Abstract
Ceftobiprole and ceftobiprole medocaril did not promote growth of or toxin production by Clostridium difficile in mouse cecal contents, whereas ceftazidime, cefoxitin, ceftriaxone, cefotaxime, and ertapenem did. The relatively low propensity of ceftobiprole to promote C. difficile was attributable to inhibitory activity against C. difficile and sparing of anaerobic microflora.
INTRODUCTION
Antimicrobial therapy promotes Clostridium difficile infection (CDI) due to disruption of the indigenous microflora of the colon, thereby allowing C. difficile to grow to high concentrations with production of toxin (13, 15). Nearly all classes of antibiotics have been associated with CDI, but clindamycin, broad-spectrum cephalosporins, and penicillins have traditionally been considered the agents that pose the greatest risk (3, 6, 13). With the emergence of the North American pulsed-field gel electrophoresis type 1 (NAP1) epidemic strain of C. difficile with increased resistance to fluoroquinolone antibiotics, fluoroquinolones have also been associated with CDI in multiple studies (6, 13). However, there remains some controversy regarding the relative importance of fluoroquinolones as a risk factor for CDI because these agents cause only minor disruption of intestinal anaerobes (1, 6, 13). There is some evidence that antibiotics with inhibitory activity against C. difficile (e.g., piperacillin-tazobactam and tigecycline) may be less likely to promote CDI, presumably because they inhibit growth of C. difficile in the colon during therapy (1, 6, 8, 9, 16). In mice, we demonstrated that piperacillin-tazobactam prevented the establishment of colonization by C. difficile during treatment but facilitated growth and toxin production when exposure to spores occurred after treatment during the period of recovery of the indigenous microflora (15).
Although cephalosporins are considered high-risk agents for promotion of C. difficile, there are significant differences among cephalosporins with regard to biliary excretion and anti-anaerobic activity (18). In general, the greatest risk for promotion of C. difficile has been associated with extended-spectrum cephalosporins that are excreted in significant concentrations into the intestinal tract (e.g., ceftriaxone and ceftazidime) (3, 18). In mice, we found that ceftriaxone promoted overgrowth and toxin production by C. difficile, whereas cefepime, an agent that is almost entirely excreted via the kidneys, did not (15). Because of the differences in routes of elimination and in vitro activity among cephalosporins, it is important that new cephalosporins be evaluated to assess their propensity to promote C. difficile and other healthcare-associated pathogens.
Ceftobiprole is a novel, broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus (2, 5, 7, 10). Ceftobiprole has the potential to promote C. difficile infection to a lesser degree than many other broad-spectrum cephalosporins for two reasons. First, ceftobiprole is primarily excreted via the kidneys, resulting in relatively low levels of intestinal exposure and only minor disruption of intestinal anaerobes (2, 10). Second, in comparison to other cephalosporins, ceftobiprole demonstrated relatively good activity against Clostridium species (MIC90 for Clostridium spp. = 4 μg/ml for ceftobiprole, 64 μg/ml for cefepime, >128 μg/ml for ceftazidime, 32 μg/ml for cefotaxime, and 16 μg/ml for cefoxitin), including some strains of C. difficile (5, 7). Here, we used a mouse model to test the hypothesis that ceftobiprole is less likely than other cephalosporins to promote establishment of colonization by C. difficile.
MATERIALS AND METHODS
C. difficile strains.
Four strains of C. difficile were studied. ATCC 43593 was a nontoxigenic strain from the American Type Culture Collection. The other strains were cultured from patients with CDI in Cleveland, OH. VA 17 and VA 20 were epidemic North American pulsed-field gel electrophoresis type 1 (NAP1) strains. VA 11 was a restriction endonuclease analysis (REA) J-type strain. Table 1 shows the MICs of the test antibiotics, as determined by broth dilution (11), for each of the strains.
Table 1.
MICs for the four C. difficile test strains
| Antibiotic | MIC (μg/ml)a |
|||
|---|---|---|---|---|
| ATCC 43599 | VA 11 | VA 17 | VA 20 | |
| Piperacillin-tazobactam | 2 | 2 | 8 | 8 |
| Ertapenem | 4 | 8 | 8 | 8 |
| Ceftobiprole | 1 | 1 | 2 | 1 |
| Ceftobiprole medocaril | 1 | 2 | 2 | 2 |
| Ceftazidime | 128 | >256 | 64 | 64 |
| Ceftriaxone | 8 | 128 | 64 | 64 |
| Cefotaxime | 16 | 128 | 64 | 64 |
| Cefoxitin | 128 | >256 | >256 | >256 |
| Clindamycin | >256 | >256 | >256 | >256 |
MICs were determined by broth dilution.
Growth of C. difficile in mouse cecal contents.
