig. 8.
Representative electron photomicrographs of kidney biopsy specimens from Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water at the termination of the 90-day dosing phase. (A) AZT; (B) PNA at 100 mg/kg/day; (C) PNA at 50 mg/kg/day; (D) control. Bars show 1 μm. Mitochondrial cytopathy with partial loss of the cristae was observed in the AZT-treated group. Conversely, no obvious mitochondrial cytopathy is observed in the PNA-treated and control groups. Original magnification, ×18,500. In each sample, more than 10 fields were examined under low or high magnification.
Enzyme assay.
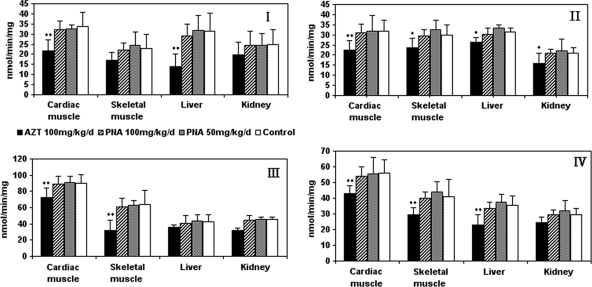
Mitochondrial respiratory chain complex enzyme levels were measured in extracts from liver, kidney, skeletal, and cardiac muscle tissues obtained from Himalayan marmots treated with PNA for 90 days (Fig. 9). No significant differences were observed between the enzyme levels from the PNA treatment groups and control groups. Interestingly, enzyme levels in tissue extracts from the group treated with PNA at 100 mg/kg/day trended lower than those in the group treated with the 50 mg/kg, but this difference was not statistically significant. However, statistically significant differences (P < 0.05 or P < 0.01) were observed between respiratory chain complex enzyme levels in skeletal muscle and liver tissues obtained from AZT-treated animals and those in tissues from control animals. In addition, mitochondrial respiratory chain complex enzyme levels were statistically different (P < 0.01) between cardiac muscle samples from control and AZT-treated animals. In skeletal muscle, significant differences in mitochondrial respiratory chain complex enzymes II, III, and IV were observed between the AZT-treated group and the control group (P < 0.01 or P < 0.05). In liver, mitochondrial respiratory chain complex enzymes I, II, and IV in the AZT-treated group were significantly affected compared with those in control groups (P < 0.01 or P < 0.05). In the kidney, with the exception of reduced mitochondrial respiratory chain complex enzyme II (P < 0.05), there were no differences between the AZT-treated group and control group in terms of the observed mitochondrial respiratory chain complex enzymes.
Fig. 9.
Enzyme activities of mitochondrial respiratory chain complexes I to IV in cardiac and skeletal muscle, liver, and kidney of Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days. Mitochondria were then extracted as described in Materials and Methods. Results are expressed as means ± standard deviations. *, P < 0.05; **, P < 0.01 (compared to results for controls, by Dunnett's multiple-comparison test).
Measurement of mtDNA content in tissues.
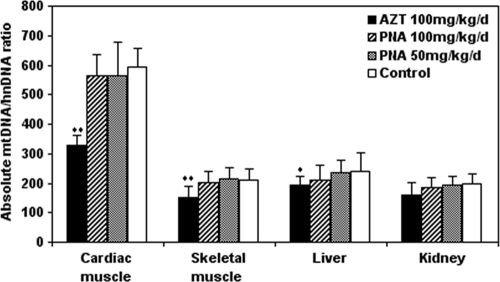
As a criterion for measurement of mtDNA content and mtDNA damage, the cytochrome b/actin ratio in DNA extracts from cardiac and skeletal muscle, liver, and kidney from treated and control Himalayan marmots was measured (Fig. 10). Generally, the mtDNA contents in cardiac and skeletal muscle, liver, and kidney were similar in the control and PNA-treated groups. However, the mtDNA contents for all tissues in the group treated with PNA at 100 mg/kg/day were less than those for control or 50 mg/kg/day PNA-treated animals. Conversely, in cardiac and skeletal muscle, significant differences in mtDNA content were observed between the AZT-treated group and the control group (P < 0.01). The mtDNA content in liver tissues from the AZT-treated group also was significantly lower than that in the control group (P < 0.05). In kidney tissues, there was a minor decrease in mtDNA content in the AZT-treated group compared with the control group, but this difference was not statistically significant.
Fig. 10.
mtDNA contents in cardiac and skeletal muscle, liver, and kidney of Himalayan marmots treated with metacavir (PNA), zidovudine (AZT), or water for 90 days. The ratios of mtDNA to nuclear DNA were determined by real-time PCR as described in Materials and Methods. Results are expressed as means ± standard deviations. *, P < 0.05; **, P < 0.01 (compared to results for controls, by Dunnett's multiple-comparison test).
