Abstract
Alpha-melanocyte-stimulating hormone (α-MSH) is an endogenous neuropeptide that is known for its anti-inflammatory and antipyretic activities. We recently demonstrated that α-MSH possesses staphylocidal activity and causes bacterial membrane damage. To understand the role of its amino acid sequences in the staphylocidal mechanism, in the present study we investigated the antimicrobial activities of different fragments of α-MSH, i.e., α-MSH(6-13), α-MSH(11-13), and α-MSH(1-5), and compared them with that of the entire peptide. Our results showed that peptides containing the C-terminal region of α-MSH, namely, α-MSH(6-13) and α-MSH(11-13), efficiently killed >90% of both methicillin-sensitive and -resistant Staphylococcus aureus cells in the micromolar range and ∼50% of these cells in the nanomolar range; their efficiency was comparable to that of the entire α-MSH, whereas the peptide containing the N-terminal region, α-MSH(1-5), was found to be ineffective against S. aureus. The antimicrobial activity of α-MSH and its C-terminal fragments was not affected by the presence of NaCl or even divalent cations such as Ca2+ and Mg2+. Similar to the case for the parent peptide, α-MSH(6-13) and α-MSH(11-13) also depolarized and permeabilized Staphylococcus cells (∼70 to 80% of the cells were depolarized and lysed after 2 h of peptide exposure at micromolar concentrations). Furthermore, scanning and transmission electron microscopy showed remarkable morphological and ultrastructural changes on S. aureus cell surface due to exposure to α-MSH-based peptides. Thus, our observations indicate that C-terminal fragments of α-MSH retain the antimicrobial activity of entire peptide and that their mechanism of action is similar to that of full-length peptide. These observations are important and are critical in the rational design of α-MSH-based therapeutics with optimal efficacy.
INTRODUCTION
The increasing drug resistance among bacterial pathogens such as Staphylococcus aureus has created a need for the development of new antimicrobial agents with novel mechanisms of action. Therefore, scientists from all over the world have been trying to solve this serious problem by applying different approaches.
One such approach is the use of natural host defense antimicrobial peptides, which are abundant in nature and which have been conserved across evolution as effective defense tools (45). These peptides are typically produced by immune cells, barrier cells, neutrophils, and epithelial cells (17, 20, 40), and in the host body they act by modulating the innate and adaptive immune responses (21). Despite considerable variation in characteristics such as size, structural motifs, and presence of disulfide bonds, host defense antimicrobial peptides generally are cationic and amphipathic in nature (16). Though different peptides kill pathogens by different mechanisms, their cationic and amphipathic nature allows them to interact with negatively charged microbial membranes (3, 6, 11, 26). Microbial membrane interaction with such peptides leads to several events, such as (i) formation of multimeric pores in the lipid bilayer of the cell membrane (11, 44), (ii) loss of ionic balance (7), and (iii) interaction with DNA or RNA (4, 5, 30, 37), all eventually resulting in cell death. Their broad-spectrum activity and unique mode of action and the fact that resistance against host defense antimicrobial peptides is very unlikely to develop make them promising candidates for a new class of antibiotics (35, 45).
Bacterial infections are most often accompanied by inflammation and fever, and therefore intrusion of a peptide which combines antimicrobial and anti-inflammatory properties could be of high benefit. One such antimicrobial peptide is alpha-melanocyte-stimulating hormone (α-MSH), an ancient linear tridecapeptide that is well recognized for its endogenous anti-inflammatory and melanogenic properties (8, 15, 29, 36). The peptide is a cleavage product of pro-opiomelanocortic precursor (POMC), which is expressed in the pituitary gland and in other cells such as neutrophils, monocytes, melanocytes, fibroblasts, and keratinocytes (9, 23, 24). The sequence of this neuropeptide is Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2. It controls inflammation and high temperature through immunomodulation, downregulates several proinflammatory cytokines, and stimulates production of anti-inflammatory cytokines such as interleukin-10 (IL-10) (2, 14, 23). The widespread distribution of this peptide and its receptor in many barrier cells such as keratinocytes and in various immune cells suggests its potential role in host defense. α-MSH also shares a number of similarities with other natural host defense antimicrobial peptides. For example, (i) it is cationic in nature, (ii) it adopts a helical structure in a membrane mimetic environment (32), (iii) it causes membrane permeabilization like known antimicrobial peptides (31), and (iv) it has broad spectrum of antimicrobial activity (10, 12, 18, 31).
Several reports, including ours, have recently demonstrated that α-MSH and its C-terminal sequence [Lys-Pro-Val, i.e., α-MSH(11-13)] have strong and rapid antimicrobial activity against methicillin-sensitive S. aureus (MSSA), methicillin-resistant S. aureus (MRSA), Escherichia coli, and Candida albicans (10, 12, 31). In our previous study we showed that α-MSH is active against staphylococcal biofilm and can retain its activity in whole blood, plasma, and serum (31). Our study also demonstrated that α-MSH damages the S. aureus membrane in a dose dependent manner (31). Studies by others have revealed that the candidacidal effect of α-MSH might be mediated through the induction of cyclic AMP (cAMP) (12). In addition, an analogue of α-MSH is already being studied in clinical trials to treat candidial vaginitis (18). A recent study suggests that the antibacterial activity of the C-terminal sequence of α-MSH is dependent not on the presence of a cationic Lys residue but on the Pro and Val residues (10). However, detailed studies on the contribution of different structural regions of α-MSH toward its antimicrobial activity, particularly the role of the core sequence α-MSH(6-9), which is common to all melanocortin peptides and responsible for binding to melanocortin receptors, have not yet been reported. In the present study, our main aim was to determine the role of the amino acid sequences, particularly the contributions of N-terminal and C-terminal regions, in the staphylocidal mechanism of α-MSH. We selected four sequences of α-MSH, i.e., the entire α-MSH, α-MSH(1-5), α-MSH(6-13), and α-MSH(11-13), and studied in detail their antistaphylococcal activities against both MSSA and MRSA strains. The study was also extended to understand the effect of NaCl and different ions (Mg2+ and Ca2+) on the killing efficacy of peptides against S. aureus. In our previous study we suggested that membrane damage might be partially responsible for staphylocidal activity of α-MSH (31). In the present study we investigated how the chosen fragments of α-MSH kill S. aureus and whether their mode of antibacterial activity recapitulates that of the entire peptide. Finally, we also examined the nature of the morphological and ultrastructural changes in S. aureus caused by exposure to these peptides in order to enhance our knowledge regarding the antimicrobial mechanism of action of α-MSH-based peptides. Such analyses are important for the rational design of α-MSH-based antimicrobial peptides with maximum potential.