The mouse model was adapted from the hamster model of colonization resistance to CDI developed by Borriello et al. (4). These investigators demonstrated that antibiotics that promoted growth of and toxin production by C. difficile in cecal emulsions of hamsters also caused CDI in hamsters, whereas antibiotics that did not promote growth and toxin production in cecal contents did not cause disease (4). We have previously found that this model yields similar results in mice (1, 9, 15). The Animal Care Committee of the Cleveland Veterans Affairs Medical Center approved the experimental protocol.
Female CF-1 mice (8 per group) weighing 25 to 30 g (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages. Mice received daily subcutaneous injections (0.2-ml total volume) of saline, ceftobiprole (0.75 mg/day), ceftobiprole medocaril (i.e., the prodrug of ceftobiprole) (1.5 mg/day), ceftriaxone (2 mg/day), ceftazidime (3 mg/day), cefoxitin (3 mg/day), ertapenem (0.5 mg/day), clindamycin (1.4 mg/day), or piperacillin-tazobactam (8 mg/day) for 5 days. In some experiments, mice received daily ceftobiprole or ceftobiprole medocaril in combination with clindamycin. The antibiotic doses were equal to the usual human doses administered over a 24-h period (milligrams of antibiotic per gram of body weight).
Mice were killed by CO2 asphyxiation 6 h after the final antibiotic dose. The ceca were removed and opened longitudinally, and the contents were collected and transferred to an anaerobic chamber (Coy Laboratories, Grass Lake, MI) within 5 min. The cecal contents were diluted 3-fold (volume/volume) with sterile prereduced phosphate-buffered saline (PBS). A final concentration of 104 CFU/ml of each C. difficile strain was added to separate aliquots of cecal contents from individual mice. The C. difficile strains were prepared for inoculation by serially diluting 72-h broth cultures in sterile prereduced PBS. To quantify C. difficile, after incubation for 24 h, samples were diluted in sterile PBS and plated on prereduced cycloserine-cefoxitin-brucella agar containing 0.1% taurocholic acid and lysozyme at 5 mg/ml (12). To determine toxin production in cecal contents, a C. difficile Tox A/B II (Wampole Laboratories, Princeton, NJ) test kit was used according to the manufacturer's instructions.
Effect of antibiotic treatment on the concentrations of total anaerobes.
Aliquots of cecal contents collected 6 h after completion of the final dose of 4 days of subcutaneous antibiotic treatment were transferred to the anaerobic chamber. The contents were serially diluted in prereduced PBS and plated onto brucella agar (Becton Dickinson) to measure concentrations of total anaerobes. The lower limit of detection was ∼4 log10 CFU/g of stool.
Statistical analysis.
Data analyses were performed with Stata software (version 6.0; Stata, College Station, TX). A one-way analysis of variance was performed to compare the groups with P values adjusted for multiple comparisons using the Scheffe correction. Because each of the four strains yielded similar results, the data for the four strains were pooled for analysis for each antibiotic group.
RESULTS
Growth of C. difficile in mouse cecal contents.
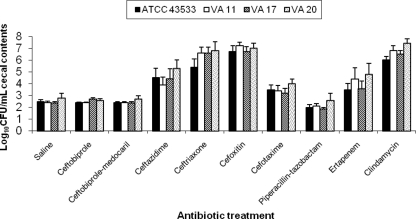
Figure 1 shows the effects of antibiotic treatment on growth of C. difficile in cecal contents collected 6 h after the final antibiotic dose. In comparison to saline controls, ceftazidime, ceftriaxone, cefoxitin, cefotaxime, ertapenem, and clindamycin promoted growth of C. difficile (P ≤ 0.01), whereas ceftobiprole, ceftobiprole medocaril, and piperacillin-tazobactam did not (P ≥ 0.68). For the toxigenic strains, C. difficile toxin was detected in ≥75% of all cecal content samples of mice treated with antibiotics that promoted growth of C. difficile, whereas none of the mice in the ceftobiprole, ceftobiprole medocaril, or piperacillin-tazobactam groups had detectable levels of toxin in cecal contents.
Fig. 1.
Effect of antibiotic treatment on growth of C. difficile in the cecal contents of mice. Mice received daily subcutaneous antibiotic treatment for 5 days. At 6 h after the final antibiotic dose, the cecal contents were collected and inoculated with 104 CFU of the C. difficile strains/ml. Samples were incubated anaerobically for 24 h, and then serial dilutions were plated onto selective media for quantification of C. difficile and assayed for toxin production. If C. difficile was not detected, the lower limit of detection (∼2 log10 CFU/g) was assigned. Error bars represent standard errors.
