Abstract
RC-101 is a synthetic microbicide analog of retrocyclin, which has shown in vitro activity against X4 and R5 HIV-1. In an effort to develop a safe and effective RC-101 vaginal microbicide product, we assessed safety in ex vivo macaque and human models and efficacy using in vitro and ex vivo models. A polyvinyl-alcohol vaginal film containing RC-101 (100 μg/film) was developed. Formulation assessment was conducted by evaluating disintegration, drug content, mechanical properties, and stability. Efficacy was evaluated by in vitro peripheral blood mononuclear cells (PBMC) assay and ex vivo human ectocervical tissue explant model. Ex vivo safety studies were conducted by exposing RC-101 to an excised monkey reproductive tract and excised human ectocervical tissue. RC-101 100 μg films were shown to be safe to human and monkey tissue and effective against HIV-1 in vitro and ex vivo in human ectocervical tissue. The 90% inhibitory concentration (IC90) for RC-101 films at 2,000 μg (IC90 = 57.5 μM) using an ex vivo model was 10-fold higher than the IC90 observed using an in vitro model (IC90 = 5.0 μM). RC-101 films were stable for 1 month at 25°C, with in vitro bioactivity maintained for up to 6 months. RC-101 was developed in a quick-dissolve film formulation that was shown to be safe in an ex vivo model and effective in in vitro and ex vivo models. RC-101 film formulations were shown to maintain bioactivity for a period of 6 months. Findings from the present study contribute to the development of a safe and effective topical microbicide product.
INTRODUCTION
The HIV/AIDS pandemic is rapidly increasing among women. Nearly 6 of every 10 infections in sub-Saharan Africa occur in women (28). Current strategies to prevent transmission of HIV are not completely successful. Considering that many men are often unwilling to use condoms and women are more susceptible to HIV infection, emerging strategies to prevent heterosexual transmission of HIV must include female-controlled methods (21). One such approach is the development of a topical microbicide to prophylactically inhibit transmission of sexually transmitted infections (STIs), including HIV infection (12, 17, 30).
Retrocyclins are tetracyclic 18-residue peptides with three cysteine disulfide bonds. Specifically, RC-101 is a retrocyclin analog synthesized by solid-phase peptide synthesis that has shown activity against X4 and R5 strains of HIV-1 in vitro (4). The anti-HIV mechanism occurs by preventing six-helix bundle formation in gp41, a major HIV surface binding protein (2). As a result, RC-101 has been identified as a potential topical microbicide agent to prevent mucosal transmission of HIV-1 (4, 31).
Further development of RC-101 as a vaginal microbicide product requires its formulation into a viable dosage form that is suitable for the intended route of administration, acceptable to the patient, and able to maintain chemical stability of the active peptide in the biological environment during its shelf-life. Most vaginal microbicides in development are formulated as hydrogels (1, 29). More recently, new dosage forms have been considered such as vaginal rings, tablets, ovules, and polymeric films (13, 14, 18, 19, 25). The choice of dosage form is dependent on the physical and chemical characteristics of the microbicide drug as well as patient acceptability, maintenance of drug efficacy, and drug targeting (25).
The successful formulation of biopharmaceuticals (proteins and peptides) is often challenging. Due to their high susceptibility to degradation, protective strategies in the formulation process may be needed to prevent peptide degradation and preserve the activity of the product during its shelf-life and in the biological environment. Semisolids are the most frequently used vaginal dosage form. However, this would not be suitable for RC-101 because an aqueous semisolid dosage form would expose RC-101 to a significant amount of water, accelerating peptide degradation. Forced degradation studies conducted in our laboratory (data not published) to identify the degradation pathways of RC-101 and to qualify the analytical methods used for stability have indicated that RC-101 is susceptible to oxidation. In addition, it was shown that the peptide is stable for 2 weeks over a range of pH from 3 to 7. These data indicate that RC-101 is stable in the short term; however, long-term stability in the final product still needs to be evaluated. As an alternative, a nonaqueous semisolid product could be formulated; however, the product would likely have low patient acceptability (16) and difficulty with miscibility in the fluids encountered in the vaginal lumen.
Based on the characteristics of RC-101, a quick-dissolving film formulation was investigated as an alternative dosage form. Polymeric films contain less water compared to traditional gel formulations and can provide rapid release of the active drug from the delivery system upon contact with cervicovaginal fluids. In addition, polymeric films have several other advantages: convenient use without the need for applicators, lower cost per dose, lack of messiness relative to current vaginal gel products, and increased drug stability (8, 9, 13, 14, 25).
In the present study, RC-101 was formulated into a quick-dissolve polymeric film. Film assessment, ex vivo safety, and both in vitro and ex vivo efficacy studies were conducted. The stability of the formulated film product over time was also assessed.
MATERIALS AND METHODS
Materials.
RC-101 was synthesized by the Peptide Synthesis Facility at the University of Pittsburgh (Pittsburgh, PA), ca. 60% concentration determined by AU-PAGE. Polyvinyl alcohol (PVA) was obtained from Kuraray America, Inc. (New York, NY). Glycerin was obtained from Dow Chemical Company (Midland, MI). Hydroxypropyl methylcellulose (HPMC) type 6 cps was obtained from Sigma-Aldrich (St. Louis, MO). All other materials were obtained from Fisher Scientific (Fair Lawn, NJ) unless specified otherwise.
Detection of RC-101 by HPLC.