(This study was presented in part at the International Conference on Physics Biology Interface, 13 to 16 December 2009, Saha Institute of Nuclear Physics, Kolkata, India.)
MATERIALS AND METHODS
Antimicrobial peptides.
α-MSH, α-MSH(1-5), α-MSH(6-13), α-MSH(11-13), gramicidin D, and calcein-AM were purchased/custom synthesized from Sigma-Aldrich (St. Louis, MO). DiBAC4(3) [bis-(1,3-dibarbituric acid)-trimethine oxanol] was purchased from Invitrogen. Glutaraldehyde was purchased from Merck (Germany). The purity of all peptides was 97%, and the concentrations of α-MSH, α-MSH(6-13), α-MSH(11-13), and α-MSH(1-5) were determined spectrophometrically (Cary 100 Bio/Varian) (31).
Bacterial strains.
MSSA strain ATCC 29213 and MRSA strain ATCC 33591 (39) were used in this study. The strains were stored at −70°C in 15% (vol/vol) glycerol until subcultured onto brain heart infusion (BHI) (Himedia Laboratories, India) agar plates or BHI broth for further analysis. Cultured cells were collected by centrifugation, washed with phosphate-buffered saline (PBS) (10 mM Na-K phosphate buffer with 150 mM NaCl, pH 7.4), and adjusted to the desired inoculum spectrophotometrically (an optical density at 600 nm [OD600] of 0.5 corresponds to 108 CFU/ml). The amount of dead bacteria was estimated microscopically using the Live/Dead BacLight bacterial viability assay kit (Invitrogen) (31).
Staphylocidal activities.
To determine the staphylocidal activities of peptides, mid-logarithmic-phase S. aureus cells were used. After being harvested, cells were washed in PBS (10 mM Na-K phosphate buffer with 150 mM NaCl, pH 7.4), and the cell density was adjusted to 104 to 107 CFU/ml, as desired, in the same buffer. All peptides studied were dissolved in PBS. Cells were then exposed to peptides at various concentrations as described elsewhere (31, 42). At selected time points, aliquots were plated on BHI agar and were incubated at 37°C overnight. Survival of S. aureus was determined by quantitative counting of the colonies and was expressed as mean percentage of survival versus that for a non-peptide-treated control (set at 100% survival). To determine the effect of Ca2+ and Mg2+ ions on the antibacterial activity of α-MSH and its fragments α-MSH(6-13) and α-MSH(11-13), physiological concentrations of CaCl2 (i.e., 2 mM) and MgCl2 (i.e., 1 mM) were included in phosphate buffer (PB) (10 mM Na-K phosphate buffer), pH 7.4 (28). Each experiment was performed at least three times in triplicates independently.
Membrane permeabilization.
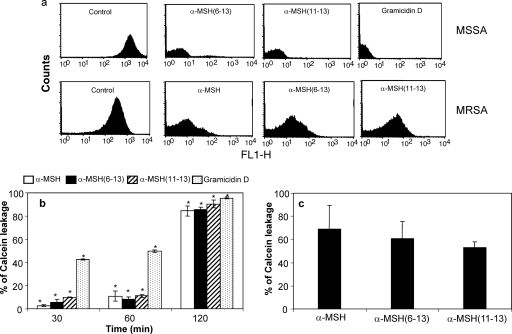
S. aureus membrane permeabilization following peptide exposure was measured via release of calcein using flow cytometry (BD FACSCalibur) as described in our previous report (31). In short, S. aureus cells were first loaded with calcein (2 μg/ml), and then calcein-loaded S. aureus cells were diluted to 106 CFU/ml and exposed to peptides (each at 1 μM) for 30 min, 60 min, and 120 min separately. Gramicidin D, a well-known membrane-targeting peptide (27), was used as a positive control. A total of 10,000 cells were acquired for each flow cytometry analysis. Cells at or above a threshold of 10 fluorescence units (FL1 units) were considered to have retained calcein, indicative of an intact cytoplasmic membrane; those cells exhibiting <10 FL1 units were interpreted to have lost calcein as a result of peptide-induced membrane permeabilization. Experiments were repeated at least three times independently on separate days.
Membrane depolarization.
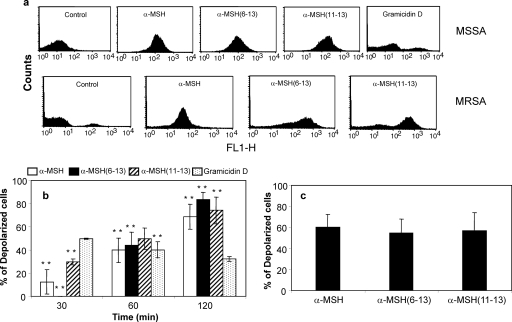
Mid-log-phase bacterial cells were diluted to 106 CFU/ml and incubated with α-MSH-based peptides (each at 1 μM) and gramicidin D (20 μg/ml) at 37°C for 30 min, 60 min, and 120 min. Cells were pelleted out, washed once with PBS (pH 7.4), and then incubated for 5 to 10 min at room temperature in dark with a membrane potential-sensitive dye, DiBAC4(3) (final concentration, 1 μM), whose excitation and emission wavelengths are 490 nm and 520 nm, respectively. DiBAC4(3) is an anionic lipophilic bis-oxonol dye which can sense the change in membrane potential and enters into depolarized cells. When the membrane potential decreases, oxonol fluorescence increases, and hence a shift in the fluorescence peak is obtained. A total of 10,000 cells were analyzed in each sample, using the Cell Quest software (BD FACSCalibur) for data acquisition and analysis (37). Experiments were repeated at least three times independently on separate days.
SEM.
Bacterial cells were first grown to mid-log phase, harvested by centrifugation at 4,000 rpm for 10 min, and resuspended in PBS (pH 7.4) to yield 108 CFU/ml. As a higher density of cells was required for cell imaging, different concentrations of peptides were used to ensure lethal activity of the studied peptides. For comparison, two different concentrations of gramicidin D were used. Thus, the bacterial suspension was incubated with 12 μM and 50 μM α-MSH-based peptides and 2 μg/ml and 20 μg/ml of gramicidin D in PBS (pH 7.4) for 2 h. After incubation, cells were spun down at 6,000 rpm for 10 min, washed with PBS (pH 7.4) several times, and fixed with 2.5% glutaraldehyde in the same buffer overnight at 4°C. After fixation, cells were washed two or three times in 0.1 M PB (pH 7.4), and dehydrated in series of graded ethanol solutions (30% to 100%), and finally dried in a desiccator under vacuum. An automatic sputter coater (Polaron OM-SC7640) was used for coating the specimens with 20-nm gold particles. Then samples were viewed via scanning electron microscopy (SEM) (EVO 40; Carl Zeiss, Germany) (13).
