Abstract
We explored the properties of corallopyronin A (CorA), a poorly characterized inhibitor of bacterial RNA polymerase (RNAP). It displayed a 50% inhibitory concentration of 0.73 μM against RNAP, compared with 11.5 nM for rifampin. The antibacterial activity of CorA was also inferior to rifampin, and resistant mutants of Staphylococcus aureus were easily selected. The mutations conferring resistance resided in the rpoB and rpoC subunits of RNAP. We conclude that CorA is not a promising antibacterial drug candidate.
INTRODUCTION
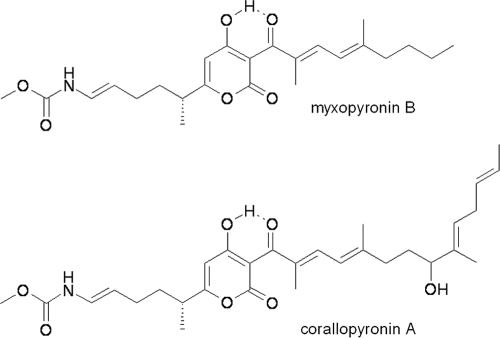
Antibiotics of the rifamycin class (e.g., rifampin) are currently the only inhibitors of bacterial RNA polymerase (RNAP) that are approved for clinical use (3). Bacterial RNAP therefore remains an attractive target for the discovery and development of new antibacterial agents (3). Corallopyronin A (CorA) and myxopyronin B (MyxB) (Fig. 1) are structurally related α-pyrone antibiotics first described around 30 years ago (8, 9). Both antibiotics are active against Gram-positive bacteria and inhibit bacterial RNAP in vitro (8, 9). Furthermore, MyxB has recently been cocrystallized with the beta-prime subunit of RNAP (2, 12). However, whether these antibiotics have the potential to be developed as clinical candidates requires further information. In this study, we examined the potency of CorA against RNAP, its mechanism of action, and its antibacterial spectrum. We also determined the frequency of selection of spontaneous resistance to CorA in Staphylococcus aureus and the nature of the mutations conferring resistance.
Fig. 1.
Structures of CorA and MyxB.
CorA was provided by G. Hofle, Helmholtz Centre for Infection Research, Germany. MyxB was a gift from T. Moy, Cubist Pharmaceuticals, Lexington, MA. Rifampin was obtained from Sigma-Aldrich (Poole, United Kingdom). Yeast Pol II was a kind gift from D. Bushnell, Stanford University, Stanford, CA.
The activities of CorA and rifampin against Escherichia coli RNAP in vitro were compared using the Kool NC-45 universal RNAP template (Epicentre, Madison, WI) (11). Although CorA inhibited E. coli RNAP, rifampin was a more potent inhibitor of the enzyme (Table 1). The activity of CorA against E. coli RNAP that we observed (50% inhibitory concentration [IC50] of 0.73 μM) is similar to the previously published value of 2 μg/ml (3.8 μM) (9). The activities of CorA and rifampin against yeast Pol II in vitro were compared in a modified nonspecific transcription assay (D. Bushnell personal communication) using Polyribose C template (Sigma, Poole, United Kingdom) and SYBR Green I (Invitrogen, Paisley, United Kingdom) detection. The addition of CorA at 100 μM inhibited yeast Pol II activity by 10.1% ± 3.2%, compared with 37.3% ± 2.6% for rifampin. The lack of activity of CorA against eukaryotic cells correlates with previous studies showing only 7% inhibition of wheat germ Pol II by a 40 μg/ml (75.8 μM) concentration of the antibiotic (9).
Table 1.