A high-performance liquid chromatography (HPLC) system (Waters Corp., Milford, MA) was used for the analysis of RC-101. The HPLC system was equipped with an autoinjector model 717 (Waters), a quaternary pump model 600 (Waters), and a UV detector model 2487 (Waters). Separation of RC-101 from degradant products was achieved using a Phenomenex Jupiter 5μ C5 300 Å (4.6-mm internal diameter and 250-mm length) column (Phenomenex, Torrance, CA) protected by a Widepore C5 (4-mm internal diameter and 3.0-mm length) guard cartridge (Phenomenex). The gradient consisted of mobile phase A (0.1% [vol/vol] trifluoroacetic acid in water) and mobile phase B (0.07% [vol/vol] trifluoroacetic acid in acetonitrile) pumped at a flow rate of 1.0 ml/min. Elution conditions started at A:B (80:20) and linearly changed to A:B (60:40) for the first 12 min, followed by a 4-min isocratic condition at A:B (60:40) and a 10-min isocratic condition at A:B (80:20) to allow equilibration of the system before the next sample was injected. Retention time of RC-101 was around 10 min, and the total run time was 26 min. Empower PRO, Empower 2 software (Waters Corp.), was used to control the HPLC system.
Formulation development: the RC-101 polymeric vaginal film manufacturing process.
Vaginal films were prepared by casting a polymeric film solution into an 8-well plate (dimensions, 27.5 by 33.5 mm/well). Three film formulations were prepared: an initial film formulation (RC_F+LA) containing 0.8% (wt/wt) lactic acid to simulate the conditions found in the vaginal environment, a second formulation without lactic acid (RC_F), and a final optimized formulation (RC_F+EDTA) containing 0.05% (wt/wt) EDTA in an effort to protect RC-101 against oxidation. Preformulation evaluations conducted (data not shown) included forced degradation studies that indicated susceptibility to oxidative degradation upon exposure to hydrogen peroxide conditions. Given that the vagina contains lactobacilli that produce hydrogen peroxide, this is important for vaginal delivery from the standpoint of stability at the biological compartment level. Incorporation of EDTA in the RC-101 film was based on the results found in the preformulation stability studies. All three formulations contained a polymeric solution base. This film base was prepared by adding 40 ml of Milli-Q water into a beaker. PVA (3.0 g) was slowly added to water with mixing, using a magnetic stir bar, followed by the addition of 60 mg of HPMC. The solution was heated for 20 min at 95°C for complete dissolution of the polymers. After the solution cooled to room temperature under mixing, 1.5 g of glycerin was added. For RC_F+LA, 0.8% lactic acid was added, and the films were prepared at a dose of 100 μg of RC-101/film. The second formulation, without lactic acid, was prepared at two dose levels: 100 μg/film (RC_F100) and 2,000 μg/film (RC_F2000). The optimized formulation (RC_F+EDTA) was prepared by adding 0.05% EDTA, and RC-101 at a dose of 100 μg of RC-101/film. The completed formulation solution (2.4 g) containing RC-101 was poured into each well of the eight-well plate. The plate was placed into a vacuum oven at 30 ± 2°C for 20 ± 4 h for drying. After this period of time, the films were removed from the plate. Placebo films were prepared according to an identical protocol, without the addition of RC-101. All films were removed from the plates, weighed, and stored in PET/aluminum foil pouches (Amcor Flexibles Healthcare, Inc., Mundelein, IL) until further analysis.
Formulation assessment.
The dimensions of the film (thickness, length, and width) were determined by using a digital micrometer caliper (Fisher Scientific, Fair Lawn, NJ). Disintegration was determined by placing the film into 3 ml of Milli-Q water in a crystallization dish (50 by 35 mm). The crystallization dish was rotated on an orbital shaker Rotomix Type 48200 (Thermolyne, Dubuque, IA) at 90 rpm, at room temperature, and the time to visual disintegration of the film was recorded. Determination of the water content of the film was obtained by Karl Fisher titration using a 758 KFD Titrino Metrohm (Brinkmann Instruments, Inc., Westbury, NY). Films were initially dissolved in 3 ml of dimethyl sulfoxide (DMSO) for a period of 20 h. An aliquot (0.5 ml) of the dissolved film solution was weighed and added to the Karl Fisher titration vessel for moisture determination. The water content of the DMSO was determined as a blank, and the result was subtracted from the sample results. Water content was expressed as a percentage (wt/wt).
The tensile strength of the films was evaluated by using the texture analyzer TA.XT2 (Texture Technologies, Scarsdale, NY). Films were held between two clamps positioned at a distance of 4.85 mm. During measurement, the films were pulled by the top clamp at a rate of 3 mm/s until breakpoint. The force at the breakpoint was recorded. Tensile strength was calculated as follows: tensile strength (N/mm2) = breaking force (N)/cross-sectional area of sample (mm2).
Stability studies were conducted to evaluate RC-101 content throughout the manufacturing process and storage. The storage conditions used were as follows: (i) room temperature (25 ± 2°C) and 60% ± 5% relative humidity (RH) and (ii) 40 ± 2°C and 75% ± 5% RH. At predetermined time points (0, 15, 30, 60, and 90 days), films (n = 3) were removed from the packaging container, cut into six pieces to obtain content uniformity information, and dissolved into 0.5 ml of Milli-Q water. Each solution was analyzed in duplicate by the HPLC-UV method described above. The total quantity of RC-101 in a single film was obtained by combining results obtained for the six individual pieces. The results for drug quantity for each film were expressed as a percentage of RC-101 detected compared to label claim.