TEM.
Transmission electron microscopy (TEM) was performed according to the procedure described elsewhere with little modification (1, 22). In brief, S. aureus cells (ATCC 29231) were grown to mid-log phase and harvested, and the cell density was adjusted to 109 CFU/ml (OD600 = 1) in PBS. The cell suspension was incubated with a final concentration of 50 μM each of α-MSH-based peptide and 20 μg/ml of gramicidin D at 37°C for 2 h. After peptide treatment, cells were spun down, washed three times in 0.1 M sodium potassium phosphate buffer (pH 7.4), and fixed in 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium potassium phosphate buffer (pH 7.4) overnight at 4°C. The samples were then postfixed in 1.0% (wt/vol) osmium tetroxide, followed by staining with uranyl acetate as a heavy-metal stain. Samples were then dehydrated in graded acetone and embedded in epoxy resin (Araldite CY212). Each sample was thin sectioned using a microtome (Leica EM UC6), transferred onto a copper grid, and stained with uranyl acetate (saturated solution of uranyl acetate in 50% alcohol) followed by lead citrate. Samples were washed three times in Milli-Q (MQ) water and dried by touching Whatman filter paper. Sections were viewed in an electron microscope (Zeol-JEM 2001, Japan) at 120 keV. For each sample, three grids were prepared separately.
Statistical analysis.
All killing experiments were performed in triplicate and repeated in three independent experiments on different days, and results were plotted as mean ± standard deviation (SD). The rest of the assays were performed as three independent experiments on different days, and results were plotted as mean ± SD. Statistical analysis (multiple comparison among data sets) was performed using one-way analysis of variance (ANOVA) with Minitab (10, 31). A P value of ≤0.05 was considered significant.
RESULTS
Antibacterial activity of α-MSH-based short peptides against MSSA.
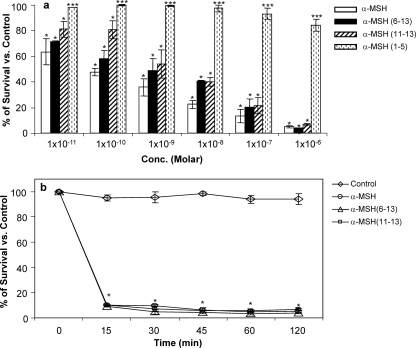
S. aureus ATCC 29213 (104 CFU/ml) was treated with a broad range of concentrations (10−11 to 10−6 M) of α-MSH, α-MSH(6-13), α-MSH(11-13), and α-MSH(1-5) for 120 min in 10 mM PBS, pH 7.4. The percentage of survival versus the control is presented in Fig. 1a. α-MSH and both of its C-terminal fragments, α-MSH(6-13) and α-MSH(11-13), exhibited substantial antimicrobial activity against S. aureus at all concentrations tested. The staphylocidal activities of these C-terminal fragments were comparable to those of the entire peptide α-MSH in the micromolar range (≥95%), while at picomolar concentrations the full-length peptide was found to be more active. For example, 1 μM α-MSH, 1 μM α-MSH(6-13), and 1 μM α-MSH(11-13) killed 95% ± 0.97%, 96.2% ± 0.46%, and 97.3% ± 0.98% of the cells, respectively, in 2 h (P < 0.001), whereas 10 pM α-MSH, 10 pM α-MSH(6-13), and 10 pM α-MSH(11-13) exhibited 35% ± 9.98%, 29% ± 0.65%, and 19% ± 6.18% bacterial killing, respectively (P < 0.001). In contrast, the N-terminal fragment α-MSH(1-5) could show only 16% ± 4.3% bacterial killing in the micromolar range, while 100% bacterial survival was obtained in the picomolar range. The killing by α-MSH(1-5) was significantly lower than those observed with α-MSH and its C-terminal fragments (P < 0.001). In addition to killing potency, to get an idea about the time required for killing of bacteria by the peptides, Staphylococcus cells were incubated with α-MSH and C-terminal fragments α-MSH(6-13) and α-MSH(11-13) for the indicated times (0 to120 min) (Fig. 1b). The data suggest that the killing activities of all the peptides were extremely rapid, and ∼90% of cells were killed within 15 min of incubation. Interestingly, incubation for longer periods of time did not substantially increase the staphylocidal activity (P < 0.001 compared to PBS control). There was no significant loss in viability of S. aureus cells in control PBS over the 2-h period of incubation (Fig. 1b).
Fig. 1.
(a) Killing activities of α-MSH and its fragments, at different concentrations, against logarithmic-phase MSSA ATCC 29213 cells after 120 min of peptide administration. These data represent the means (±SDs) from three independent experiments. Multiple comparisons among data sets indicate significant changes (*, P < 0.001; **, P < 0.01;***, P < 0.05). (b) Killing activities of α-MSH and its C-terminal fragments (each at 1 μM), at different time points from 0 min to 120 min, against MSSA ATCC 29213. These data represent the means (±SDs) from three independent experiments. *, P < 0.001 compared to PBS control.
Activity of α-MSH and its C-terminal fragments against MRSA.
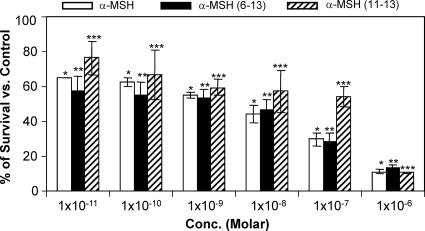
Staphylocidal activities of α-MSH and its C-terminal fragments against prototype MRSA strain ATCC 33591 (39) were determined (Fig. 2). Similar to the case for the MSSA strain, the MRSA strain was also found to be highly susceptible to the studied peptides in the entire concentration range. For example, ∼90% of MRSA cells were killed by α-MSH and its C-terminal fragments after 2 h of incubation with 1 μM of each peptide. With a lower concentration of peptide, e.g., 10 pM, the percentages of survival were 64 ± 0.06, 57 ± 8.8, and 76 ± 9.3 for α-MSH, α-MSH(6-13), and α-MSH(11-13), respectively. The observed killing was found to be significant for the entire range of concentrations of each peptide (P < 0.05).
Fig. 2.
