Abstract
The discovery of the human immunodeficiency virus type 1 (HIV-1) in 1982 soon led to the identification and development of antiviral compounds to be used in treatment strategies for infected patients. Early in the epidemic, drug monotherapies frequently led to treatment failures because the virus quickly developed resistance to the single drug. Following the advent of highly active antiretroviral therapy (HAART) in 1995, dramatic improvements in HIV-1-infected patient health and survival were realized as more refined combination therapies resulted in reductions in viral loads and increases in CD4+ T-cell counts. In the absence of an effective vaccine, prevention of HIV-1 infection has also gained traction as an approach to curbing the pandemic. The development of compounds as safe and effective microbicides has intensified and has focused on blocking the transmission of HIV-1 during all forms of sexual intercourse. Initial preclinical investigations and clinical trials of microbicides focused on single compounds effective against HIV-1. However, the remarkable successes achieved using combination therapy to treat systemic HIV-1 infection have subsequently stimulated the study and development of combination microbicides that will simultaneously inhibit multiple aspects of the HIV-1 transmission process by targeting incoming viral particles, virus-infected cells, and cells susceptible to HIV-1 infection. This review focuses on existing and developing combination therapies, covering preclinical development, in vitro and in vivo efficacy studies, and subsequent clinical trials. The shift in focus within the microbicide development field from single compounds to combination approaches is also explored.
TEXT
In the areas of disease prevention and treatment, the use of existing drugs in combination has become an important complement to the continued development of new therapeutic agents. Effective combinations of two or more drugs have been used to treat cancer as well as diseases associated with infectious pathogens (4, 5, 9, 10, 88, 89, 99). Other agents, such as insecticides, fungicides, and other poisons, have also been used in combination to increase or broaden their effectiveness (42). However, investigations into the combined effects of two or more agents began long before the inception of combination drug development. Early studies involving combinations of inhibitors originated in the field of biochemistry, focusing on the effective inhibition of enzymatic activity. As a result of these studies, several mathematical approaches were developed to adequately describe the combined activities of multiple participants in an enzymatic reaction. In fact, current approaches used to analyze drug combinations have their roots in these biochemical investigations.
At their simplest, combinatorial investigations focus solely on the effect produced by the combined agents (42). These studies are generally used to identify combinations of agents that are more effective than either of the partners used in isolation. However, not all agents or drugs can be combined in an effective manner; chemical incompatibilities or interference between agents may diminish their combined activity relative to the expected sum of their individual activities. This effect, which is termed antagonism, may arise if two compounds act competitively on the same target, thereby reducing their overall combined activity. The potential for antagonism was recognized early in studies designed to determine antagonistic potential between compounds (101, 107). Numerous mathematical equations have been derived to describe the activity associated with each of the individual components of a combination and the activity associated with the combination (13, 37). From these equations, common terms evolved to describe the nature of the combined activity, including synergism, antagonism, additive action, similar action, and independent action. As combination testing expanded, more analytic methods were derived, and, with expanded analyses, the common terms developed multiple definitions. Despite variations in the definitions, it is universally recognized that synergism refers to a level of combined activity that exceeds the sum of the individual activities. It is also understood that synergy is generally a desirable attribute in an agent combination.
Realistically, the development of therapeutic drug combinations must take into account effects that are beyond the desired activity. Combining two drugs may permit reductions in the amounts of agents used without compromising the desired outcome or effect, thereby reducing potential side effects attributable to drug toxicity. However, the use of two drugs may instead result in additional toxicity not associated with the use of either drug alone. Another aspect to consider in the development of therapeutic combinations is drug resistance. By combining two drugs that achieve the same effect through different mechanisms of action, the development of resistance to a single drug in the combination may be less likely to occur, and when it does occur, it may have a lower impact on the therapeutic outcome. Furthermore, reductions in concentration made possible by the combined effects of multiple agents may lessen the probability or delay the development of drug resistance. Conversely, the therapeutic efficacy of a drug combination may be greatly diminished if a single resistance mechanism affects both drugs in the combination.