In vitro efficacy studies using TZM-bl cells.
The baseline anti-HIV activity from the formulation components was evaluated by testing the in vitro activity against HIV-1. Excipients samples were prepared in water at the concentration equivalent to 1 film dissolved in 1 ml of water. Then each sample was further diluted with growth medium (GM) at ratios of 1:10, 1:20, and 1:40 (vol/vol). The activity of the RC-101 drug substance against HIV-1BaL was determined using TZM-bl reporter cells and quantification of luciferase expression as previously described (3). Briefly, cells (5,000/well; 96-well plates) were treated with 50 μl of either plain GM or GM containing the samples. To each well, 50 μl of GM containing HIV-1BaL (2 ng of p24/ml, multiplicity of infection = 0.02) was added. Supernatants were then removed, and cells were lysed with 100 μl of 1× Glo lysis buffer (Promega Corp., Madison, WI). The luciferase activity was measured by using a Bright Glo luciferase assay buffer (Promega). Protection from infection was measured as the percentage of reduction in luciferase activity, relative light units, compared to the HIV-1BaL-infected control (vehicle only, no peptide). To confirm that the observed reductions in luciferase activity were not due to nonspecific effects of RC-101 on the cells, the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays were performed on identically treated TZM-bl cells. As a control, RC-101 films at 100 μg (RC_F100) and RC-101 films at 2,000 μg (RC_F2000) were dissolved in 10 ml of GM and then processed as described above.
Ex vivo efficacy studies using human ectocervical tissue.
Human ectocervical tissues were obtained from premenopausal women undergoing hysterectomy. The ex vivo efficacy studies were set up using an organ culture system previously described (6, 15), except phytohemagglutinin-stimulated CD8-depleted peripheral blood mononuclear cells (PBMC; 5 × 105) were placed in the bottom chamber of the Transwell system (Corning, Inc., Corning, NY). A Transwell with agarose only in the top chamber served as a negative control, while a Transwell with the membrane only served as a positive control. To study the effect of RC-101 film on HIV-1 transmission, 100 μl of dissolved film was added to the tissue well for 1 h, after which cell-free HIV-1 BAL (50% tissue culture infective dose, 106) was added to the top chambers of the tissue and control wells, followed by incubation at 37°C for 3 to 4 days. After incubation, the top chamber of the well was removed, and culture of CD8-depleted cells in the bottom chamber was continued for an additional 10 days. Viral growth was monitored by measuring HIV-1 p24 antigen levels in the culture supernatant in the bottom chamber of the tissue well. A ≥3-fold increase in HIV-1 p24 during the 2-week period was considered a positive virus infection of the CD8-depleted cells. The transmission of virus in the tissue-containing wells was determined in comparison to the amount of virus passaged through the membrane in the control wells containing medium alone. Suppression of viral transmission by RC-101 was calculated by dividing transmission in the presence of RC-101 by that occurring in the absence of the drug. Suppression of viral transmission, expressed as the percentage of viral content in the control, was measured by monitoring viral infectivity of transmitted HIV-1 in the bottom well, determined at day 14 of culture.
Ex vivo toxicity studies. (i) Excised macaque reproductive tract.
Reproductive tracts from sexually mature female Macaca nemestrina were obtained from the tissue distribution program at the Washington National Primate Research Center. Prior approval for use of monkeys in this protocol was obtained from the Institutional Animal Care and Use Committee at the University of Washington. Animals were handled humanely, and experiments were performed within the National Institutes of Health's laboratory animal use guidelines. Whole reproductive tracts were collected at necropsy and transported to Magee-Womens Research Institute (Pittsburgh, PA) on ice within 24 h. Upon arrival, the tissue was warmed to 37°C and immediately used for the experiments.
(ii) Ex vivo toxicity experimental setup for monkey tissue.
The toxicities of placebo films and RC-101 films (100 μg; RC_F+LA) were evaluated in the fully excised monkey reproductive tract. The protocol was designed to simulate the experimental conditions used in an in vivo setting, wherein animals are sedated and remain in a horizontal position for about 30 min after microbicide application. For that reason, freshly excised monkey reproductive tissues (n = 2) were maintained in a horizontal orientation for the first 30 min after insertion of the film. After that time, the tissues were oriented vertically for another 90 min. For these assessments, excised monkey reproductive tracts were warmed to 37°C and placed in a 150-by-15-mm petri dish containing 2 ml of Milli-Q water. The film products were inserted into the vaginal cavity with a glass rod. Intact reproductive tract tissues were incubated for a total period of 2 h at 37°C. The tissues were then removed from the incubator and washed (4 ml of water, 10 times) by inserting a 5-ml syringe in the vaginal opening. A cytobrush was inserted and gently rotated six times into the cervical os. The cytobrush was then placed in 4 ml of water and vortexed. The solution from the cytobrush was tested for RC-101 content by HPLC; however, the RC-101 concentrations were below the detection limit of the assay. The tissues were cut open, and 6-mm biopsy samples (n = 3) were collected from each part of the tissue (lower vagina, middle vagina, upper vagina, transformation zone, endocervix, ectocervix, and uterus) and stained with hematoxylin and eosin (H&E) for histology.
(iii) Excised human ectocervical tissue.