Killing activities of α-MSH and its fragments, at different concentrations, against logarithmic-phase MRSA ATCC 33591 cells after 120 min of peptide administration. These data represent the means (±SDs) from three independent experiments. Multiple comparisons among data sets indicate significant changes (*, P < 0.001; **, P < 0.01;***, P < 0.05).
Staphylocidal activity with varying bacterial cell density.
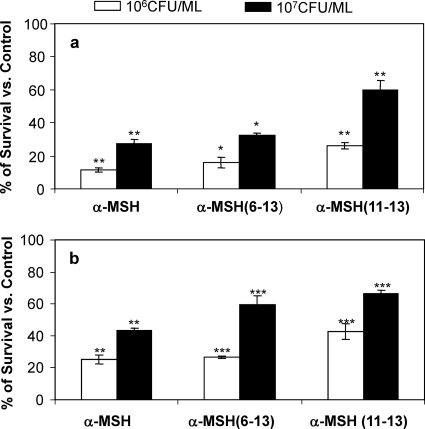
The initial antimicrobial assay was performed with a lower S. aureus cell density (104 CFU/ml) (Fig. 1 and 2). In order to investigate whether the antibacterial effect of α-MSH was influenced by bacterial cell number, higher densities of MSSA and MRSA cells (106 and 107 CFU/ml) were incubated for 2 h with different α-MSH peptides in PBS, pH 7.4 (Fig. 3a and b). As can be seen in Fig. 3, the bactericidal effects of α-MSH, α-MSH(6-13), and α-MSH(11-13) were decreased when higher numbers of bacterial cells were used. Thus, it was observed that there was ≥95% killing by all α-MSH-based peptides (each at 1 μM) when 104 CFU/ml MSSA cells were used (Fig. 1a). However, 73% ± 2.7%, 67% ± 1.04%, and 40% ± 5.7% inhibition was observed when 107 CFU/ml MSSA cells were exposed to same concentration of α-MSH, α-MSH(6-13), and α-MSH(11-13), respectively (Fig. 3a). Similarly, in the case of the MRSA strain, the killing by all the peptides was ∼90% when 104 CFU/ml was used (Fig. 2), while the killing was reduced to 75% ± 3%, 74% ± 0.66%, and 68% ± 4.9% with α-MSH, α-MSH(6-13), and α-MSH(11-13), respectively, for 106 CFU/ml MRSA cells and to 67% ± 1.4%, 41% ± 5.5%, and 33% ± 1.9% with α-MSH, α-MSH(6-13), and α-MSH(11-13), respectively, for 107 CFU/ml (Fig. 3b). These differences reached statistical significance for all the tested peptides (P < 0.005, comparing data for 107 CFU/ml versus 106 CFU/ml versus 104 CFU/ml for both MSSA and MRSA).
Fig. 3.
Killing activities of α-MSH, α-MSH(6-13), and α-MSH(11-13) against logarithmic-phase S. aureus MSSA ATCC 29213 (a) and MRSA ATCC 33591 (b) cells at bacterial densities of 106 and 107 CFU/ml. These data represent the means (±SDs) from three independent experiments. *, P < 0.001; **, P < 0.01,***, P < 0.05 (comparing data for 107CFU/ml versus 106 CFU/ml versus 104 CFU/ml).
Antibacterial activity in the presence of different salts.
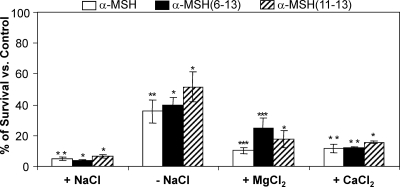
We previously showed that the antibacterial activity of α-MSH was not inhibited in the presence of physiological concentrations of NaCl (31). In this study, the antibacterial activities of α-MSH, α-MSH(6-13), and α-MSH(11-13) were examined in 10 mM Na-K phosphate buffer with no salt and in the presence of 150 mM NaCl (i.e., PBS), 1 mM Mg2+, and 2 mM Ca2+ (Fig. 4). It was observed that all the peptides were potentially active in the presence of physiological concentrations of salts, whereas killing was comparatively lower on the removal of salt (Fig. 4). Thus, in PBS killing was ≥95% for all the peptides, and in the presence of 1 mM Mg2+, α-MSH, α-MSH(6-13), and α-MSH(11-13) it was 90% ± 1.8% (P = 0.035), 81.6% ± 5.3% (P = 0.036), and 85% ± 1.3% (P < 0.001), respectively. Similarly in the presence of 2 mM Ca2+, α-MSH, α-MSH(6-13), and α-MSH(11-13) demonstrated 88.5% ± 2.9% (P = 0.017), 88% ± 0.32% (P = 0.008), and 84.33% ± 0.72% (P < 0.001) killing, respectively. In the absence of salt, killing was reduced by ∼30% for all the peptides. Taken together, the data suggested that all three peptides possess potential killing activity in the presence of physiological concentrations of NaCl, Mg2+, and Ca2+.
Fig. 4.
Killing efficacies of α-MSH and its C-terminal fragments, under different microenvironmental conditions, against MSSA cells after 2 h of incubation with 1 μM peptide. Killing activities of α-MSH, α-MSH(6-13), and α-MSH(11-13) in phosphate buffer without salt (−NaCl), with 150 mM NaCl (i.e., PBS) (+NaCl), with 1 mM Mg2+ (+MgCl2), and with 2 mM Ca2+ (+CaCl2) were measured. Each experiment was done thrice on independent days, and values represent means ± SDs. *, P < 0.001; **, P < 0.01;***, P < 0.05 (comparing data for different salts with those for phosphate buffer).
Membrane permeabilization by the C-terminal fragments α-MSH(6-13) and α-MSH(11-13).
