Abstract
Several benzimidazole nucleoside analogs, including 1H-β-d-ribofuranosyl-2-bromo-5,6-dichlorobenzimidazole (BDCRB) and 1H-β-l-ribofuranosyl-2-isopropylamino-5,6-dichlorobenzimidazole (maribavir [MBV]), inhibit the replication of human cytomegalovirus. Neither analog inhibited the related betaherpesvirus human herpesvirus 6 (HHV-6). Additional analogs of these compounds were evaluated against both variants of HHV-6, and two l-analogs of BDCRB had good antiviral activity against HHV-6A, as well as more modest inhibition of HHV-6B replication.
INTRODUCTION
Two variants of human herpesvirus 6 (HHV-6A and HHV-6B) have been described and are highly prevalent in the population, with the B variant being the most common (29). Primary infection with HHV-6B has been shown to be the cause of exanthem subitum in infants (27). Reactivation of HHV-6 frequently occurs during immune suppression and is associated with fever, rash, pneumonia, encephalitis, bone marrow suppression, and graft rejection (2, 28). The susceptibility of HHV-6 to antiviral drugs is distinct from that of cytomegalovirus (CMV), although cidofovir (CDV), foscarnet, and ganciclovir (GCV) inhibit virus replication in vitro with very modest efficacy (4, 5, 15, 21, 26). Limited clinical data suggest that these compounds may provide some therapeutic benefit, but controlled studies are lacking (13). More effective agents are in various stages of development and include CMX001 (26), cyclopropavir (CPV), other methylenecyclopropane analogs (8, 12), arylsulfone derivatives (16), A-5021 (17), CMV423, and S2242 (3, 5, 15). However, there is an unmet medical need for more effective therapies as immunocompromised populations increase and a greater appreciation for diseases associated with HHV-6 is acquired.
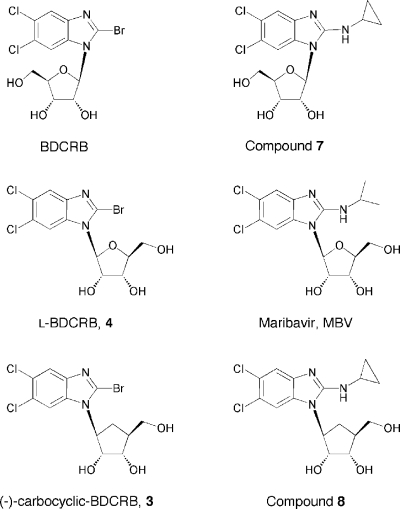
A number of benzimidazole analogs have been shown to be potent inhibitors of CMV replication (1, 6, 14, 22–25). One analog, 1H-β-d-ribofuranosyl-2-bromo-5,6-dichlorobenzimidazole (BDCRB), inhibits CMV genome cleavage/packaging, and mutations associated with resistance map to genes involved in this process, including UL56, UL89, and possibly UL104 (9–11, 24). A related d-ribopyranosyl analog, GW275175X, also appears to exert its antiviral effects against CMV by a similar mechanism (23). However, a structurally related l analog, 1H-β-l-ribofuranosyl-2-isopropylamino-5,6-dichlorobenzimidazole (maribavir [MBV]), inhibits viral replication through the specific inhibition of the CMV UL97 protein kinase. We hypothesized that other compounds in this series might have greater antiviral activity against HHV-6, so molecules related structurally to both MBV and BDCRB were evaluated against both HHV-6A and HHV-6B (Table 1). Structures of representative compounds are shown in Fig. 1.
Table 1.