The various definitions associated with synergistic, antagonistic, and additive activity make up only a part of the confusion associated with combination testing. In addition to multiple definitions to these seemingly simple terms, multiple models, methods of analysis, and methods of interpretation exist that can be applied to studies of multiple compounds. Therefore, arriving at the meaning and mechanism of synergy can be a less than straightforward process. A variety of techniques are available for application to combination studies, including the isobologram method introduced by Loewe (56, 57), Bliss independence for independence criterion (106), the Chou and Talalay median-effect principle (22–24), and, finally, the response surface method described by Prichard and Shipman in their three-dimensional model of drug-drug interactions (78, 79).
There are two methods of analysis that have been widely used in recent years in investigations involving drug combinations. The first, the method of Chou and Talalay, is based on the median-effect principle, which for mutually exclusive agents is based on Loewe additivity and for mutually nonexclusive agents resembles Bliss independence. This method calculates combination indices (CI), which indicate the nature of the combined activity. For both mutually exclusive and mutually nonexclusive drug combinations, CI values of less than 1 indicate levels of synergy, CI values equal to 1 indicate additive activity or summation, and CI values greater than 1 indicate levels of antagonism. The second is the method proposed by Prichard and Shipman, which uses the Bliss independence mathematical definition of expected effects for drug-drug interactions (59). For brief reviews of the Loewe isobologram method and Bliss independence, as well as more detailed information on the median-effect principle of Chou and Talalay and the response surface model described by Shipman and Pritchard, please refer to the supplemental material available for this review.
RATIONALE FOR COMBINATION THERAPIES FOR HIV-1
HIV-1, which is the etiologic agent of the AIDS, has been the subject of intense studies focused on understanding the virus and the pathogenesis associated with HIV-1 infection. Our greater understanding of HIV-1 virology has permitted the development of numerous drugs that can interrupt specific events critical to continued viral replication. The current armamentarium of therapeutic drugs provides substantial benefits in terms of delayed disease progression and significant reductions in disease-associated mortality. However, no therapeutic approach available to date offers a cure for HIV-1 infection.
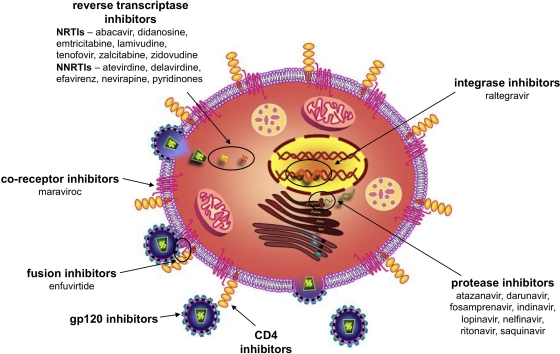
Multiple points within the HIV-1 replication cycle provide opportunities for therapeutic intervention (Fig. 1). Initial efforts to interrupt HIV-1 replication focused on HIV-1 reverse transcriptase (RT), which is responsible for converting the single-stranded viral RNA into complementary double-stranded proviral DNA. This enzyme was an obvious target for selective therapeutic intervention because RT is essential for retrovirus replication but is not found within HIV-1-susceptible host cells (77). Drug development efforts over the past 2 to 3 decades have produced nucleoside reverse transcriptase inhibitors (NRTIs) such as zidovudine (AZT) (85), zalcitabine (ddC) (64), and didanosine (ddI) (1) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) such as nevirapine (NVP) (61), delavirdine (DLV) (31), efavirenz (EFV), and etravirine (ETV) (43) (Table 1). HIV-1 protease, which participates in the assembly of HIV-1 virions, provided another selective target for drug development (60). Protease inhibitors (PIs) are now part of highly effective combination therapies for HIV-1 infection. HIV-1 integrase, which serves to integrate the proviral DNA into the genome of the infected cell, has also been exploited as a drug development opportunity (21, 76). Recent drug development efforts also led to the identification of HIV-1 binding and entry inhibitors, which act on HIV-1 gp120-mediated interactions with the cellular receptor CD4 and coreceptors CCR5 or CXCR4. These efforts have resulted in the approval of the first CCR5-specific coreceptor inhibitor, maraviroc ([MVC] Selzentry) for clinical use (35, 48, 104).
Fig. 1.