Freshly excised human ectocervical tissue was obtained from the Tissue Procurement Facility at Magee-Womens Hospital (Pittsburgh, PA) according to the approved IRB protocol 0503103. Tissue was obtained from premenopausal women undergoing hysterectomy for benign conditions. No specimens were used when there was evidence of tissue abnormality that could influence the state of the mucosa. All tissue specimens were obtained within 1 h of surgical excision and held at 4°C in Dulbecco modified Eagle medium (DMEM; Mediatech, Inc., Herndon, VA) during transfer from surgery to the laboratory. Portions of tissue samples were retained for histological evaluation for comparison before and after the experiment (n = 2).
(iv) Ex vivo safety toxicity experimental setup for human tissue.
Ex vivo safety studies were conducted using a Franz cell system (Fig. 1) (10), a two-compartment system consisting of an upper chamber (donor compartment) and a lower chamber (receptor compartment). The Franz cells were water jacketed, and the temperature was maintained at 37°C throughout the experiment via a circulating water bath. DMEM was used in the receptor chamber, continuously stirred by a magnetic stir bar. Human ectocervical tissue was sandwiched between the two compartments with the epithelial surface facing the donor solution. The tissue was equilibrated with DMEM in the donor compartment for 5 min prior to the initiation of the safety study. After the equilibration period, DMEM was removed from the donor compartment and replaced with 100 μl of vaginal fluid simulant (22) and a 6-mm punch biopsy specimen of an RC-101-containing film (RC_F+LA, RC_F100, or RC_F+EDTA). After a 2-h period, tissue samples were removed and processed for histological evaluations.
Fig. 1.

Representation of a Franz cell system.
(v) Histological evaluations.
Histological evaluations were conducted on all tissue specimens tested. Retained tissue from each test specimen was fixed for histology and processed to compare with postexperimental histology. Tissue was fixed in Clark's solution (ethanol-acetic acid [75:25]) for 24 h, transferred to ethanol, incubated for 24 h, and subsequently embedded in paraffin. Tissue sections of 5 μm were cut and stained with H&E. Histology was conducted to evaluate gross alterations in tissue morphology caused by exposure to film product. Gross alterations that indicate damage to the tissue are characterized by a decrease in the number of nuclei, a reduction of squamous epithelium layers, and shrinking of epithelial cell size, as they have been observed in the positive control solution 4% nonoxynol-9 (data not shown).
Statistical analysis.
HPLC data obtained from the stability studies were expressed as the average percentage of the initial RC-101 concentration ± the standard deviation (n = 3). The results were analyzed by one-way analysis of variance comparing each time point to time zero values using a Dunnett correction to detect significant differences after adjustment for multiple comparisons. P values of ≤0.05 were considered to be statistically significant, unless specified otherwise. Bioactivity data were expressed as the average percent suppression of the virus (n = 2).
RESULTS
Formulation assessment.
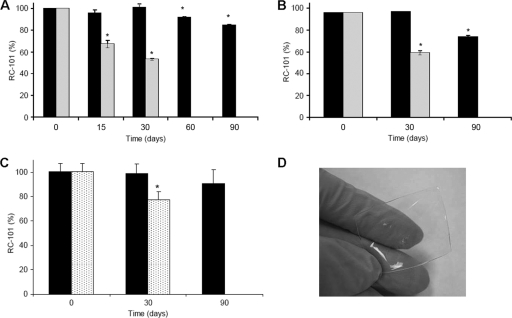
The appearance of all films (placebo, RC_F+LA, RC_F100, RC_F2000, and RC_F+EDTA) was clear, without bubbles, flexible, without sharp edges, and with a smooth surface. A summary of results of the films assessment tests for placebo, RC_F100, and RC_F2000 is presented in Table 1. An HPLC method was used to determine the concentration of RC-101 in each film. Figure 2A to C shows the percentages of initial RC-101 content (average ± the standard deviation) resulting from the stability study for RC_F+LA, RC_F100, and RC_F+EDTA films, respectively. In RC_F+LA and RC_F100, when stored at 40°C and 75% RH, a decrease in RC-101 was observed within the first month. In the films containing lactic acid (RC_F+LA), a 15% loss was observed after 90 days when stored at room temperature, and 50% loss of RC-101 was observed after 30 days when stored at 40°C and 75% RH. No RC-101 was detected in the films stored for more than 30 days at 40°C and 75% RH (Fig. 2A). In the film formulation without lactic acid (RC_F100), a 25% loss was observed after 90 days when stored at room temperature, and a 40% loss of RC-101 was observed after 30 days stored at 40°C and 75% RH. No RC-101 was detected in the films stored for more than 30 days at 40°C and 75% RH (Fig. 2B). In the film formulation containing EDTA (RC_F+EDTA), a 10% loss of RC-101 was observed after 90 days when stored at room temperature, and a 23% loss of RC-101 was observed after 30 days stored at 40°C and 75% RH. No RC-101 was detected in the films stored at 90 days at 40°C and 75% RH (Fig. 2C). It should be noted that although loss of the parent peak (RC-101) occurred over time, no secondary peaks were generated in the HPLC.
Table 1.