Inhibition of RNAP by rifampin and CorA in vitro, the susceptibility of bacteria to these antibiotics, and their effects on the cytoplasmic membrane of S. aureus
CorA is reported primarily to inhibit the growth of Gram-positive bacteria (9). We determined its activity against several species (Table 1) by microdilution in Mueller-Hinton broth according to British Society for Antimicrobial Chemotherapy guidelines (1). In agreement with previously published work (9), the antibacterial activity of CorA was limited to the Gram-positive bacteria tested, i.e., S. aureus SH1000 (6) and Bacillus subtilis (17). Both wild-type and AcrAB efflux pump-deficient strains of E. coli (14) proved insusceptible to CorA (Table 1), the latter result suggesting that CorA is not a substrate for the AcrAB-TolC efflux system. However, an E. coli tolC knockout mutant (16) was >32-fold more susceptible to CorA than the wild type (Table 1), indicating that CorA may be a substrate for TolC efflux systems that are linked to membrane fusion proteins and cytoplasmic membrane transporters other than AcrAB.
Although CorA inhibits RNAP, it is not known whether this alone accounts for its antibacterial activity. To examine the mechanism of action of CorA, we utilized B. subtilis antibiotic biosensors containing promoter-luciferase reporter constructs which are induced by conditions of antibiotic stress (10, 11, 17). Rifampin and CorA induced the biosensor (yvgS) responsive to inhibition of RNA synthesis (10). Although rifampin only induced yvgS (data not shown), CorA also induced the fabHB biosensor that is responsive to inhibition of fatty acid biosynthesis. (10). However, induction was only just above the published threshold for this biosensor (17) and 3-fold lower than that exhibited by triclosan, a known inhibitor of fatty acid biosynthesis (17). Therefore, although CorA might possess an additional mechanism of action involving inhibition of fatty acid synthesis, further studies are required to confirm this suggestion. Nevertheless, inhibition of fatty acid biosynthesis has been observed with other antimicrobial agents that contain an α-pyrone moiety (5).
With the notable exception of daptomycin, inhibitors that damage the bacterial cytoplasmic membrane tend to be unsuitable for further development as drug candidates (13). Consequently, the ability of CorA to damage the membrane of S. aureus was investigated using the BacLight assay (11). Neither CorA nor rifampin caused membrane damage, as the cells essentially maintained 100% membrane integrity after exposure for 10 min to the antibiotics at 4× MIC (Table 1).
Mutations conferring resistance to CorA in E. coli have been selected by random mutagenesis of the genes encoding RNAP (12). However, the frequency of spontaneous mutation to CorA resistance and the nature of mutants arising by this route have not been determined. The mutation frequency of resistance to CorA in S. aureus SH1000 at a selective concentration of 4× MIC was 7.2 × 10−8 ± 2.4 × 10−8 (number of mutants per total number of viable bacteria), which was comparable to that of rifampin at 1.4 × 10−7 ± 2.2 × 10−7. Eight independent mutants of S. aureus SH1000 displaying resistance to CorA (Table 2) were selected for analysis of cross-resistance to a panel of agents representing the major antibiotic classes, including rifampin. Cross-resistance did not occur (data not shown). However, cross-resistance to the related α-pyrone antibiotic MyxB was observed (Table 2), suggesting, by analogy to the random mutagenesis study (12), that the spontaneous CorA-resistant mutants probably possessed changes in RNAP. Accordingly, the genes encoding the RNAP α, β, and β′ subunits (rpoA, rpoB, and rpoC, respectively) in the CorA mutants were subjected to PCR amplification and DNA sequencing as previously described (15). Mutations were found in either rpoB or rpoC (Table 2). Some resistant mutants contained identical mutations (e.g., Cor2/Cor8 and Cor3/Cor4) or mutations affecting the same amino acid residue (e.g., Cor1, Cor3, Cor4). The remaining two mutants (Cor5, Cor6) contained mutations that were independent of each other and the mutants described above (Table 2). The introduction of mutations in E. coli RNAP corresponding to those in Cor1, Cor3, Cor4, Cor5, and Cor7 (i.e., at S1322, E1279, and L1326 of wild-type E. coli rpoB) and Cor6 (i.e., K345 of wild-type E. coli rpoC) have previously been reported to confer resistance to both Myx and CorA in E. coli (12).
Table 2.