Inhibition of HIV-1 replication can be accomplished at different steps in the viral life cycle. Within the HIV-1 replication cycle, multiple events can be targeted by systemic inhibitors as well as topically applied microbicides. The first compounds developed with anti-HIV-1 activity were reverse transcriptase inhibitors (RTIs). RTIs are currently being used therapeutically to treat HIV-1-infected individuals and are also being investigated as candidate microbicides. Protease inhibitors, which inhibit the activity of HIV-1 protease, are currently part of the recommended standard of care for HIV-1-infected patients. Additional mechanisms of inhibition can involve inactivating the virus or inhibiting viral binding and entry through direct interactions with viral proteins (gp120 and gp41) or with cell surface molecules that interact with the virus (CD4, CXCR4, or CCR5). Maraviroc (MVC) is a recently approved inhibitor that acts by inhibiting viral binding to CCR5. Although approved for use in HIV-1-infected patients, it is used mainly as a salvage therapy once the patient has developed resistance to established treatment regimens. Raltegravir is the first HIV-1 integrase inhibitor approved for clinical use.
Table 1.
Nomenclature of commonly used/commercially available antiviral compounds
| Compound type and generic namea | Commercial name | Common abbreviation | Target |
|---|---|---|---|
| NRTIs | |||
| Azidothymidine or zidovudine | Retrovir | AZT | HIV-1 reverse transcriptase |
| Zalcitabine | Hivid | ddC | |
| Didanosine | Videx | ddI | |
| Lamivudine | Zeffix | 3TC | |
| Stavudine | Zerit | D4T | |
| Abacavir | Ziagen | ABC | |
| Emtricitabine | Emtriva | FTC | |
| NNRTIs | |||
| Nevirapine | Viramune | NVP | HIV-1 reverse transcriptase |
| Delavirdine | Rescriptor | DLV | |
| Efavirenz | Sustiva | EFV | |
| Etravirine | Intelence | ETV | |
| Tenofovir | Viread | TDF | |
| PIs | |||
| Amprenavir | Agenerase | APV | HIV-1 protease |
| Indinavir | Crixivan | IDV | |
| Nelfinavir | Viracept | NFV | |
| Atazanavir | Reyataz | ATV | |
| Lopinavir | Kaletra or Aluvia | LPV | |
| Saquinavir | Invirase or Fortovase | SQV | |
| Darunavir | Prezista | DRV | |
| Fosamprenavir | Lexiva or Telzir | FPV | |
| Ritonavir | Norvir | RTV | Targets HIV-1 protease and also inhibits cytochrome P450-3A4, which normally metabolizes protease inhibitors |
| INSTI | |||
| Raltegravir | Isentress | RAL | HIV-1 integrase |
| Binding and entry inhibitors | |||
| Maraviroc | Selzentry or Celsentri | MVC | CCR5 in its role as an HIV-1 coreceptor |
| Enfuvirtide | Fuzeon | T-20 | gp41-mediated fusion |
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor.
The principal impetus behind the development of combination therapies for HIV-1 (HAART) was the emergence of drug resistance following the therapeutic use of single antiretroviral drugs such as AZT. The development of HIV-1 drug resistance has been attributed to the enzymatic activity of RT. HIV-1 RT proofreading errors lead to a forward mutation rate of 3.4 × 10−5 per base pair per replication cycle (58, 66). It has been suggested that every single point mutation within the 9.7-kb proviral genome occurs in excess of 10,000 times per day in infected patients (26, 66). This mutation rate results in extensive genotypic variation within even a single infected individual. Some mutations severely reduce viral fitness or render the virus noninfectious, whereas others have no discernible effect on viral phenotype. In some circumstances, viral fitness may be enhanced, either on a global scale or within specific compartments or tissues. For resistance to a therapeutic agent to emerge, the coding region for the drug target must be mutated in such a way that the target protein retains its function while in the presence of the inhibitor. Resistance mutations may be transmitted from one person to another, and drug-resistant isolates have been identified in individuals who have not yet received antiviral therapy (68, 69). Mutations that confer resistance against a number of NRTIs, NNRTIs, and PIs are a significant clinical problem (47). The effects of these mutations were especially evident early in the HIV-1 epidemic when antiretroviral monotherapy was commonly used. Monotherapy is more likely to result in the emergence of resistant isolates. To combat the growing problem of drug resistance and to preserve the usefulness of many of the early antiretroviral drugs, many drugs were evaluated in vitro and clinically for their use as drug combination partners.