DISCUSSION
Using the Himalayan marmot animal model, we have investigated the effects induced by a 90-day PNA treatment on mitochondrial respiratory chain complex enzyme activities, mitochondrial DNA content, plasma lactate concentrations, histology, and mitochondrial morphology. Referring to the time required to produce mitochondrial toxicity in woodchucks (Marmota monax) with fialuridine treatment (33, 36), the dosing period of 90 days is sufficient to induce clinical mitochondrial toxicity in Himalayan marmots. The measured mitochondrial parameters in cardiac and skeletal muscle, livers, and kidneys were not statistically different between the PNA-treated and control animals. There was a tendency for a small decrease in mtDNA content and enzyme levels in all tissues examined from the animals treated with PNA at 100 mg/kg/day compared with the control group, but these differences were not statistically significant. Our preclinical studies showed that hepatic tissue was the predominant target of PNA toxicity in rats, consistent with the present findings of increased liver/body weight ratio in the animals treated with PNA at 100 mg/kg/day. Histologic examination and electron microscopy studies also provided an indication of hepatotoxic effects with PNA treatment at the 100-mg/kg/day dose. These observations may in part explain why PNA toxicity was observed predominantly in the livers of rats in the preclinical studies. Therefore, more attention should be paid to potential mitochondrial hepatotoxicity induced by PNA in clinical trials.
Conversely, AZT did increase serum plasma lactate levels after 60 days of treatment compared with those at the start of treatment or in the control group. After 3 months of treatment, AZT altered mitochondrial respiratory chain complex enzyme activities, mitochondrial DNA contents, histologic and mitochondrial morphologies, and plasma lactate concentrations compared with those in the control group. Based on plasma lactate concentrations, measurement of mtDNA and respiratory chain complex enzyme levels, histologic alterations, and mitochondrial morphology changes, there was marked evidence of clinical toxicity in Himalayan marmots treated by oral administration (for 90 days) with AZT at 100 mg/kg/day. These results are similar to previous reports of AZT toxicity in clinical patients (12, 13, 16–18).
A limitation of this study is the absence of a recovery phase, so we did not explore the reversibility of the adverse effects induced by PNA treatment with drug withdrawal in this new animal model. However, in animal and human studies, mitochondrial injury sufficient to cause injury to cells and clinical toxicity requires an mtDNA reduction of greater than 70% (14, 29, 40). Therefore, the less than 10% reduction of mtDNA content observed in the animals treated with PNA at 100 mg/kg/day might not induce significant mitochondrial injury. Compared with the adverse effect induced by AZT, the extent of the hepatic effect of PNA at 100 mg/kg/day was much lower, because this group maintained more than 90% of the control levels of mtDNA. Therefore, we propose that these mild injuries induced by the highest dose of PNA should be reversible with drug withdrawal, as observed in rhesus monkey (41).
AZT is a well-known inhibitor of human immunodeficiency viruses (1, 2). Several clinical studies have demonstrated that AZT induces significant mitochondrial toxicity with long-term therapy (1, 12, 16–18, 22–24, 34). However, Korba et al. did not find obvious treatment-related hematological or serological indications of toxicity, including drug-related hepatic toxicity (based on alanine aminotransferase [ALT], aspartate transaminase [AST], and sodium dehydrogenase [SDH] levels) in woodchucks (Marmota monax) treated with AZT at 15 mg/kg/day for 28 days (21). This discrepancy could be related to the short-term, low-dose treatment or to sensitivity of the species to the toxicity. Notably, our current data showed that the toxicity profiles of AZT used in Himalayan marmots were very similar to those observed in patients treated with this nucleoside. This difference might correlate with the relative sensitivity of this animal species to mitochondrial toxicity (29).
In a previous study with rhesus monkeys, intravenous administration of PNA at the dose of 120 mg/kg/day resulted in moderate mitochondrial injury, which was most prominent in mtDNA content and mitochondrial morphology in skeletal muscle and liver after 90 days of treatment (41). The lack of a significant mitochondrial toxicity after exposure to PNA at 100 mg/kg/day in Himalayan marmots in our study may be explained by the different administration method and the relatively lower dose. Furthermore, species differences in PNA absorption, metabolism, and distribution may contribute to the differences in mitochondrial toxicity.