To analyze whether the C-terminal fragments α-MSH(6-13) and α-MSH(11-13) killed S. aureus by membrane permeabilization like the entire α-MSH, flow cytometry studies using calcein-AM were performed with both MSSA and MRSA cells (Fig. 5). As demonstrated in Fig. 5a, exposure of MSSA cells to C-terminal peptides α-MSH(6-13) and α-MSH(11-13) caused a shift in peak similar to that with the entire α-MSH and gramicidin D, indicating release of calcein due to membrane perturbation by both peptides. MRSA strains also exhibited leakage of calcein when exposed to α-MSH and its C-terminal peptides (Fig. 5a). Consistent with our previous observations (31), treatment of MSSA ATCC 29213 with 1 μM α-MSH for 2 h caused 84% calcein leakage compared to that for untreated calcein-loaded control samples. Interestingly, the time-dependent profiles (30 min to 120 min) of calcein leakage from MSSA cells caused by all the three peptides (each at 1 μM) showed different amounts of calcein release (Fig. 5b). For instance, 2.6% ± 0.6%, 5.8% ± 2.2%, and 9.92% ± 0.2% calcein was released after 30 min of α-MSH, α-MSH(6-13), and α-MSH(11-13) treatment, respectively, and after an increase in incubation time from 30 min to 60 min there was little increase in calcein leakage. However, a 120-min exposure to all three peptides caused a dramatic increase in calcein release from S. aureus cells, comparable to that with gramicidin D. Thus, after 120 min of peptide exposure, 84% ± 4.1%, 85% ± 2.1%, 90% ± 3.3%, and 95.4% ± 0.79% calcein release was observed for α-MSH, α-MSH(6-13), α-MSH(11-13), and gramicidin D, respectively. These differences in the percentage of membrane permeabilization over time were found to be statistically significant for each peptide (P < 0.001, comparing data for 30 min versus 60 min versus 120 min). As shown in Fig. 5c, the calcein leakage from MRSA ATCC 33591 cells on exposure to 1 μM α-MSH-based peptides ranged from 50 to 70%.
Fig. 5.
Membrane permeabilization of S. aureus ATCC 29213 by α-MSH(6-13) and α-MSH(11-13). (a) Calcein leakage assay. Logarithmic-phase MSSA ATCC 29213 and MRSA ATCC 33591 cells were labeled with calcein and analyzed for membrane permeabilization after incubation with α-MSH-based peptides and gramicidin D. A total of 10,000 cells were acquired for each flow cytometry analysis. Cells at or above a threshold of 10 fluorescence units (FL1 units) were considered to have retained calcein, indicative of an intact cytoplasmic membrane; those cells exhibiting <10 FL1 units were interpreted to have lost calcein as a result of α-MSH-induced membrane permeabilization. (b) Time-dependent changes in membrane permeabilization of MSSA ATCC 29213 by α-MSH, α-MSH(6-13), α-MSH(11-13), and gramicidin D as quantified by calcein leakage using flow cytometry. The data show that 30-min and 60-min treatment with all the tested peptide could cause only ∼10% calcein leakage, which was followed by sharp increase in calcein release after 120 min of peptide treatment. These data represent the means (±SDs) from three independent experiments. *, P < 0.001 (comparing data for 30 min versus 60 min versus 120 min). (c) Percentage of calcein leakage from MRSA ATCC 33591 on exposure to 1 μM α-MSH, α-MSH(6-13) and α-MSH(11-13). These data represent the means (±SDs) from three independent experiments.
Membrane depolarization by α-MSH-based antimicrobial peptides.
Previous studies have reported that various membrane-targeting antimicrobial peptides, such as nisin LL-37, human neutrophil peptide-1 (HNP-1), and gramicidin D, also result in membrane depolarization (19, 34, 43). In order to analyze whether α-MSH and its C-terminal fragments cause membrane depolarization, peptide-treated S. aureus cells were incubated with the membrane potential-sensitive dye DiBAC4 (3) and compared with untreated control cells. As shown in Fig. 6a, exposure of all three peptides to both MSSA and MRSA strains for 120 min caused uptake of DiBAC4 (3) inside the cells and resulted in an increase in the fluorescence signal, indicating depolarization of the bacterial membrane. It was also observed that all three peptides led to a change in the membrane potential of MSSA cells over time (Fig. 6b). Thus, 1 μM α-MSH caused 13%, 40%, and 68% depolarized bacteria after 30, 60, and 120 min of incubation, respectively. Similarly, both C-terminal fragments caused 0 to 30% depolarization after 30 min of incubation, whereas the percentage of depolarized cells was enhanced to approximately 70 to 83% after 120 min of incubation. Similar peptide-induced membrane depolarizations were also observed for the MRSA strain as well (Fig. 6c). All three α-MSH-based peptides (each at 1 μM) caused 55 to 60% depolarized cells after 2 h of exposure. This indicated that like other membrane-targeting peptides (e.g., HNP-1 [37]), α-MSH and its C-terminal fragments α-MSH(6-13) and α-MSH(11-13) also depolarized the S. aureus cell membrane along with causing membrane permeabilization. In contrast, gramicidin D caused less depolarization at longer time points, although membrane depolarization at 30 min was higher than that for α-MSH-based peptides. These data reflect an immediate depolarization of the staphylococcal membrane upon peptide binding followed by complete leakage of cell material, which was also revealed by SEM and TEM studies (see below). The difference in depolarization with time was found to be statistically significant for each peptide (P < 0.05, comparing data for 30 min versus 60 min versus 120 min).
Fig. 6.
Membrane depolarization of S. aureus by α-MSH, α-MSH(6-13), and α-MSH(11-13). (a) Depolarization of the bacterial membrane leads to uptake of the anionic dye DiBAC4(3), resulting in increase in the fluorescence signal. MSSA and MRSA were incubated without peptide (control) or with a 1 μM concentration of each peptide for 2 h and then incubated with DiBAC4(3) and analyzed by flow cytometry, with a total of 10,000 cells being acquired for analysis. Cells below 10 FL1 units were considered unloaded, and those above 10 FL1 units were considered loaded. Histograms show uptake of DiBAC4(3) by both MSSA and MRSA cells treated with α-MSH, α-MSH(6-13), and α-MSH(11-13) compared to an untreated control and demonstrate the shift of the fluorescence peak in case of peptide-treated loaded cells. (b) Time-dependent changes in MSSA bacterial membrane potential, expressed as percentage of depolarized cells after 30 min, 60 min, and 120 min of peptide treatment compared to untreated control S. aureus. These data represent the means (±SDs) from three independent experiments. **, P ≤ 0.01 (comparing data for 30 min versus 60 min versus 120 min). (c) Percentage of depolarization occurring in MRSA ATCC 33591 on exposure to α-MSH, α-MSH(6-13), and α-MSH(11-13) at 1 μM each for 2 h. These data represent the means (±SDs) from three independent experiments.
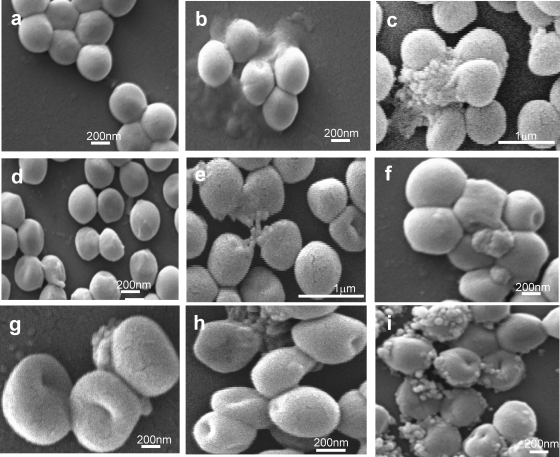
Morphological changes in S. aureus on peptide exposure observed using SEM.