IN VITRO STUDIES OF COMBINATION THERAPIES
One of the first drugs used to treat HIV-1 infection was the NRTI AZT. Early combination studies combined AZT with ddI, as well as with alpha interferon (IFN-α) and dideoxycytidine (ddC). Prior to and following extended AZT monotherapy, AZT-sensitive and AZT-resistant viruses were isolated from an HIV-1-infected patient and used in vitro to evaluate combination treatment efficacy. Synergy, using the median-effect principle, was demonstrated when AZT was combined with ddI, IFN-α, or ddC and when ddI was combined with IFN-α (32, 50). Experiments were also performed on combinations of NRTIs and NNRTIs. The combination of AZT and the NNRTI atevirdine was synergistically active against AZT-resistant isolates and additive against AZT-susceptible strains (19). In contrast, the combination of atevirdine and ddI provided additive activity against both ddI-susceptible and ddI-resistant strains (19).
Interestingly, the latter studies illustrate the point that the emergence of resistance to a partner in a drug combination does not necessarily negate the efficacy of the combination. Drug-resistant viruses, like those used in the above studies (19), are not completely unaffected by the drug but, rather, are characterized by reduced drug susceptibility relative to viruses that do not harbor the resistance mutation(s). The low but effective activity of the drug to which the virus is resistant may simply add to the activity of the more effective partner in the combination. Alternatively, synergy subsequent to the development of drug resistance may be achieved through multiple mechanisms. Potential explanations for the synergistic activity of AZT and atevirdine against AZT-resistant clinical isolates included the following: (i) increased activity of AZT as a result of interactions between atevirdine and RT, (ii) enhanced activity of atevirdine attributed to alterations in RT conformation that accompanied the changes in RT responsible for AZT resistance, and (iii) increases in AZT uptake/activation or decreases in AZT degradation in the presence of atevirdine (19). Although combination synergy has also been attributed to differential antiviral activities against viral quasispecies that differ in their sensitivities to drugs in the combination (50), mathematical modeling involving a hypothetical mixed viral isolate suggested that a combined drug application would result only in additive activity (19).
As new anti-HIV-1 drugs were developed, the scope of combination research was expanded. Whereas the use of the PI amprenavir (APV) combined with another PI ritonavir (RTV) resulted in additive activity against wild-type, susceptible strains, the use of this drug pair against AZT-resistant and multinucleoside-resistant isolates revealed slightly antagonistic antiviral activity (92). This result was particularly interesting because all of the isolates examined in these experiments should have been susceptible to these inhibitors. These results indicate the importance of using a variety of HIV-1 isolates during combination experiments to establish a more complete picture of combined antiviral activity.
Combinatorial analyses have also been performed with new compounds in various stages of preclinical development. PRO 542 and T-20 are two such compounds. PRO 542 is a tetravalent CD4-immunoglobulin fusion protein that is thought to neutralize primary HIV-1 isolates (3, 49). In contrast, T-20 is a peptide derived from the C-terminal ectodomain of gp41 that is considered to be a fusion inhibitor (52). Using the median-effect principle and analyses provided by CalcuSyn, the combination of PRO 542 and T-20 potently inhibited virus-cell fusion mediated by JR-FL (a CCR5-utilizing virus) and DH123 (a CXCR4-utilizing virus), with CI values ranging from 0.14 to 0.39 and from 0.36 to 1.1 for JR-FL and DH123, respectively (67). In addition to blocking virus-cell fusion, the combination was also able to block cell-cell fusion at three different combination ratios, with CI values ranging from 0.27 to 0.76 in a ratio-dependent manner. These studies concluded that the combination of PRO 542 and T-20 provides potent synergistic antiviral activity against two different strains of virus (67).