Properties and nature of CorA-resistant mutants of S. aureus SH1000 isolated in this study
| Strain | CorA MIC (μg/ml) | MyxB MIC (μg/ml) | Avg generation time (min) ± SD | Amino acid substitution (codon change)a |
|
|---|---|---|---|---|---|
| β subunit | β′ subunit | ||||
| SH1000 | 2 | 2 | 37.2 ± 3.1 | – | – |
| SH1000-Cor1 | >128 | >128 | 45.3 ± 3.1 | S1127P (TCA-CCA) | None |
| SH1000-Cor2 | 64 | >128 | 46.9 ± 3.6 | None | L1165R (CTT-CGT) |
| SH1000-Cor3 | >128 | >128 | 41.1 ± 4.9 | S1127L (TCA-TTA) | None |
| SH1000-Cor4 | >128 | >128 | 42.7 ± 2.2 | S1127L (TCA-TTA) | None |
| SH1000-Cor5 | 64 | >128 | 36.7 ± 3.7 | E1084K (GAA-AAA) | None |
| SH1000-Cor6 | >128 | >128 | 38.1 ± 4.4 | None | K334N (AAA-AAC) |
| SH1000-Cor7 | 128 | 128 | 44.5 ± 8.0 | L1131F (TTG-TTT) | None |
| SH1000-Cor8 | 64 | >128 | 47.8 ± 1.1 | None | L1165R (CTT-CGT) |
S. aureus numbering.
The S. aureus Cor mutants described here exhibited only a slight loss of fitness compared to strain SH1000 when mean generation times were measured (7) (Table 2). This suggests that if such mutants arose in the clinical setting they might not suffer a competitive disadvantage compared to CorA-sensitive strains; i.e., CorA-resistant mutants could persist in natural populations even in the absence of CorA selection pressure.
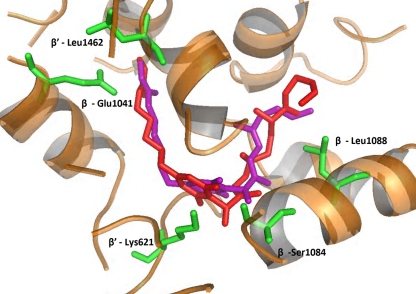
Docking of CorA in the MyxB binding site of Thermus thermophilus RNAP was performed using eHITS (18). This involved exhaustive docking of CorA in the MyxB binding site and selection of a binding conformation using a number of criteria. These included satisfying key hydrogen bonding interactions (i.e., with G344, W1276, and E1279), a good predicted binding affinity (calculated via a combination of hydrogen bonding, van der Waals interactions, hydrophobic contacts, and space-filling properties), and a visual assessment of the overall fit within the site. The altered residues in the S. aureus Cor mutants, by aligning the S. aureus and T. thermophilus RNAP subunit sequences (4), were all located directly within the myxopyronin binding site as previously described (Fig. 2) (2, 12). The occurrence of spontaneous mutations at this site (the switch region) strongly supports the hypothesis that CorA also binds to RNAP, inhibiting transcription.
Fig. 2.
Model of myxopyronin (purple) and CorA (red) bound to the beta subunits of RNAP, showing the proximity to the mutated residues in the RNAP of S. aureus strains SH1000-Cor1 to -Cor8. The residue numbers are those of T. thermophilus RNAP (4).
In summary, we have shown that CorA inhibits RNA biosynthesis at the level of RNAP and does not damage the bacterial cell membrane. Its activity both as an RNAP inhibitor and as a antibacterial agent is inferior to that of rifampin. CorA has a limited spectrum of antibacterial activity and, like rifampin, a high propensity for selection of resistance. Therefore, CorA is of limited interest for development as a future drug candidate, especially for monotherapy. However, if derivatives of CorA with improved features can be developed, these could be future antibiotic candidates. Indeed, close inspection of the myxopyronin-RNA cocrystal structure (2, 12) indicates that it may be possible to produce synthetic hybrid Myx-CorA variants with increased potency.