Activity of benzimidazole analogs against HHV-6A and HHV-6B
| Compounda no. or name | 2-position | Isomer | HHV-6A (HSB-2 cells) |
HHV-6B (MOLT-3 cells) |
||||
|---|---|---|---|---|---|---|---|---|
| EC50b | CC50c | SId | EC50 | CC50 | SI | |||
| CDV | − | − | 0.33 ± 0.09 | 6.8 ± 3.4 | 21 | 2.3 ± 0.3 | 8.1 ± 4.1 | 3.5 |
| BDCRB | Br | d | 15 ± 5.2 | 32 ± 11 | 2.1 | >36 ± 22 | 50 ± 11 | <1.4 |
| 1 | Br | d, le | 31 ± 19 | 62 ± 6.5 | 2.0 | 45 ± 29 | 62 ± 18 | 1.4 |
| 2 | Br | d | 34 ± 24 | 59 ± 7.2 | 1.7 | 62 ± 3.8 | 67 ± 8.1 | 1.1 |
| 3 | Br | l | 5.5 ± 5.1 | 25 ± 7.6 | 4.5 | 15 ± 1.7 | 32 ± 28 | 2.1 |
| 4 | Br | l | 2.8 ± 0.7 | 11 ± 1.1 | 3.9 | 9.7 ± 1.5 | 14 ± 2.1 | 1.4 |
| 5 | Br | d | 23 ± 32 | 43 ± 50 | 1.9 | >30 ± 17.3 | 44 ± 27 | <1.5 |
| 11 | Br | d | >10 | 17 | <1.7 | 72 | 85 | 1.2 |
| 13 | Br | de | 10 | 15 | 1.5 | 55 | 80 | 1.5 |
| MBV | iPrNH2 | l | 60 ± 2.6 | >100 | >1.7 | >20 | 57 | <2.9 |
| 6 | cPrNH2 | d | >100 | >100 | 1 | >100 | >100 | 1 |
| 7 | cPrNH2 | d | 44 | 90 | 2.0 | >100 | >100 | 1 |
| 8 | cPrNH2 | l | >20 | 82 | <4.1 | >100 | >100 | 1 |
| 9 | cPrNH2 | d | >100 | >100 | 1 | >100 | >100 | 1 |
| 10 | iPrNH2 | d | >20 | 28 | <1.4 | 83 | >100 | >1.2 |
| 12 | iPrNH2 | df | >10 | 17 | <1.7 | >20 | 64 | <3.2 |
| 14 | iPrNH2 | lg | 62 | 68 | 1.1 | >100 | >100 | 1 |
Compounds evaluated (with compound number in parentheses) were 2-bromo-5,6-dichloro-1-([±]-carbocyclic)benzimidazole (1), 2-bromo-5,6-dichloro-1-([+]-carbocyclic)benzimidazole (2), 2-bromo-5,6-dichloro-1-([−]-carbocyclic)benzimidazole (3), 2-bromo-5,6-dichloro-1-(β-l-ribofuranosyl)benzimidazole (4), 2-bromo-5,6-dichloro-1-(β-d-ribopyranosyl)benzimidazole (5), 2-cyclopropylamino-5,6-dichloro-1-(β-d-5′-deoxyribofuranosyl)benzimidazole (6), 2-cyclopropylamino-5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole (7), 2-cyclopropylamino-5,6-dichloro-1-([−]-carbocyclic)benzimidazole (8), 2-cyclopropylamino-5,6-dichloro-1-(β-d-erythrofuranosyl)benzimidazole (9), 2-isopropylamino-5,6-dichloro-1-(β-d-erythrofuranosyl)benzimidazole (10), 2-bromo-5,6-dichloro-1-(β-d-erythrofuranosyl) benzimidazole (11), 2-isopropylamino-5,6-dichloro-1-(α-l-5′-deoxylyxofuranosyl)benzimidazole (12), 2-bromo-5,6-dichloro-1-(α-l-5′-deoxylyxofuranosyl)benzimidazole (13), 2-isopropylamino-5,6-dichloro-1-(α-d-5′-deoxylyxofuranosyl)benzimidazole (14). BDCRB and compounds 6, 7, 9, 12, 13, and 14 were synthesized in the laboratory of L. B. Townsend, University of Michigan, Ann Arbor, MI (6, 14, 22). The remaining experimental compounds were synthesized in the Burroughs Wellcome Research Laboratories, Research Triangle Park, NC, and provided via an agreement with the University of Michigan.