Summary of physical and chemical assessment of film formulation
| Parameter | Avg ± SDa |
||
|---|---|---|---|
| Placebo film | RC_F100 | RC-101_F2000 | |
| Dimension (mm) | 27.5 by 33.5 | 27.5 by 33.5 | 27.5 by 33.5 |
| Thickness (mm) | 0.192 ± 0.003 | 0.208 ± 0.005 | 0.10 ± 0.012 |
| Wt (mg) | 238.2 ± 9.3 | 240.2 ± 3.7 | 223.8 ± 1.5 |
| Disintegration (min) | 3.1 ± 0.5 | 4.8 ± 1.1 | 2.0 ± 0.1 |
| Water content (%) | 0.30 ± 0.04 | 0.46 ± 0.06 | 0.35 ± 0.06 |
| Tensile strength (N/mm2) | 5.22 ± 1.88 | 6.38 ± 1.30 | 3.98 ± 0.31 |
| Amt of RC-101 (% of label claim) | NA | 96.2 ± 3.8 | 89.6 ± 8.2 |
Results show averages ± standard deviations (n = 3) except for the dimension data. NA, not applicable.
Fig. 2.
Stability studies of RC-101 100 μg film over time. Samples were stored at 25°C (black) and at 40°C and 75% RH (dotted or gray). The graphs represent averages ± the standard deviation (SD). (A) RC_F+LA; (B) RC_F100. (C) RC_F+ EDTA. RC-101 films are stable for 1 month under 25°C storage condition. The results were analyzed by HPLC (n = 3, mean ± the SD). No RC-101 was detected at 60 and 90 days stored at 40°C and 75% RH. *, P < 0.002 (Dunnett analysis for multiple comparisons). (D) Representative picture of RC-101 100 μg film.
In vitro efficacy evaluations.
The toxicity and bioactivity of each excipient used in the film formulation and RC_F2000 was tested against TZM-bl cells. MTT results were expressed as the percent viability for each excipient used in the film formulation. The control for these studies was growth medium (GM). RC_F2000 film showed an MTT result greater than 90%. Most of the excipients showed no difference compared to the blank vehicle (water) used to dissolve the excipients. However, lactic acid showed toxicity compared to control by the MTT assay, evidenced by percentage viability in <30% of TZM-bl cells (data not shown). The bioactivity of each excipient was demonstrated by the percentage of HIV-1 suppression, an average of two determinations (n = 2). Duplicates did not differ by more than 10%. PVA and lactic acid were shown to be active against HIV, with HIV-1 suppression >70% for PVA and >95% for lactic acid for the initial dilution.
RC_F100, RC_F2000, and placebo films were tested in vitro against HIV using the TZM-bl cell model. RC_F100 films were dissolved and tested at 5 and 10 μg/ml (0.0026 and 0.0052 μM), and normal antiviral activity was observed. The percent HIV suppression results are shown in Fig. 3A for the RC_F2000 film. A second graph was plotted (Fig. 3B) using the log of the final RC-101 concentration (μM) of each dilution versus the percent suppression of HIV. Since the RC-101 synthesized for our studies was later determined to have a 40% lower concentration than the RC-101 utilized in earlier studies, the 90% inhibitory concentration (IC90) and the IC50 determined were calculated accounting for the 60% purity. The IC90 and IC50 values were determined from the graph as the concentrations that suppressed 90 and 50% of the HIV-1, respectively. Log calculations estimated an IC90 of 3.0 μM and an IC50 of 1.3 μM. RC-101_F2000 films after 1, 2, and 6 months were tested at the full concentration range (1.25 to 20 μg/ml), and normal antiviral activity was observed.
Fig. 3.
HIV-1 suppression (%) in vitro. (A) TZM-bl cell in vitro model for RC_F2000 (solid line) and placebo (dashed line) films (n = 3). Dilution factor represents how many times the sample was diluted in GM. (B) HIV-1 suppression (%) versus log RC-101 concentration (μM). RC_F2000 using TZM-bl cell in vitro model. (C) Human ectocervical tissue ex vivo model for the determination of activity of RC_F2000 and placebo film (n = 1). (D) HIV-1 suppression (%) versus log RC-101 concentration (μM). RC_F2000 using an ex vivo ectocervical tissue model. Graphs represent averages ± the SD.
Ex vivo efficacy evaluations.
RC_F2000 films were also tested using the ex vivo explant model of human ectocervical tissue, and the results are shown in Fig. 3C. Activity of RC_F2000 was observed up to a 20-fold dilution of the films. RC_F2000 films at 1:100 dilution still demonstrated some effectiveness against HIV-1. However, viral suppression at 1:100 dilution was ca. 60%, which is below the minimal suppression level required to be considered active against HIV-1 when using this ex vivo explant method (24, 26). A second graph was constructed by plotting the suppression of HIV-1 (%) versus the log of the final RC-101 concentration (μM) of each dilution (Fig. 3D). Since the suppression values were not lower than 60%, it was not possible to estimate the IC50; thus, only IC90 was calculated (56.2 μM). Notably, the IC90 result for RC_F2000 film using an ex vivo model represents a >18-fold increase over the IC90 observed for the same film when tested using an in vitro model (IC90 = 3.0 μM), which is likely a result of the 100-fold differences in the viral inocula between the two models. In addition, since the ex vivo model represents a more complex cellular environment than that present in the in vitro cell culture assay, like TZM-bl, it is expected that the IC90 value obtained in tissue models will be higher than that obtained in an in vitro cell culture. A similar situation is observed in in vivo evaluation where obtained IC90 values could increase up to 1,000-fold compared to that observed in cell culture.
Ex vivo toxicity evaluations.