Acknowledgments
This work was supported by project grant G0600810 awarded to I.C. by the United Kingdom Medical Research Council and a CASE Ph.D. studentship awarded to K.R.M. by the United Kingdom Biological and Biosciences Research Council.
We thank G. Hofle and T. Moy for providing, respectively, CorA and MyxB.
Footnotes
Published ahead of print on 14 February 2011.
REFERENCES
- 1. Anonymous. 1991. A guide to sensitivity testing. Report of the Working Party on Antibiotic Sensitivity Testing of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 27(Suppl. D):1–50 [PubMed] [Google Scholar]
- 2. Belogurov G. A., et al. 2009. Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457:332–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chopra I. 2007. Bacterial RNA polymerase: a promising target for the discovery of new antimicrobial agents. Curr. Opin. Invest. Drugs 8:600–607 [PubMed] [Google Scholar]
- 4. Gasteiger E., et al. 2003. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giddens A. C., et al. 2008. Natural product inhibitors of fatty acid biosynthesis: synthesis of the marine microbial metabolites pseudopyronines A and B and evaluation of their anti-infective activities. Tetrahedron 64:1242–1249 [Google Scholar]
- 6. Horsburgh M. J., et al. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurdle J. G., O'Neill A. J., Chopra I. 2004. The isoleucyl-tRNA synthetase mutation V588F conferring mupirocin resistance in glycopeptides-intermediate Staphylococcus aureus is not associated with a significant fitness burden. J. Antimicrob. Chemother. 53:102–104 [DOI] [PubMed] [Google Scholar]
- 8. Irschik H., Gerth K., Hofle G., Kohl W., Reichenbach H. 1983. The myxopyronins, new inhibitors of bacterial RNA synthesis from Myxococcus fulvus (Myxobacterales). J. Antibiot. 36:1651–1658 [DOI] [PubMed] [Google Scholar]
- 9. Irschik H., Jansen R., Hofle G., Gerth K., Reichenbach H. 1985. The corallopyronins, new inhibitors of bacterial RNA synthesis from myxobacteria. J. Antibiot. 38:145–152 [DOI] [PubMed] [Google Scholar]
- 10. Mariner K. R., Ooi N., Roebuck D., O'Neill A. J., Chopra I. 2011. Further characterization of Bacillus subtilis antibiotic biosensors and their use for antibacterial mode-of-action studies. Antimicrob. Agents. Chemother. 55:1784–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariner K., et al. 2010. Furanyl-rhodanines: unattractive drug candidates for development as inhibitors of bacterial RNA polymerase. Antimicrob. Agents Chemother. 54:4506–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukhopadhyay J., et al. 2008. The RNA polymerase ‘switch region’ is a target for inhibitors. Cell 135:295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neill A. J., Chopra I. 2004. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Invest. Drugs 13:1045–1063 [DOI] [PubMed] [Google Scholar]
- 14. O'Neill A. J., Bostock J., Morais-Moita A., Chopra I. 2002. Antimicrobial activity and mechanisms of resistance to cephalosporin P1, an antibiotic related to fusidic acid. J. Antimicrob. Chemother. 50:839–848 [DOI] [PubMed] [Google Scholar]
- 15. O'Neill A. J., Huovinen T., Fishwick C. W. G., Chopra I. 2006. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob. Agents Chemother. 50:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro E., Baneyx F. 2002. Stress-based identification and classification of antibacterial agents: second-generation Escherichia coli reporter strains and optimization of detection. Antimicrob. Agents Chemother. 46:2490–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Urban A., et al. 2007. Novel whole-cell antibiotic biosensors for compound discovery. Appl. Environ. Microbiol. 73:6436–6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zsoldos Z., Reid D., Aniko, Sadjad S. B. S., Johnson P. A. 2006. eHiTS: an innovative approach to the docking and scoring function problems. Curr. Protein Pept. Sci. 7:421–435 [DOI] [PubMed] [Google Scholar]