All values represent the concentration of compound (μM) required to reduce viral replication by 50% (EC50) obtained in at least two independent experiments, with standard deviation values shown.
Concentration (μM) sufficient to reduce cell viability by 50% (CC50) was determined in at least two independent experiments, with the standard deviation values shown.
The selectivity index (SI) is defined as the ratio of CC50 to EC50.
The conformation of “carbocyclic” nucleosides is properly designated “+” or “−.” d and l are used here for ease of comparison.
The sugar conformation of this α-l-xylosyl nucleoside resembles the β-d-conformation of a ribosyl nucleoside except that the orientation of the 5′-CH2OH is the same as the 2′- and 3′-OH groups, not the opposite as in BDCRB.
The sugar conformation of compound 14 resembles a β-l-ribosyl nucleoside such as MBV.
Fig. 1.
Structures of selected benzimidazole nucleoside analogs. Compound names and numerical designations are given in Table 1.
Antiviral activity was determined by methods reported previously (26). Briefly, uninfected HSB-2 or MOLT-3 cells were seeded into 96-well plates at a concentration of 104 cells/well in media supplemented with a range of compound concentrations and the infection was initiated with 103 HHV-6A-infected HSB-2 cells or HHV-6B-infected MOLT-3 cells. Cultures were incubated for 7 days, and a DNA hybridization assay was used to quantify viral replication. Cytotoxicity was evaluated under the same conditions using a CellTiter-Glo assay.
Results from in vitro antiviral studies identified two active compounds that were similar to BDCRB, with bromine in the 2-position but with modifications in the sugar moiety. The l-carbocyclic compound 3 as well as the l-ribosyl compound 4 exhibited good antiviral activity against HHV-6A and were the most active molecules against HHV-6B (Table 1). Both compounds were also active against CMV (50% effective concentrations [EC50s] of 1.3 and 3.8 μM, respectively), well tolerated in HSB-2 cells and MOLT-3 cells, and less toxic than CDV. The modest selective indices exhibited by these compounds, as well as the CDV control, are a characteristic of the cell lines used, which in our experience are very sensitive to cytotoxicity. The d-nucleoside analogs of BDCRB with sugar moieties other than ribose (compounds 5, 11, and 13) were essentially inactive against HHV-6 even though they have a 2-bromo substituent and are active against CMV (6). A second series that had a 2-iso- or 2-cyclopropyl amine in the 2-position, similarly to MBV, was evaluated, but they differed in the sugar moiety. None of the compounds in this series exhibited significant antiviral activity (EC50 < 20 μM) against HHV-6A or HHV-6B (Table 1).
Inhibition of HHV-6A DNA synthesis was evaluated further in the context of a high multiplicity of infection (MOI). Limited quantities of compound 4 precluded additional assays, although a sufficient quantity of compound 3 was available for further studies. The accumulation of viral DNA was quantified by real-time PCR using primers 5′-GTT AGG ATA TAC CGA TGT GCG TGA T-3′ and 5′-TAC AGA TAC GGA GGC AAT AGA TTT G-3′, with probe 5′-6FAM-TCC GAA ACA ACT GTC TGA CTG GCA AAA-TAMRA-3′ and plasmid pMP219 to provide absolute quantification. The CDV control inhibited viral DNA replication, with an EC50 of 1.4 ± 0.65 μM, whereas MBV was essentially inactive. Compound 3 also inhibited the accumulation of viral DNA, with an EC50 of 9.0 ± 2.4 μM suggesting that it inhibited viral replication by interfering with viral DNA synthesis.