Toxicity studies were conducted in excised M. nemestrina tissues. Figure 4A shows the full reproductive tract of the excised monkey tissue. Figure 4B shows the H&E-stained biopsy specimens obtained after exposure to RC_F+LA. No gross morphological changes were observed in any part of the reproductive tract after exposure to the films, suggesting that RC-101 and placebo formulations are safe to intact monkey reproductive tract tissues. In addition, no exposed and RC-101 exposed middle vaginal tissues are shown in Fig. 4C. H&E-stained human ectocervical tissues after 2 h exposure to RC_F100 and RC_F+EDTA films are shown in Fig. 5. No gross changes in morphology were observed for both samples compared to the preexposure tissue or DMEM exposure (negative control), suggesting that the RC-101 formulations may be safe to human ectocervical tissue.
Fig. 4.
Safety studies in excised Macaca nemestrina reproductive tract tissue. (A) Picture of a full reproductive tract of M. nemestrina before the experiments were conducted. (B) Histological evaluations of excised M. nemestrina reproductive tract tissue after exposure for 2 h with RC_F+LA. H&E staining was done. No gross changes in tissue morphology were observed after exposure to RC-101 film. (C) Samples not exposed and RC-101-exposed middle macaque vaginal tissue.
Fig. 5.
H&E-stained human ectocervical tissue after exposure to films using the Franz cell system. Images tissues are shown: preexposure, postexposure (2 h) to DMEM (negative control), postexposure (2 h) to RC_F100 film, postexposure (2 h) to RC_F+EDTA film, and postexposure (2 h) to 4% Nonoxynol-9 (positive control). No gross changes in tissue morphology were observed after exposure to RC-101 films, as evidenced by the integrity of the epithelium and the presence of the nuclei.
DISCUSSION
Film formulation for vaginal microbicide drug delivery has several advantages over alternative formulations. Compared to semisolid gels, film products are easier to apply, more quickly release the active drug, and can improve stability of drugs that are sensitive to water; they also do not suffer from the problems commonly associated with conventional vaginal gel dosage forms, including poor retention in the vaginal lumen, leakage, and messiness (8, 9, 13, 14, 25). There are, however, several drawbacks associated with film dosage forms. The predominant dosage form utilized for vaginal delivery of drugs are gel and cream products, and for this reason a greater level of familiarity exists for women with this dosage form. A second limitation to thin film drug delivery is an upper limit to drug loading in this dosage form. However, the peptide of interest in the present study is highly potent and furthermore there may be incompatibilities of peptide drugs with some conventional dosage forms. Based on these issues, as well as the advantages of vaginal films as a drug delivery system for microbicides, a polymeric film was selected as the final dosage form of RC-101.
RC-101 was formulated into a quick-dissolving PVA film. PVA is a polymer prepared from polyvinyl acetate, primarily used in topical pharmaceutical formulations. It is generally regarded as nontoxic and is approved by the U.S. Food and Drug Administration for vaginal use. RC-101 film formulation was optimized to obtain the most suitable formulation that would assure RC-101 stability over the desired shelf-life required for these studies. Initial development of a vaginal film included lactic acid to maintain the acidic pH in the vagina (RC_F+LA); however, no tests were conducted to confirm this idea. There is a certain limitation for a polymeric film dosage form to maintain the vaginal pH. In contrast to vaginal gels, the distribution and final volume of a film is dependent on the fluids present in the vaginal lumen.
Despite the fact that the film formulation was safe to be used in human ectocervical tissue, films proved to be toxic when used with TZM-bl cells for determination of bioactivity. When decomposition of the gel was conducted and individual excipients were analyzed, it was found that lactic acid was the component that was cytotoxic. Lactic acid was removed from the formulation to avoid any toxicity to the cells during evaluation of in vitro activity against HIV-1. Since RC-101 was shown to be stable over a range of pH 3 to 7 (data not published), there were no detrimental effects on drug stability in the formulation without lactic acid.
Evaluation of the stability of RC-101 in the film upon storage at 25°C and 40°C and 75% RH showed a loss of RC-101 content over time, indicating a short-term stability of this formulation. The second formulation prepared without lactic acid (RC_F100) also demonstrated short-term stability of RC-101 over time, at 25°C and at 40°C and 75% RH. A third formulation was prepared with the addition of EDTA as a chelating agent to delay the oxidation process of RC-101. EDTA is a common pharmaceutical excipient that sequesters metal ions with its carboxylate and amine groups. If the loss of RC-101 observed over time is due to the oxidation, EDTA would be able to protect against oxidation. Films prepared in the present study (RC_F+EDTA) contained a concentration of 0.05% EDTA and displayed no toxicity when exposed to human ectocervical tissue using single exposure in an ex vivo model. However, chronic exposure to human ectocervical tissue was not investigated and, depending on the dose regimen for this product (coital dependence), chronic exposure to the films should be evaluated. Preliminary studies in our laboratory have shown that prolonged exposure to EDTA is toxic to the human ectocervical tissue in vitro and may affect normal vaginal flora in a concentration of 0.1% in solution. Films prepared with EDTA (RC_F+EDTA) were more stable than the films without EDTA. However, a 10% loss of RC-101 was observed for films stored at room temperature for 90 days, which is remarkably stable for a peptide or protein.