Early HIV-1-associated disease is characterized by a predominance of replicating viruses that use CCR5 as the coreceptor. As a result, CCR5 antagonists are being investigated as therapeutic and preventive agents. One such compound is SCH-C (SCH 351125). This compound is a potent inhibitor of HIV-1 infection, with in vitro activities in the nanomolar range (51, 90, 95). In experiments designed to evaluate drug combinations containing CCR5 inhibitors, SCH-C was combined with the anti-CCR5 monoclonal antibody, PA14 (73). Similarly, PA14 was combined with TAK779 (6), which is another potent CCR5 antagonist. The CCR5 antagonists and PA14 inhibit HIV-1 infection by different mechanisms of action, and the combination of these different mechanisms of action would be presumed to reduce the risk of the emergence of coreceptor inhibitor-resistant viruses. Combination analyses using CalcuSyn, which established CI values in the ranges of 0.06 to 0.17 for the SCH-C–PA14 combination and 0.25 to 0.57 for the TAK779-PA14 combination, indicated that the combinations were synergistic (83). Further studies were performed to examine the combined activity of SCH-C and compounds in clinical use, including two NRTIs (AZT and lamivudine [3TC]), an NNRTI (EFV), and a PI (indinavir [IDV]) (93). Additionally, SCH-C was examined in combination with the fusion inhibitor, enfuvirtide (T-20). These experiments were performed to evaluate combinations of drugs with dissimilar mechanisms of action. SCH-C partnered with all of these drugs to produce synergistic activities against HIV-1, with CI values ranging from 0.23 to 0.77. The combination of SCH-C and T-20 was further tested against a panel of CCR5-using viruses, including several viruses resistant to select RTIs and PIs. The SCH-C–T-20 combination was synergistically active against all but one virus, against which the combination was additive at the lowest concentration examined. These results suggested that SCH-C might be a useful compound to include in combination therapy and might be particularly useful when combined with a fusion inhibitor in the treatment of multidrug-resistant viruses (93).
In 2007, MVC became the first CCR5-specific entry inhibitor to be approved by the U.S. Food and Drug Administration for use in treatment-experienced patients. Prior to its approval, MVC was the subject of extensive in vitro studies to determine not only the activity of the compound as a single agent but also its activity in combination with other previously approved antiretroviral agents, including 3TC, EFV, nelfinavir (NFV), T-20, and others (29). Cell line-based combination studies, analyzed using MacSynergy, demonstrated moderate synergy in experiments involving atazanavir (ATV), IDV, and T-20 but additive effects when these experiments were repeated. In assays using more relevant primary blood leukocytes, minor synergy was observed when MVC was tested in combination with EFV and NFV. In all of the combination assays performed with MVC and other antiretroviral compounds, the results suggested additive to mildly synergistic interactions. In summary, these studies implied potential gains in therapeutic efficacy as a result of combining MVC with previously approved RTIs.
The targeting of CD4 is thought to provide potent antiviral activity against a broad spectrum of HIV-1 isolates. One compound that targets the use of CD4 by HIV-1 is the synthetic macrocycle cyclotriazadisulfonamide (CADA) (102), which functions as an inhibitor by downmodulating cell surface CD4. Because of its unique mechanism of action, CADA was examined as a combination partner with numerous RTIs (AZT, stavudine [d4T], 3TC, ddC, ddI, abacavir [ABC], tenofovir [TDF], NVP, DLV, and EFV), multiple PIs (lopinavir [LPV], saquinavir [SQV], IDV, NFV, APV, and RTV), a gp41 fusion inhibitor (T-20), and a CXCR4 antagonist (AMD3100) (103). CalcuSyn analyses established CI values between 0.34 and 0.96 for combinations containing the RTIs, indicating that the combinations ranged from mildly synergistic to synergistic. This synergism was apparent even when the combinations were examined at multiple fixed ratios. When CADA was combined with the PIs at multiple fixed ratios, mild to moderate synergy was again observed (CI values ranging between 0.37 and 0.99). Finally, combinations containing CADA–T-20 or CADA-AMD3100 provided CI values ranging between 0.56 and 0.80, indicating, again, synergistic interactions. When analyzed at multiple fixed ratios, however, these combinations were characterized as moderately synergistic to additive with respect to inhibition of HIV-1. There was no indication of antagonism with any combination examined. Interestingly, this study indicated that a compound that specifically down-modulates the CD4 receptor can act synergistically with numerous antiretroviral drugs that act several steps later in the replication cycle.