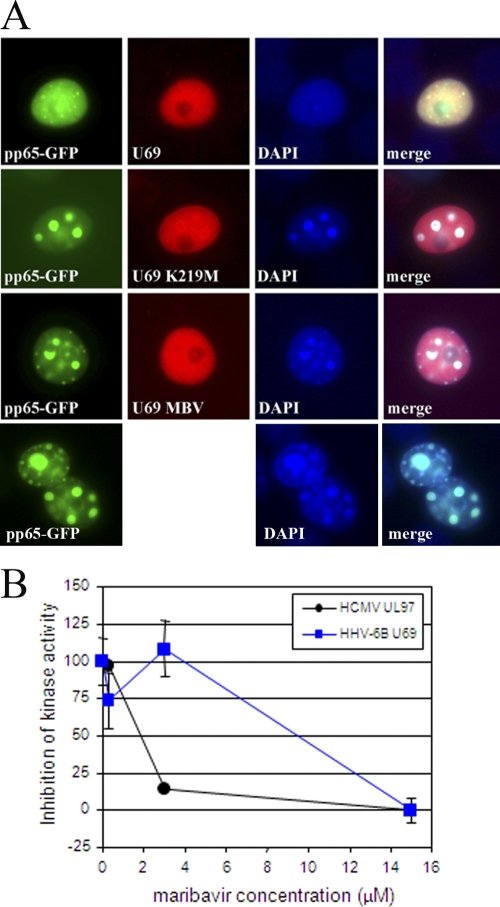
Inhibition of HHV-6 U69 protein kinase activity was also assessed using methods similar to those used to evaluate the CMV UL97 protein kinase (18, 20). The U69 kinase open reading frame was amplified from the Z29 strain of HHV-6B and cloned into pcDNA3.1 such that it contained a V5 epitope tag fused to the amino terminus (pMP258). The invariant lysine required for enzymatic activity (K219) was mutagenized to a methionine to generate a U69 protein without kinase activity (pMP344). Expression of U69 was confirmed using a V5 monoclonal antibody (Invitrogen, Carlsbad, CA), and aggresome formation was evaluated under a fluorescent microscope by determining the proportion of cells with aggresomes in at least 10 separate fields (18, 19).
Coexpression of U69 kinase with CMV pp65-GFP inhibited the formation of nuclear aggresomes, and this activity was indistinguishable from that exhibited by the CMV UL97 kinase (Fig. 2A, row 1 compared with row 4). This activity was also dependent on U69 kinase enzymatic activity, since the mutation of the invariant lysine (K219M) eliminated the ability of the kinase to inhibit aggresome formation (Fig. 2A, row 2). The addition of 15 μM MBV to cells expressing the active form of the U69 kinase also eliminated the ability of the kinase to inhibit aggresome formation, indicating that it inhibited kinase activity (Fig. 2A, row 3). Thus, both genetic inactivation and pharmacologic inhibition of U69 kinase confirmed that the assay was performing as expected. Its activity was investigated further in a dose-response study, and the results indicated that the inhibition of the U69 kinase assay required slightly higher concentrations of MBV than those required to inhibit UL97 (Fig. 2B). If the HHV-6 U69 kinase fulfills the same function as the CMV UL97 kinase, then it should be critical for viral replication and MBV would be predicted to be active. But since MBV is less active against CMV in proliferating cells, it is possible that MBV might exhibit improved activity against HHV-6 in resting lymphocytes rather than proliferating lymphocytes used in the standard antiviral assay. Indeed, the antiviral activity of MBV against HHV-6A in reduced serum concentrations (2% and 5%) was significantly improved, whereas the efficacy of CDV was totally unaffected (data not presented). These data indicated that MBV can inhibit the U69 kinase but that its impact on the replication of HHV-6 in rapidly proliferating lymphocytes is minimal. In contrast, compounds 3 and 4 did not inhibit U69 kinase activity.
Fig. 2.