In summary, our results indicate that the mitochondrial damage induced by PNA in Himalayan marmots at doses 50 times the recommended human dose over a period of 90 days was very moderate compared with that induced by AZT (50 mg/kg/day). Our results suggest that PNA could pose a very low risk for potential mitochondrion-related effects in clinical trials. However, continued attention is required for the assessment of potential PNA-associated hepatotoxicity. Further studies exploring the mitochondrial toxicity of PNA are required to determine the mechanisms of mitochondrial insult and whether the combination of PNA with other nucleoside analogue drugs results in increased toxicity.
ACKNOWLEDGMENTS
We thank Kathy Gauthier, Christopher Roberts, Ling Zhong, and Wei Wang (Medical College of Wisconsin) for help in modifying the syntax of the manuscript.
This work was supported by the Mega-Projects of Science Research for the 11th Five-Year Plan: Standardized Platform Construction and Scientific Application in Technologies for New Drug Screening (no. 2009ZX09302-002).
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1. Arnaudo E., Dalakas M., Shanske S., Moraes C. T., DiMauro S., Schon E. A. 1991. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337:508–510 [DOI] [PubMed] [Google Scholar]
- 2. Ayers K. M., Tucker W. E., Jr., Hajian G., de Miranda P. 1996. Nonclinical toxicity studies with zidovudine: acute, subacute, and chronic toxicity in rodents, dogs, and monkeys. Fundam. Appl. Toxicol. 32:129–139 [DOI] [PubMed] [Google Scholar]
- 3. Benbrik E., et al. 1997. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J. Neurol.Sci. 149:19–25 [DOI] [PubMed] [Google Scholar]
- 4. Biesecker G., et al. 2003. Evaluation of mitochondrial DNA content and enzyme levels in tenofovir DF-treated rats, rhesus monkeys and woodchucks. Antiviral Res. 58:217–225 [DOI] [PubMed] [Google Scholar]
- 5. Birkus G., Hitchcock M. J., Cihlar T. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birkus G., Gibbs C. S., Cihlar T. 2003. Comparative effects of adefovir and selected nucleoside inhibitors of hepatitis B virus DNA polymerase on mitochondrial DNA in liver and skeletal muscle cells. J. Viral Hepat. 10:50–54 [DOI] [PubMed] [Google Scholar]
- 7. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 8. Brinkman K., ter Hofstede H. J., Burger D. M., Smeitink J. A., Koopmans P. P. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735–1744 [DOI] [PubMed] [Google Scholar]
- 9. Brinkman K., ter Hofstede H. J. M. 1999. Mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors: lactic acidosis, risk factors and therapeutic options. AIDS Rev. 1:140–146 [Google Scholar]
- 10. Carr A., Miller J., Law M., Cooper D. A. 2000. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 14:25–32 [DOI] [PubMed] [Google Scholar]
- 11. Carr A., Cooper D. A. 2000. Adverse effects of antiretroviral therapy. Lancet 356:1423–1430 [DOI] [PubMed] [Google Scholar]
- 12. Casademont J., et al. 1996. The effect of zidovudine on skeletal muscle mtDNA in HIV-1 infected patients with mild or no muscle dysfunction. Brain 119:1357–1364 [DOI] [PubMed] [Google Scholar]
- 13. Chan S. S., et al. 2007. Mitochondrial toxicity in hearts of CD-1 mice following perinatal exposure to AZT, 3TC, or AZT/3TC in combination. Environ. Mol. Mutagen. 48:190–200 [DOI] [PubMed] [Google Scholar]
- 14. Cote H. C., et al. 2002. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N. Engl. J. Med. 346:811–820 [DOI] [PubMed] [Google Scholar]
- 15. Cui L., Yoon S., Schinazi R. S., Sommadossi J. P. 1995. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil in human liver cells. J. Clin. Invest. 95:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalakas M. C., et al. 1990. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 322:1098–1105 [DOI] [PubMed] [Google Scholar]
- 17. Dalakas M. C., Leon-Monzon M. E., Bernardini I., Gahl W. A., Jay C. A. 1994. Zidovudine-induced mitochondrial myopathy is associated with muscle carnitine deficiency and lipid storage. Ann. Neurol. 35:482–487 [DOI] [PubMed] [Google Scholar]
- 18. de la Asuncion J., del Olmo M. L., Sastre J., Pallardo F. V., Vina J. 1999. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology 29:985–987 [DOI] [PubMed] [Google Scholar]
- 19. Divi R. L., et al. 2007. Erythrocebus patas monkey offspring exposed perinatally to NRTIs sustain skeletal muscle mitochondrial compromise at birth and at 1 year of age. Toxicol. Sci. 99:203–213 [DOI] [PubMed] [Google Scholar]