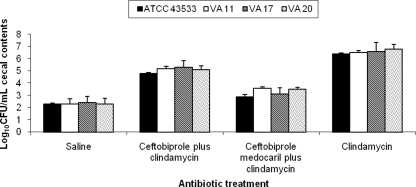
As shown in Fig. 2, administration of ceftobiprole and ceftobiprole medocaril in combination with clindamycin reduced clindamycin-induced overgrowth of C. difficile in cecal contents collected 6 h after the last dose of antibiotics (P < 0.001). Concentrations of C. difficile were significantly reduced in the ceftobiprole medocaril plus clindamycin versus the ceftobiprole plus clindamycin group (P < 0.01).
Fig. 2.
Effect of antibiotic treatment on growth of C. difficile in the cecal contents of mice. Mice received daily subcutaneous antibiotic treatment for 5 days. At 6 h after the final antibiotic dose, the cecal contents were collected and inoculated with 104 CFU of the C. difficile strains/ml. Samples were incubated anaerobically for 24 h, and then serial dilutions were plated onto selective media for quantification of C. difficile and assayed for toxin production. If C. difficile was not detected, the lower limit of detection (∼2 log10 CFU/g) was assigned. Error bars represent standard errors.
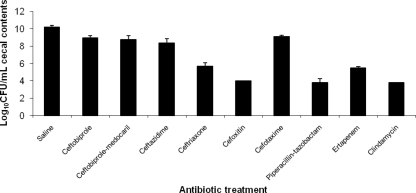
Figure 3 shows the effects of antibiotic treatment on concentrations of total anaerobes in cecal contents. The concentrations of total anaerobes were reduced significantly in each of the antibiotic treatment groups in comparison to the saline control group (P ≤ 0.02). In comparison to the ceftobiprole and ceftobiprole medocaril groups, concentrations of total anaerobes were significantly reduced in the ceftriaxone, cefoxitin, piperacillin-tazobactam, ertapenem, and clindamycin groups (P < 0.003), but not in the cefotaxime or ceftazidime groups (P ≥ 0.08).
Fig. 3.
Effect of subcutaneous antibiotic treatment on concentrations of total anaerobes in cecal contents of mice. Mice received daily subcutaneous antibiotic treatment for 5 days. At 6 h after the final antibiotic dose, the cecal contents were collected, transferred to an anaerobic chamber, and plated onto brucella agar to determine bacterial densities. Identical aliquots were plated onto brucella agar and incubated in room air to confirm than anaerobes were being measured. If organisms were not detected in stool, the lower limit of detection (4 log10 CFU/g) was assigned.
DISCUSSION
We found that ceftobiprole and the prodrug ceftobiprole medocaril did not promote growth of or toxin production by C. difficile in cecal contents of mice, whereas ceftazidime, cefoxitin, ceftriaxone, cefotaxime, and ertapenem did. In comparison to the other cephalosporins, ceftobiprole and ceftobiprole medocaril had much lower MICs for the C. difficile test strains (1 to 2 μg/ml versus 8 to >256 μg/ml). In addition, when ceftobiprole or ceftobiprole medocaril were combined with clindamycin, clindamycin-induced overgrowth of C. difficile was significantly reduced. Ceftobiprole and ceftobiprole medocaril also caused less suppression of total anaerobes in the cecal contents than did cefoxitin or ceftriaxone. These findings suggest that ceftobiprole may have a relatively low propensity to promote CDI in comparison to many other broad-spectrum cephalosporins due to greater inhibitory activity against C. difficile and reduced disruption of the anaerobic microflora.
The effects of the antibiotics studied on total anaerobes in the intestinal tract are consistent with findings of previous studies in human volunteers and patients (18). Cefoxitin and ceftriaxone achieve higher concentrations in the intestinal tract and/or have greater anti-anaerobic activity than do cefotaxime and ceftazidime and cause greater suppression of intestinal anaerobes measured in stool samples from volunteers or patients (18). The effects of ertapenem, piperacillin-tazobactam, and clindamycin on concentrations of anaerobes are consistent with previous studies in humans and in mice (9, 15, 18). Ceftobiprole was not detectable in stool of human volunteers and caused only minor suppression of intestinal anaerobes (2).