Morphological changes in S. aureus due to peptide exposure were examined by scanning electron microscopy (SEM). As the cell density has to be sufficiently high (108 CFU/ml) for this technique, two different concentrations of α-MSH-based peptides (12 μM and 50 μM) and of gramicidin D (2 μg/ml and 20 μg/ml) were used. As shown in Fig. 7a, untreated S. aureus cells looked round, smooth, and intact. Upon exposure of the bacterial cells to 2 μg/ml of gramicidin D (Fig. 7 h), there was clear indication of surface perturbation, including a distorted appearance, rupture lines, depression, and dents, while hole formation and complete leakage of cell material was visible on incubation with 20 μg/ml gramicidin D (Fig. 7i) (22). Similar morphological changes were also observed for S. aureus cells exposed to α-MSH, α-MSH(6-13), and α-MSH(11-13) (Fig. 7b to g). It is evident from Fig. 7b, d, and f that exposure to 12 μM α-MSH, α-MSH(6-13), and α-MSH(11-13) caused a rough and damaged surface, depression, rupture lines, and hole formation. On incubation of S. aureus cells with higher concentrations of α-MSH-based peptides (each at 50 μM), cell bursting and leakage were prominent (Fig. 7c, e, and g). Surface damage caused by the α-MSH-based peptides used in this study was similar to but less extensive than that with gramicidin D, particularly at higher concentrations.
Fig. 7.
Scanning electron microscopic images of S. aureus of untreated control cells (a) and of cells after 2 h exposure to 12 μM α-MSH (b), 50 μM α-MSH (c), 12 μM α-MSH(6-13) (d), 50 μM α-MSH(6-13) (e), 12 μM α-MSH(11-13) (f), 50 μM α-MSH(11-13) (g), 2 μg/ml gramicidin D (h), or 20 μg/ml gramicidin D (i). S. aureus cells exposed to the lower concentration of all peptide show morphological changes including surface roughness, depression, and dent formation, whereas incubation at the higher dose caused leakage of cell material which was absent in untreated control cells. Similar appearances were found in separate experiments on different days.
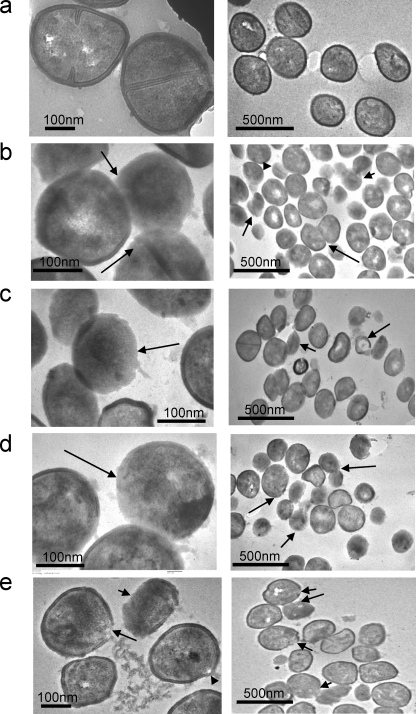
Ultrastructural changes in S. aureus on peptide exposure observed using TEM.
To examine the effect of α-MSH-based peptides on bacteria more accurately, transmission electron microscopy (TEM) was performed on peptide-treated and untreated bacterial samples. For comparison, gramicidin D was used. As indicated in Fig. 8a, the untreated control samples had an intact cell membrane and cell wall, and the cells were uniformly shaped. However, huge changes were observed in the images of peptide-treated cells. Figure 8b, c, and d represent the images of α-MSH-, α-MSH(6-13)- and α-MSH(11-13)-treated S. aureus cells, respectively, and Fig. 8e shows gramicidin D-treated bacterial cells. Incubation with α-MSH and its short peptides for 2 h caused distortion in the cell boundary; many cells (called ghost cells) were devoid of their cell walls, while some cells had remnants of the cell wall and cell membrane. These results consistent with the peptide-induced ultrastructural changes reported earlier (1). Gramicidin D-treated cells (Fig. 8e) did not have many ghost cells, but clear envelop gaps were found, through which the cell cytoplasmic contents were leaking out.
Fig. 8.
Transmission electron microscopic images of S. aureus cells treated with no peptide (control) (a), 50 μM α-MSH (b), 50 μM α-MSH(6-13) (c), 50 μM α-MSH(11-13) (d), or 20 μg/ml gramicidin D (e) for 2 h. Left and right panels are images of the same sample with different magnifications. Arrows point to the peptide-treated cells without cell walls, to cell leakage, and to cells without cytoplasmic content.
DISCUSSION
In our previous study, we had demonstrated that α-MSH is very active against S. aureus in the biofilm form (31). To further our investigation, the structure-activity relationship of α-MSH, particularly the contribution of its amino acid sequences to the staphylocidal mechanism, was analyzed in the present study. We chose certain fragments of α-MSH from both the C-terminal and N-terminal regions, i.e., α-MSH(6-13), α-MSH(11-13), and α-MSH(1-5), and we studied their activity and mechanism of action against MSSA and MRSA and compared them with those for the entire sequence of α-MSH. Since in our previous study α-MSH had been found to cause damage to the S. aureus membrane (31), in the present work we investigated the loss of bacterial membrane potential, if any, due to exposure to the studied peptides and also performed membrane permeabilization studies. Finally, membrane perturbation of S. aureus on peptide exposure was confirmed by investigating morphological and ultrastructural changes using SEM and TEM.
Several encouraging results were obtained from the current study. First, our activity assay clearly indicated that both the C-terminal fragments, α-MSH(6-13) and α-MSH(11-13), retained the in vitro antibacterial activity of entire peptide, α-MSH (Fig. 1a) in the picomolar to micromolar concentration range, and the killing was found to be very rapid for all the peptides (Fig. 1b). In contrast to earlier observations (26, 38), the presence of physiological concentrations of salts did not affect the killing potency of any of the studied peptides (Fig. 4). Of note, the activities of C-terminal fragments of α-MSH against both MSSA and MRSA were comparable to that of the full-length peptide in the micromolar range, while at picomolar concentrations the entire peptide, α-MSH, was most effective. Our study also indicated that peptides containing the core sequence (amino acids 6 to 9), such as the entire α-MSH and α-MSH(6-13), were more active than α-MSH(11-13), suggesting an important role of the core sequence in antistaphylococcal activity. Further, the N-terminal region of α-MSH did not show any significant antibacterial activity at any of the concentrations tested (Fig. 1a), suggesting the importance of C-terminal fragments in the staphylocidal mechanism of α-MSH.