Inhibition of aggresome formation by the HHV-6B U69 kinase activity. Inhibition of the HHV-6B U69 kinase activity was evaluated in a transient aggresome formation assay in COS7 cells (A). The formation of aggresomes induced by the CMV pp65-GFP fusion protein is inhibited by the enzymatic activity of the U69 kinase coexpressed in the cells (compare cotransfected cells in row 1 to those expressing only pp65-GFP in row 4). The kinase null K219M mutant was unable to inhibit aggresome formation (row 2). MBV at a concentration of 15 μM also eliminated the ability of the HHV-6B U69 kinase to inhibit aggresome formation and indicated that the drug inhibited its enzymatic activity (row 3). Both genetic and pharmacologic evidence shows that kinase activity is required to inhibit aggresome formation. (B) The proportion of cells with aggresomes was quantified by counting at least 10 separate fields at the concentrations shown, with error bars representing the standard deviation values. MBV inhibited both the HHV-6B U69 kinase and the CMV UL97 kinase but exhibited greater efficacy against the UL97 kinase.
These two l-ribosyl analogs of BDCRB (3 and 4) that had antiviral activity against HHV-6A also exhibited the best antiviral activity of the series against HHV-6B. Although the compounds were not as potent as CDV, they were less toxic and confirmed that this series of compounds may contain a compound that might be useful for the therapy of HHV-6 infections. Interestingly, both active compounds were similar to BDCRB in that they have bromine in the 2-position but differed since they are l-, not d-nucleoside analogs (Fig. 1). This clearly establishes a different structure-activity relationship (SAR) for HHV-6 than for CMV and predicted a different mechanism of action. This was confirmed in high-MOI studies with compound 3 which indicated that it inhibited viral DNA synthesis but did not inhibit the U69 kinase. However, these studies do not necessarily suggest that the DNA polymerase is the molecular target of compound 3 and it is possible that this is an indirect effect. Additional studies will be required to further define its activities and mechanism of action when more material becomes available.
Studies with the U69 kinase indicated that MBV was the only molecule that inhibited its activity. It is possible that the apparent inactivity of MBV against HHV-6 might be related to the high levels of cyclin-dependent kinases in the rapidly replicating lymphocytes. Cellular kinases are known to compensate for the loss of function of the CMV UL97 kinase, and this has been reported to impact the activity of MBV (7). The improved efficacy of MBV against HHV-6A in reduced serum conditions is consistent with this interpretation of the data, and it is possible that the drug might exhibit some activity in persons infected with HHV-6.
The results presented here confirm that both the sugar moiety and the substituent in the 2-position of the heterocycle impact the antiviral activity of benzimidazole nucleosides against the betaherpesviruses. The precise targets of the l-analogs of BDCRB in HHV-6 remain undefined, but the activity is clearly dependent of the conformation of the ribose. The further evaluation of additional analogs in this series will likely identify better inhibitors of HHV-6 replication and is a next logical step in the development of novel inhibitors of HHV-6 replication.
Acknowledgments
The compounds were provided and evaluated with support from Public Health Service contracts N01-AI-30049 and HHSN2722011000010C from the NIAID, NIH.