Our study has several limitations. First, growth of C. difficile in cecal contents was assessed at a single time point 6 h after the final dose of a 5-day course of treatment. The risk for establishment of colonization by C. difficile may vary depending on the timing of exposure in relationship to antibiotic dosing, the duration of antibiotic treatment, and the time required for recovery of the indigenous microflora (6, 13). Therefore, it is possible that our results could differ in clinical settings where the timing of exposure to C. difficile spores is unpredictable and duration of treatment is variable. Second, the pharmacokinetics of antibiotics differs significantly in mice and humans such that higher and more frequent dosing of mice is required to achieve equivalent systemic drug exposures (15). However, we have previously found that equivalent doses (mg/kg of body weight) result in similar levels of antibiotics in stool of mice and humans with similar effects on the indigenous microflora (1, 9, 15, 17). Third, the effects of antibiotics on C. difficile colonization and infection depends on multiple factors, including the antibacterial spectrum of activity against the indigenous microflora, the amount of excretion into the intestinal tract, the half-life of the agent, and the agent's activity against C. difficile (6, 13). The relative importance of each of these factors in patients has not been determined conclusively. In particular, data are needed to clarify whether systemic antibiotics with in vitro inhibitory activity against C. difficile (e.g., piperacillin-tazobactam, tigecycline, and ceftobiprole) are able to inhibit colonization by C. difficile in patients. Finally, the commercial enzyme immunoassay for toxins A and B used to evaluate toxin production in cecal contents has suboptimal sensitivity in comparison to cell culture cytotoxicity assays and also has less than ideal specificity (14).
In summary, we found that ceftobiprole was less likely than other broad-spectrum cephalosporins to promote the growth of and toxin production by C. difficile in the cecal contents of mice. If ceftobiprole is licensed for clinical use, further studies will be needed to evaluate its impact on colonization and infection with C. difficile in patients.
ACKNOWLEDGMENTS
This study was supported by a grant from Ortho-McNeil Pharmaceutical and by the Department of Veterans Affairs.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Adams D. C., Riggs M. M., Donskey C. J. 2007. Effect of fluoroquinolone treatment on growth of and toxin production by epidemic and non-epidemic Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 51:2674–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backstrom T., et al. 2010. Effect of ceftobiprole on the normal human intestinal microflora. Int. J. Antimicrob. Agents 36:537–541 [DOI] [PubMed] [Google Scholar]
- 3. Bignardi G. E. 1998. Risk factors for Clostridium difficile infection. J. Hosp. Infect. 40:1–15 [DOI] [PubMed] [Google Scholar]
- 4. Borriello S. P., Barclay F. E., Welch A. R. 1988. Evaluation of the predictive capability of an in-vitro model of colonization resistance to Clostridium difficile infection. Microb. Ecol. Health Dis. 1:61–64 [Google Scholar]
- 5. Ednie L., Shapiro S., Appelbaum P. C. 2007. Antianaerobe activity of ceftobiprole, a new broad-spectrum cephalosporin. Diagn. Microbiol. Infect. Dis. 58:133–136 [DOI] [PubMed] [Google Scholar]
- 6. Gerding D. N. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin. Infect. Dis. 38:646–648 [DOI] [PubMed] [Google Scholar]
- 7. Goldstein E. J. C., et al. 2006. In vitro activity of ceftobiprole against aerobic and anaerobic strains isolated from diabetic foot infections. Antimicrob. Agents Chemother. 50:3959–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson S., et al. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645–1651 [DOI] [PubMed] [Google Scholar]
- 9. Jump R. L., Li Y., Pultz M. J., Kypriotakis G., Donskey C. J. 2011. Tigecycline exhibits inhibitory activity against Clostridium difficile in the colon of mice and does not promote growth of or toxin production. Antimicrob. Agents Chemother. 55:546–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murthy B., Schmitt-Hoffmann A. 2008. Pharmacokinetics and pharmacodynamics of ceftobiprole, and anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 47:21–33 [DOI] [PubMed] [Google Scholar]
- 11. National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow anaerobically, 5th ed. Approved standard M7–A5. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 12. Nerandzic M. M., Donskey C. J. 2009. An effective and reduced cost modified selective medium for isolation of Clostridium difficile. J. Clin. Microbiol. 47:397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owens R. C., Donskey C. J., Gaynes R. P., Loo V. G., Muto C. A. 2008. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1):S19–S31 [DOI] [PubMed] [Google Scholar]
- 14. Peterson L. R., Robicsek A. 2009. Does my patient have Clostridium difficile infection? Ann. Intern. Med. 151:176–179 [DOI] [PubMed] [Google Scholar]
- 15. Pultz N. J., Donskey C. J. 2005. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 49:3529–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Settle C. D., Wilcox M. H., Fawley W. N., Corrado O. J. O. J., Hawkey P. M. 1998. Prospective study of the risk of Clostridium difficile diarrhoea in elderly patients following treatment with cefotaxime or piperacillin-tazobactam. Aliment. Pharmacol. Ther. 12:1217–1223 [DOI] [PubMed] [Google Scholar]
- 17. Stiefel U., Pultz N. J., Donskey C. J. 2007. Effect of carbapenem administration on establishment of intestinal colonization by vancomycin-resistant enterococci and Klebsiella pneumoniae in mice. Antimicrob. Agents Chemother. 51:372–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan A., Edlund C., Nord C. E. 2001. Effect of antimicrobial agents on the ecological balance of human microbiota. Lancet Infect. Dis. 1:101–114 15 [DOI] [PubMed] [Google Scholar]