- 20. Duong Van Huyen J.-P., et al. 2003. Toxic effects of nucleoside reverse transcriptase inhibitors on the liver. Am. J. Clin. Pathol. 119:546–555 [DOI] [PubMed] [Google Scholar]
- 21. Korba B. E., Cote P., Hornbuckle W., Tennant B. C., Gerin J. L. 2000. Treatment of chronic woodchuck hepatitis virus infection in the eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 31:1165–1175 [DOI] [PubMed] [Google Scholar]
- 22. Lewis W., Gonzalez B., Chomyn A., Papoian T. 1992. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J. Clin. Invest. 89:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis W., Dalakas M. C. 1995. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1:417–422 [DOI] [PubMed] [Google Scholar]
- 24. Lewis W., Day B. J., Copeland W. C. 2003. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2:812–822 [DOI] [PubMed] [Google Scholar]
- 25. Li Z., et al. 2008. A sensitive and specific liquid chromatography-mass spectrometry method for determination of metacavir in rat plasma. J. Chromatogr. B 864:9–14 [DOI] [PubMed] [Google Scholar]
- 26. Mazzucco C. E., Hamatake R. K., Colonno R. J., Tenney D. J. 2008. Entecavir for treatment of hepatitis B virus displays no in vitro mitochondrial toxicity or DNA polymerase gamma inhibiton. Antimicrob. Agents Chemother. 52:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKenzie R., et al. 1995. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 333:1099–1105 [DOI] [PubMed] [Google Scholar]
- 28. Miller K. D., Cameron M., Wood L. V., Dalakas M. C., Kovacs J. A. 2000. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann. Intern. Med. 133:192–196 [DOI] [PubMed] [Google Scholar]
- 29. Morton D. M. 1998. Importance of species selection in drug toxicity testing. Toxicol. Lett. 102:545–550 [DOI] [PubMed] [Google Scholar]
- 30. Moyle G. 2000. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin. Ther. 22:911–936 [DOI] [PubMed] [Google Scholar]
- 31. Pan-Zhou X. R., Cui L., Zhou X. J., Sommadossi J. P., Darley-usmar V. M. 2000. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob. Agents Chemother. 44:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters B. S., Winer J., Landon D. N., Stotter A., Pinching A. J. 1993. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q. J. Med. 86:5–15 [PubMed] [Google Scholar]
- 33. Richardson F. C., et al. 1999. An evaluation of the toxicities of 2′-fluorouridine and 2′-fluorocytidine-HCl in f344 rats and woodchucks (Marmota monax). Toxicol. Pathol. 27:607–617 [DOI] [PubMed] [Google Scholar]
- 34. Ruga E., et al. 2003. Zidovudine-induced alterations in the heart and vascular smooth muscle of the rat. Cardiovasc. Res. 60:147–155 [DOI] [PubMed] [Google Scholar]
- 35. Sundar K., Suarez M., Banogon P. E., Shapiro J. M. 1997. Zidovudine induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature. Crit. Care Med. 25:1425–1430 [DOI] [PubMed] [Google Scholar]
- 36. Tennant B. C., et al. 1998. Antiviral activity and toxicity of filauridine in the woodchuck model of hepatitis B virus infection. Hepatology 28:179–191 [DOI] [PubMed] [Google Scholar]
- 37. Vickers A. E. M., Bentley P., Fisher R. L. 2006. Consequences of mitochondrial injury induced by pharmaceutical fatty acid oxidation inhibitors is characterized in human and rat liver slices. Toxicol. In Vitro 20:1173–1182 [DOI] [PubMed] [Google Scholar]
- 38. Vickers A. E. M. 2009. Characterization of hepatic mitochondrial injury induced by fatty acid oxidation inhibitors. Toxicol. Pathol. 37:78–88 [DOI] [PubMed] [Google Scholar]
- 39. Walker U. A., Setzer B., Venhoff N. 2002. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS 16:2165–2173 [DOI] [PubMed] [Google Scholar]
- 40. White A. J. 2001. Mitochondrial toxicity and HIV therapy. Sex. Transm. Infect. 77:158–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng W., et al. 2009. In vivo assessment of mitochondrial toxicity of metacavir in rhesus monkeys after three months of intravenous administration. Acta Pharmacol. Sinica 30:1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang P. H., et al. 2010. In vitro mitochondrial toxicity of metacavir, a new nucleoside reverse transcriptase inhibitor for treatment of hepatitis B virus. Antimicrob. Agents Chemother. 54:4887–4892 [DOI] [PMC free article] [PubMed] [Google Scholar]