In the HPLC analysis of all three film formulations, no additional peaks were observed that would indicate the mechanism of degradation of RC-101. The HPLC method used is a stability assay indicative of oxidative and thermal degradation pathways, suggesting that other mechanisms of decrease are happening. Interestingly, the bioactivity of RC-101 films after storage for 6 months is maintained, providing more than 90% suppression of the HIV-1 in vitro. This result indicates that the disappearance of the parent RC-101 HPLC peak can be attributed to a mechanism of degradation other than oxidation or thermal degradation and does not affect bioactivity. For example, RC-101 might be complexing or aggregating with the PVA polymer, decreasing detection of the parent peak by HPLC. Further investigations of RC-101 stability in films are being conducted to (i) confirm bioactivity of RC-101 films against HIV-1 over time to understand if modification of the drug compromises bioactivity, (ii) evaluate interaction of RC-101 with other polymers to verify compatibility, and (iii) identify other analytical methods that will facilitate extraction of RC-101 from the polymer.
In vitro HIV-1 efficacy studies have been used extensively to screen microbicide candidates. Most of the in vitro methods published are cell-based assays to determine the suppression of HIV-1 by an investigational compound (7, 24, 27). In our studies, we have investigated the efficacy of RC-101 films against HIV using an in vitro cell assay and an ex vivo human ectocervical tissue model. The results for in vitro assays showed an IC90 of 3.0 μM and an IC50 of 1.3 μM. In an earlier study that evaluated the inhibitory concentration of RC-101 tested against 27 primary HIV-1 isolates, the IC50 was reported as <1.25 μg/ml (0.66 μM) (23). Our results suggest that the formulation of RC-101 into films required a concentration two times higher than RC-101 in solution to achieve 50% viral inhibition. This increase in the IC50 may be attributed to the lower availability of RC-101 in the formulation compared to in solution.
RC-101 has shown several advantages compared to other peptide microbicides. RC-101 is not toxic and not inflammatory. The activity mechanism of RC-101, including the activity in cervicovaginal tissues, has been previously reported (2, 3, 11), and it has been shown to be between 1 and 2 μg/ml. One concern regarding the use of a peptide for a microbicide would be the cost of manufacture. When larger quantities are manufactured, the costs will go down abruptly, and so this does not represent a concern at this stage of development. Moreover, there are many aspects of RC-101 that are beneficial and might offset the costs: RC-101 has only modest levels of viral resistance, demonstrates adherence to mucosa (likely due to lectin properties), is active in vaginal fluid, does not become systemic, and retains ex vivo activity in cervicovaginal tissues and in cervical organ culture (2, 3, 5, 11).
More recently, ex vivo explant models using human ectocervical tissue (6, 20) have been investigated to assess the efficacy of microbicide candidates. In a study by Cole et al. (2), it was demonstrated that RC-101 (10 μM) suppressed HIV-1 infection by >90% in a biologically relevant explant model using cervicovaginal engineered epithelial tissue. In our studies, the formulated RC-101 film (RC_F2000) evaluated in an ex vivo model displayed an IC90 of 56.2 μM; these results suggest that formulation of RC-101 into films required a concentration approximately five times greater than RC-101 in solution in order to achieve 90% viral inhibition. Again, the increase in the IC90 may be attributed to the lower availability of RC-101 in the formulation compared to solutions, inherent differences in the tissue models utilized (infection model versus transmission model), or differences in the viral inocula for each model (∼100-fold greater in the organ-like cervical transmission model).
An important objective of pharmaceutical drug development is to correlate in vitro with in vivo results. The experiments conducted here have yielded the effective concentrations of RC-101 in the film formulation to inactivate 90% of HIV-1 in an in vitro and in an ex vivo model. The value obtained in the ex vivo model was 18-fold greater than that observed in the in vitro model. This difference in IC90 observed between the in vitro and ex vivo models is expected. In a cell-based assay (in vitro), target receptors for RC-101 are readily available, requiring a lower concentration of the peptide for the same inhibitory effect. In contrast, in the ex vivo model, RC-101 must penetrate the tissue to attach to CD4+ cells in addition to being available to attach to the glycoproteins in the virus, requiring a higher concentration to achieve the same level of inhibition. In addition, nonspecific binding of RC-101 to other proteins or to biological matrices in an ex vivo experiment may decrease the efficacy of the peptide.
Evaluation of tissue (vaginal and cervical mucosa) damage caused by-product exposure is part of the preclinical development of any successful vaginal formulation. Before the use of animal models, an in vitro or ex vivo model is necessary to assess the toxicity of a formulation. Excised macaque and human vaginal and ectocervical tissues have been extensively used in our laboratory to assess the changes in morphology after exposure to the vaginal microbicide solution or the final product formulation. None of the film formulations presented here induced gross changes in morphology in human or macaque excised ectocervical tissues, indicating the relative safety of the formulation to human tissue. Further studies are necessary to investigate the safety of the film formulation in animal models and to understand the consequences of the frequent use of the product.
Overall, we have described RC-101 film formulation development, efficacy results from a cell-based assay to an ex vivo model, and safety results from two ex vivo models. RC-101 was successfully formulated in a quick-dissolve polymeric film, which was demonstrated to be efficacious and safe in vitro and ex vivo. Stability and bioavailability of RC-101 in the film formulation over time require further investigation. Although efficacy studies in animals still need to be conducted, the findings of these studies provide a significant contribution toward the development of RC-101 into a successful topical microbicide product. In addition, the rational process of microbicide development presented here may be considered a template to be used for the systematic development of other microbicide molecules.