CLINICAL TRIALS AND COMBINATION REGIMENS IN CURRENT USE
The initial successes achieved using AZT monotherapy were soon followed by clinical failures characterized by rebounds in viral load and concomitant decreases in CD4+ cell counts. These disappointing results prompted combination investigations designed to salvage the therapeutic utility of AZT, stave off the emergence of resistant mutants, and possibly decrease the toxicity associated with several anti-HIV-1 drug regimens. Both AZT and ddC have well-documented toxicity associated with their use. However, their associated toxicities are specific to each compound and do not overlap with one another. For AZT, inhibition of the growth of granulocyte-macrophage cells and early and late erythroid progenitors is a common effect (81), whereas peripheral neuropathy is a common side effect of ddC administration (12, 38, 108). Clinical trials were therefore conducted with AZT combined with ddC in the hope that the combination would have greater efficacy, as was suggested by in vitro studies, and that the combined toxicity would be less than the toxicity of either drug alone (32). In a phase I/phase II open-label clinical trial, administration of both drugs resulted in weight gain, increased CD4 cell counts, and decreased p24 levels, with the best results occurring in those patients who received 600 mg of AZT with 0.03 mg of ddC/kg of body weight daily. Larger-scale studies were also performed with patients who received AZT monotherapy for a minimum of 6 months prior to the trial. Patients received AZT in combination with either ddC or ddI. For patients with CD4 cell counts greater than or equal to 150 cells/mm3 receiving the combination therapy, progressive disease and mortality were significantly decreased. No differences were noted in the groups receiving monotherapy. The same trend was not, however, observed in patients with lower CD4 cell counts (2). Several trials were also conducted with AZT and ddI, with the goal of confirming the drug synergy observed in vitro. In one trial, AZT and ddI were examined in five different combination ratios. No increase in toxicity was associated with the combination over AZT alone. CD4 cell counts also increased in each of the five groups (28). In a separate trial, 42% of patients receiving AZT-ddI combination therapy maintained negative cultures for 12 months compared to 8% of patients receiving AZT monotherapy (18; M. Ragni, R. Dafni, D. Amato, J. Korvick, and T. Merigan, presented at the VIII International Conference on AIDS, Amsterdam, Netherlands, 19 to 24 July 1992). Although these results demonstrated the improved efficacy of combination therapy using two drugs, these drug combinations did not appear to prevent the emergence of AZT-resistant mutants, which continued to be a confounding factor during treatment (18; R. Shafer, M. Kozal, and M. Winters, presented at the IX International Conference on AIDS, Berlin, Germany, 6 to 11 June 1993). While there was a modest benefit observed in the two-drug arm of the study versus the one-drug arm, neither NRTI monotherapy nor NRTI combination therapy provided sustainable efficacy, with viral loads rebounding under both treatment regimens.
Additional studies evaluated combinations containing three NRTIs (41, 98). Pilot studies were conducted with triple NRTI combinations of ddI-3TC-TDF, ddI-3TC-d4T, and AZT-3TC-ABC. However, only later was it determined that patients receiving monomechanistic therapy (i.e., three NRTI drugs with the same mechanism of action) typically had increased antiretroviral failure rates compared to PI- and NNRTI-containing regimens (see below). It became clear from these collective studies that combination therapy in patients must include drugs that use distinct mechanisms of action or target multiple events in the HIV-1 replication cycle (25).
Subsequent trials evaluated combinations of AZT with select NNRTI drugs in order to examine treatments using drugs with similar but distinct mechanisms of action. A small trial was conducted to examine the combination of AZT and NVP, which is an NNRTI with potent antiviral activity. NVP monotherapy has been associated with the rapid emergence of NVP-resistant viruses (20). Patients given AZT-NVP combination therapy had larger decreases in p24 levels than patients taking NVP monotherapy. Although NVP resistance was still detectable during the course of combination therapy, the resistant virus genotype differed from that of resistant viruses detected in the presence of NVP alone (82). This observation suggested that combination therapy directed at two different targets not only could permit the development of resistant viruses but also was capable of altering the absolute phenotype of the resistant virus that did develop. As such, this observation was useful because it indicated that combination therapy may have the potential to alter the course of resistance development in a clinically meaningful manner.