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Biron K. K., et al. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boeckh M., Erard V., Zerr D., Englund J. 2005. Emerging viral infections after hematopoietic cell transplantation. Pediatr. Transplant. 9(suppl. 7):48–54 [DOI] [PubMed] [Google Scholar]
- 3. De Bolle L., et al. 2004. Potent, selective and cell-mediated inhibition of human herpesvirus 6 at an early stage of viral replication by the non-nucleoside compound CMV423. Biochem. Pharmacol. 67:325–336 [DOI] [PubMed] [Google Scholar]
- 4. De Clercq E., Naesens L. 2006. In search of effective anti-HHV-6 agents. J. Clin. Virol. 37(suppl. 1):S82–S86 [DOI] [PubMed] [Google Scholar]
- 5. De Clercq E., et al. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381–395 [DOI] [PubMed] [Google Scholar]
- 6. Gudmundsson K. S., et al. 2000. Synthesis and antiviral evaluation of halogenated beta-D- and -L-erythrofuranosylbenzimidazoles. J. Med. Chem. 43:2464–2472 [DOI] [PubMed] [Google Scholar]
- 7. Hertel L., Chou S., Mocarski E. S. 2007. Viral and cell cycle-regulated kinases in cytomegalovirus-induced pseudomitosis and replication. PLoS Pathog. 3:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kern E. R., et al. 2005. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob. Agents Chemother. 49:1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komazin G., Townsend L. B., Drach J. C. 2004. Role of a mutation in human cytomegalovirus gene UL104 in resistance to benzimidazole ribonucleosides. J. Virol. 78:710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krosky P. M., et al. 2002. Phosphorylation of beta-d-ribosylbenzimidazoles is not required for activity against human cytomegalovirus. Antimicrob. Agents Chemother. 46:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krosky P. M., et al. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kushner N. L., et al. 2003. Efficacy of methylenecyclopropane analogs of nucleosides against herpesvirus replication in vitro. Nucleosides Nucleotides Nucleic Acids 22:2105–2119 [DOI] [PubMed] [Google Scholar]
- 13. Ljungman P., et al. 2007. Effectiveness of ganciclovir against human herpesvirus-6 excreted in saliva in stem cell transplant recipients. Bone Marrow Transplant. 39:497–499 [DOI] [PubMed] [Google Scholar]
- 14. Migawa M. T., et al. 1998. Design, synthesis, and antiviral activity of alpha-nucleosides: D- and L-isomers of lyxofuranosyl- and (5-deoxylyxofuranosyl)benzimidazoles. J. Med. Chem. 41:1242–1251 [DOI] [PubMed] [Google Scholar]
- 15. Naesens L., Bonnafous P., Agut H., De Clercq E. 2006. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 37(suppl. 1):S69–S75 [DOI] [PubMed] [Google Scholar]
- 16. Naesens L., et al. 2006. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antiviral Res. 72:60–67 [DOI] [PubMed] [Google Scholar]
- 17. Neyts J., Naesens L., Ying C., De Bolle L., De Clercq E. 2001. Anti-herpesvirus activity of (1′S,2′R)-9-[[1′,2′-bis(hydroxymethyl)-cycloprop-1′-yl]methyl] x guanine (A-5021) in vitro and in vivo. Antiviral Res. 49:115–120 [DOI] [PubMed] [Google Scholar]
- 18. Prichard M. N., Britt W. J., Daily S. L., Hartline C. B., Kern E. R. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494–15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prichard M. N., Daily S., Perry A. L., Kern E. R. 2008. Maribavir inhibits the replication of human herpesvirus 6 and the activity of the U69 protein kinase. Antiviral Res. 78:A29 [Google Scholar]
- 20. Prichard M. N., et al. 2008. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 82:5054–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reymen D., et al. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antiviral Res. 28:343–357 [DOI] [PubMed] [Google Scholar]
- 22. Townsend L. B., Devivar R. V., Turk S. R., Nassiri M. R., Drach J. C. 1995. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 38:4098–4105 [DOI] [PubMed] [Google Scholar]
- 23. Underwood M. R., et al. 2004. Mechanism of action of the ribopyranoside benzimidazole GW275175X against human cytomegalovirus. Antimicrob. Agents Chemother. 48:1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Underwood M. R., et al. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams S. L., et al. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams-Aziz S. L., et al. 2005. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamanishi K., et al. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065–1067 [DOI] [PubMed] [Google Scholar]
- 28. Yoshikawa T. 2003. Human herpesvirus-6 and -7 infections in transplantation. Pediatr. Transplant. 7:11–17 [DOI] [PubMed] [Google Scholar]
- 29. Zerr D. M., et al. 2005. A population-based study of primary human herpesvirus 6 infection. N. Engl. J. Med. 352:768–776 [DOI] [PubMed] [Google Scholar]