ACKNOWLEDGMENTS
We thank Kathleen J. Paul for her assistance in editing the manuscript. We thank Lindsey Ferguson for assistance in revising the manuscript. We thank Lindsay Mock from the Tissue Procurement Facility at Magee-Womens Hospital for the extra effort to obtain tissue for these studies.
This study was supported by NIH grant U19 AI065430 from the National Institute of Allergy and Infectious Diseases (NIAID).
The contents are of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID.
Footnotes
Published ahead of print on 14 February 2011.
REFERENCES
- 1. Coggins C., et al. 2000. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex. Transm. Infect. 76:480–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cole A. L., et al. 2007. The retrocyclin analogue RC-101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology 121:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole A. L., et al. 2006. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J. Immunol. 176:6900–6905 [DOI] [PubMed] [Google Scholar]
- 4. Cole A. M., et al. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. U. S. A. 99:1813–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole A. M., et al. 2010. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application on pigtailed macaques. PLoS One 5:e15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins K. B., Patterson B. K., Naus G. J., Landers D. V., Gupta P. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475–479 [DOI] [PubMed] [Google Scholar]
- 7. Dezzutti C. S., et al. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobaria N., Mashru R. 2009. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clindamycin phosphate. Pharm. Dev. Technol. 15:405–414 [DOI] [PubMed] [Google Scholar]
- 9. Dobaria N. B., Badhan A. C., Mashru R. C. 2009. A novel itraconazole bioadhesive film for vaginal delivery: design, optimization, and physicodynamic characterization. AAPS Pharm. Sci. Tech. 10:951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franz T. J. 1968. On the diffusion of tritiated water through skin. J. Invest. Dermatol. 50:260. [DOI] [PubMed] [Google Scholar]
- 11. Gallo S. A., et al. 2006. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 281:18787–18792 [DOI] [PubMed] [Google Scholar]
- 12. Garg S., et al. 2003. Development pharmaceutics of microbicide formulations. I. Preformulation considerations and challenges. AIDS Patient Care STDs 17:17–32 [DOI] [PubMed] [Google Scholar]
- 13. Garg S., et al. 2003. Development pharmaceutics of microbicide formulations. II. Formulation, evaluation, and challenges. AIDS Patient Care STDs 17:377–399 [DOI] [PubMed] [Google Scholar]
- 14. Garg S., et al. 2005. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm. Res. 22:584–595 [DOI] [PubMed] [Google Scholar]
- 15. Gupta P., et al. 2002. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 76:9868–9876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hardy E., Jimenez A. L., de Padua K. S., Zaneveld L. J. 1998. Women's preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packaging. Contraception 58:245–249 [DOI] [PubMed] [Google Scholar]
- 17. Harrison P. F., Rosenberg Z., Bowcut J. 2003. Topical microbicides for disease prevention: status and challenges. Clin. Infect. Dis. 36:1290–1294 [DOI] [PubMed] [Google Scholar]
- 18. Joglekar N. S., Joshi S. N., Navlakha S. N., Katti U. R., Mehendale S. M. 2006. Acceptability of Praneem polyherbal vaginal tablet among HIV uninfected women and their male partners in Pune, India: phase I study. Indian J. Med. Res. 123:547–552 [PubMed] [Google Scholar]
- 19. Johansson E. D., Sitruk-Ware R. 2004. New delivery systems in contraception: vaginal rings. Am J. Obstet. Gynecol. 190:554–559 [DOI] [PubMed] [Google Scholar]
- 20. Kawamura T., et al. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moench T. R., Chipato T., Padian N. S. 2001. Preventing disease by protecting the cervix: the unexplored promise of internal vaginal barrier devices. AIDS 15:1595–1602 [DOI] [PubMed] [Google Scholar]
- 22. Owen D. H., Katz D. F. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 23. Owen S. M., et al. 2004. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 20:1157–1165 [DOI] [PubMed] [Google Scholar]
- 24. Rohan L. C., Ratner D., McCullough K., Hiller S. L., Gupta P. 2004. Measurement of anti-HIV activity of marketed vaginal products and excipients using a PBMC-based in vitro assay. Sex. Transm. Dis. 31:143–148 [DOI] [PubMed] [Google Scholar]
- 25. Rohan L. C., Sassi A. B. 2009. Vaginal drug delivery systems for HIV prevention. AAPS J. 11:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sassi A. B., et al. 2007. Effects of physiological fluids on physical-chemical characteristics and activity of topical vaginal microbicide products. J. Pharm. Sci. 97:3123–3139 [DOI] [PubMed] [Google Scholar]
- 27. Turpin J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Invest. Drugs 11:1077–1097 [DOI] [PubMed] [Google Scholar]
- 28. UNAIDS. 2009. AIDS epidemic update, December 2009. World Health Organization, Geneva, Switzerland [Google Scholar]
- 29. van Damme L., et al. 2000. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex. Transm. Infect. 76:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van de Wijgert J., Coggins C. 2002. Microbicides to prevent heterosexual transmission of HIV: ten years down the road. Beta 15:23–28 [PubMed] [Google Scholar]
- 31. Wang W., Cole A. M., Hong T., Waring A. J., Lehrer R. I. 2003. Retrocyclin, an antiretroviral o-defensin, is a lectin1. J. Immunol. 170:4708–4716 [DOI] [PubMed] [Google Scholar]