Host defense cationic peptides target the bacterial membrane through electrostatic interaction with negatively charged membrane components, resulting in membrane damage and eventually cell death (33, 43, 44, 45). Our previous study suggested that the staphylocidal effect of α-MSH might be mediated through membrane disruption (31). Similarly to the case for the entire α-MSH, both α-MSH(6-13) and α-MSH(11-13) caused substantial membrane depolarization of and calcein leakage from MSSA and MRSA strains (Fig. 5 and 6) after 2 h of incubation, indicating that all of these peptides caused membrane damage. Our data also suggest that membrane depolarization occurs prior to membrane permeabilization. As observed, 30 min and 60 min of incubation of S. aureus cells with α-MSH or either of its C-terminal fragments could induce only up to 10% membrane leakage and 40% membrane depolarization, whereas >90% killing was obtained with all three peptides within 15 min. Comparison of the time kinetics of killing and those of membrane permeabilization and depolarization suggests membrane perturbation as a secondary event, following the lethal hit of α-MSH-derived peptides, rather than as a major cause of staphylocidal activity. However, membrane permeabilization and depolarization assays represent results in real time, whereas killing assays (determined by colony count) depict data after overnight incubation. There is always a possibility that the entry of the peptides into S. aureus cells may require 15 min or less and the lethal hit probably occurs during the overnight incubation period (25, 37). Loss of bacterial membrane integrity was further confirmed by morphological and ultrastructural studies (Fig. 7 and 8). Our SEM images clearly indicate severe staphylococcal membrane perturbation, including leakage of cell contents caused by all three α-MSH-based peptides. Further, the loss of cell wall and the presence of cells without cytoplasmic content were clearly visible from TEM images of peptide-treated S. aureus cells.
It has been reported that a given host defense antimicrobial peptide may use more than one mechanism for its microbicidal activity (41). It appears from our present study as well as our previous study that the bacterial membrane is the major target for staphylocidal activity of α-MSH and its C-terminal fragments. However, other targets cannot be ruled out, particularly due to the fact that the killing by α-MSH-based peptides was very rapid while substantial membrane disruption occurred at later time points. These findings suggest either that membrane damage is a secondary effect or that α-MSH-based peptides target bacterial components other than membranes, such as nucleic acids or an important enzymatic process, leading to cell death and, thereafter, membrane damage. The occurrence of all these processes simultaneously may be another possible antibacterial mechanism of action of α-MSH-based peptides. To elucidate the mode of staphylocidal action of α-MSH and its C-terminal fragments, a detailed study on inhibition of macromolecular synthesis needs to be performed and targets in the bacterial envelope need to be identified.
In conclusion, C-terminal fragments of α-MSH, including α-MSH(6-13) and tripeptide α-MSH(11-13), exhibited rapid and potent activities against both MSSA and MRSA strains, demonstrating their importance in the α-MSH-mediated staphylocidal effect. Like other host defense antimicrobial peptides, these peptides caused membrane depolarization followed by membrane permeabilization, and the mechanisms of action of full-length α-MSH and its C-terminal fragments were equivalent. Electron microscopic images of S. aureus demonstrated remarkable morphological and ultrastructural changes as a result of peptide exposure, including leakage of cell material and loss of the cell wall. Importantly, an N-terminal fragment of α-MSH, i.e., α-MSH(1-5), did not show any antimicrobial activity. It is also important to mention that the minimum sequence required for anti-inflammatory activity of α-MSH is the C-terminal tripeptide, i.e., α-MSH(11-13). Therefore, the C-terminal fragments of α-MSH having both anti-inflammatory and antibacterial properties could emerge as excellent antibacterial agents in the treatment of staphylococcal infection.
ACKNOWLEDGMENTS
This research was supported by a grant from the Indian Council of Medical Research and Department of Biotechnology, India, to K.M. M.S. acknowledges a fellowship from the University Grant Commission.
We thank G. Sainy, S. C. P. Sharma, and R. Pal of AIRF, JNU, for help during acquisition of SEM and TEM images. We are grateful to B. Dhawan (AIIMS, New Delhi, India) for providing MSSA strain ATCC 29213. We thank S. S. Komath (SLS, JNU, India) and N. Gupta (ICMR, India) for their input on the manuscript. Finally, we thank the reviewers for their insightful comments, which were very helpful in improving the manuscript.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1. Anderson R. C., Haverkamp R. G., Yu P. L. 2004. Investigation of morphological changes to Staphyloccus aureus induced by ovine-derived antimicrobial peptide using TEM and AFM. FEMS Microbiol. Lett. 240:105–110 [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj R. S., et al. 1996. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J. Immunol. 156:2517–2521 [PubMed] [Google Scholar]
- 3. Boman H. G., Agerberth B., Boman A. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and Pr-39, two antibacterial peptides from pig intestine. Infect. Immun. 61:2978–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowdish D. M. E., Davidson D. J., Hancock R. E. W. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Peptide Sci. 6:35–51 [DOI] [PubMed] [Google Scholar]
- 5. Braff M. H., Zaiou M., Fierer J., Nizet V., Gallo R. L. 2005. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 73:6771–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabiaux V., et al. 1994. Secondary structure and membrane interaction of Pr-39, a Pro+Arg-rich antibacterial peptide. Eur. J. Biochem. 224:1019–1027 [DOI] [PubMed] [Google Scholar]
- 7. Cahan R., Friman H., Nitzan Y. 2008. Antibacterial activity of Cyt1Aa from Bacillus thuringiensis subsp. israelensis. Microbiology 154:3529–3536 [DOI] [PubMed] [Google Scholar]
- 8. Catania A., Lipton J. M. 1993. α-Melanocyte stimulating hormone in the modulation of host reactions. Endocrinol. Rev. 14:564–576 [DOI] [PubMed] [Google Scholar]
- 9. Catania A., et al. 1996. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides 17:675–679 [DOI] [PubMed] [Google Scholar]
- 10. Charnley M., Moir A. J. G., Douglas C. W. I., Haycock J. W. 2008. Anti-microbial action of melanocortin peptides and identification of a novel X-Pro-d/l-Val sequence in gram-positive and gram-negative bacteria. Peptides 29:1004–1009 [DOI] [PubMed] [Google Scholar]
- 11. Cociancich S., Ghazi A., Hetru C., Hoffman J. A., Letellier L. 1993. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channel in Micrococcus luteus. J. Biol. Chem. 268:19239–19245 [PubMed] [Google Scholar]
- 12. Cutuli M., Cristiani S., Lipton J. M., Catania A. 2000. Antimicrobial effects of α-MSH peptides. J. Leukoc. Biol. 67:233–239 [DOI] [PubMed] [Google Scholar]
- 13. Dewan P. C., Anantharaman A., Chauhan V. S., Sahal D. 2009. Antimicrobial action of prototypic amphipathic cationic decapeptides and their branched dimers. Biochemistry 48:5642–5657 [DOI] [PubMed] [Google Scholar]
- 14. Donnarumma G., Paoletti I., Buommino E., Tufano M. A., Baroni A. 2004. α-MSH reduces the internalization of Staphylococcus aureus and down-regulate HSP 70, integrins and cytokine expression in human keratinocyte cell lines. Exp. Dermatol. 13:748–754 [DOI] [PubMed] [Google Scholar]
- 15. Eberle A. N. 2000. Proopiomelanocortin and the melanocortin peptides, p. 3–68 In Cone R. D. (ed.), The melanocortin receptors. Humana Press Inc., Totowa, NJ [Google Scholar]
- 16. Epand R. M., Vogel H. J. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11–28 [DOI] [PubMed] [Google Scholar]
- 17. Epand R. M., Shai Y., Segrest J. P., Anantharamaiah G. M. 1995. Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers 37:319–338 [DOI] [PubMed] [Google Scholar]
- 18. Foubister V. 2003. Superpeptide to treat Candida albicans. Drug Discov. Today 8:380–381 [DOI] [PubMed] [Google Scholar]
- 19. Friedrich C. L., Moyels D., Beveridge T. J., Hancock R. E. W. 2000. Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hancock R. E. 1997. Peptide antibiotics. Lancet 349:418–422 [DOI] [PubMed] [Google Scholar]
- 21. Hancock R. E. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. 1:156–164 [DOI] [PubMed] [Google Scholar]
- 22. Hartmann M., et al. 2010. Damage of the bacterial cell envelope by antimicrobial peptide gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 54:3132–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill R. P., MacNeil S., Haycock J. W. 2006. Melanocyte stimulating hormone peptides inhibit TNF-alpha signaling in human dermal fibroblast cells. Peptides 27:421–430 [DOI] [PubMed] [Google Scholar]
- 24. Hill R. P., Wheeler P., MacNeil S., Haycock J. W. 2005. Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells. Peptides 26:1150–1158 [DOI] [PubMed] [Google Scholar]
- 25. Jepras R. I., Paul F. E., Pearson S. C., Wilkinson M. J. 1997. Rapid assessment of antibiotic effects on Escherichia coli by bis-(1,3-dibutylbarbituric acid) trimethine oxonol and flow cytometry. Antimicrob. Agents Chemother. 41:2001–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansen C., Verheul A., Gram L., Gill T., Abee T. 1997. Protamine-induced permeabilization of cell envelopes of gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 63:1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koo S.-P., Bayer A. S., Yeaman M. R. 2001. Diversity in antistaphylococcal mechanisms among membrane-targeting antimicrobial peptides. Infect. Immun. 69:4916–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krishnakumari V., Singh S., Nagraj R. 2006. Antibacterial activities of synthetic peptides corresponding to the carboxy-terminal region of human β-defensins 1-3. Peptides 27:2607–2613 [DOI] [PubMed] [Google Scholar]
- 29. Lipton J. M., Catania A. 1997. Anti-inflammatory influence of the neuro immunomodulator alpha-MSH. Immunol. Today 18:140–145 [DOI] [PubMed] [Google Scholar]
- 30. Liu L. D., Roberts A. A., Ganz T. 2003. By IL-1 signaling, monocyte derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J. Immunol. 170:575–580 [DOI] [PubMed] [Google Scholar]
- 31. Madhuri, et al. 2009. In vitro antimicrobial activity of alpha-melanocyte stimulating hormone against major human pathogen Staphylococcus aureus. Peptides 30:1627–1635 [DOI] [PubMed] [Google Scholar]
- 32. Mukhopadhyay K., Basak S. 1998. Conformation induction in melanotropic peptides by trifluoroethanol: fluorescence and circular dichroism study. Biophys. Chem. 74:175–186 [DOI] [PubMed] [Google Scholar]
- 33. Mukhopadhyay K., et al. 2007. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbial protein-1(tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 153:1187–1197 [DOI] [PubMed] [Google Scholar]
- 34. Penyige A., Matko J., Deak E., Bodnar A., Barabas G. 2002. Depolarization of the membrane potential by β-lactams as a signal to induce autolysis. Biochem. Biophys. Res. Commun. 290:1169–1175 [DOI] [PubMed] [Google Scholar]
- 35. Reddy K. V. R., Yedery R. D., Aranha C. 2004. Antimicrobial peptides: premises and promises. Antimicrob. Agents Chemother. 24:536–547 [DOI] [PubMed] [Google Scholar]
- 36. Rousseau K., et al. 2007. Proopiomelanocortin (POMC), the ACTH/ melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB. J. 21:1844–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senyurek I., et al. 2009. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomita T., et al. 2000. Effects of ions on the antibacterial activity of human beta defensin 2. Microbiol. Immunol. 44:749–754 [DOI] [PubMed] [Google Scholar]
- 39. Turner J., Cho Y., Dinh N. N., Waring A. J., Lehrer R. I. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White S. H., Wimely W. C., Selsted M. E. 1995. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5:521–527 [DOI] [PubMed] [Google Scholar]
- 41. Wiedemann I. E., et al. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772–1779 [DOI] [PubMed] [Google Scholar]
- 42. Xiong Y. Q., Mukhopadhyay K., Yeaman M. R., Adler-Moore J., Bayer A. S. 2005. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3114–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeaman M. R., Bayer A. S., Koo S.-P., Foss W., Sullam P. M. 1998. Platelete microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 101:178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yenugu S., Hamil K. G., Radhakrishnan Y., French F. S., Hall S. H. 2004. The androgen-regulated epididymal sperm-binding protein, human β-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial β-defensin. Endocrinology 145:3165–3173 [DOI] [PubMed] [Google Scholar]
- 45. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 24:389–395 [DOI] [PubMed] [Google Scholar]