With the approval of HIV-1 PIs in late 1995, the options for highly effective triple drug regimens were greatly expanded. The incorporation of PIs into combination therapies provided, for the first time, a therapeutic approach that provided three distinct mechanisms of antiretroviral activity. As a result of the introduction of triple therapy or HAART, mortality rates associated with HIV-1 infection have declined substantially since 1995 and even more so since the beginning of the epidemic. Because of the recognized benefits of HAART, the current standard of care includes the following regimens: an NNRTI, PI, or integrase inhibitor combined with two NRTIs (74) (Table 2). Additionally, the recent approvals of the entry inhibitor MVC (44) and the integrase inhibitor raltegravir (Isentress) (46, 91) for clinical use have expanded the number of possible combination treatments available.
Table 2.
Recommended regimens for treatment-naï patients
| Regimen type | Regimen component(s)a |
|||||
|---|---|---|---|---|---|---|
| Column A |
Column B |
|||||
| NNRTI | PI | INSTI | Dual NRTI | Dual NRTI with NNRTI | Dual NRTI with PI | |
| Preferred component | Efavirenz | Atazanavir with low-dose ritonavir | Raltegravir | Tenofovir-emtricitabine (coformulated) | ||
| Darunavir with low-dose ritonavir | ||||||
| Alternative to preferred component | Nevirapine | Atazanavir with low-dose ritonavir | Abacavir-lamivudine (coformulated)b | Tenofovir-emtricitabine (coformulated) | ||
| Efavirenz | Fosamprenavir with low-dose ritonavir | Zidovudine-lamivudine (coformulated) | ||||
| Saquinavir with low-dose ritonavir | ||||||
| Lopinavir with low-dose ritonavir | ||||||
| Acceptable regimen (less preferred) | Efavirenz | Atazanavir | Didanosine with either emtricitabine or lamivudine | Abacavir-lamivudine (coformulated) | ||
According to Department of Health and Human Services guidelines, each regimen type should consist of one component selected from column A plus one component from column B. The table was adapted from reference 74.
Only if patient is HLA-B*5701 negative.
COMBINATION STUDIES OF TOPICAL MICROBICIDES EFFECTIVE AGAINST HIV-1
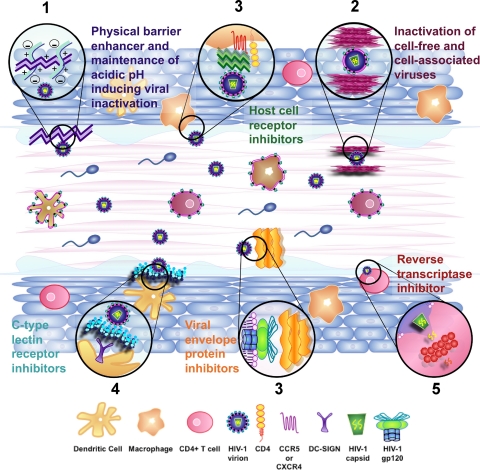
Microbicides that effectively interrupt events leading to HIV-1 transmission following topical vaginal (or rectal) application are currently being developed to provide a safe and effective method for HIV-1 prevention (8, 53). Current anti-HIV-1 microbicide development efforts are focused on numerous compounds with varied chemistries and mechanisms of action. Paralleling the development of effective monotherapies at the beginning of the HIV/AIDS epidemic, preclinical development and clinical testing of potential microbicide agents first focused on single-agent approaches (45, 62, 63, 86, 96, 97; L. Van Damme, presented at the Fourth International AIDS Society Conference on HIV Treatment and Pathogenesis, Sydney, Australia, 22 to 25 July 2007). Although many microbicides are being developed as products containing single agents (Fig. 2), increasing emphasis is being placed on the development of combination microbicides, due in part to the successes achieved using combination therapies administered systemically.
Fig. 2.
Combinations of inhibitors with different mechanisms of action may provide more complete protection from HIV-1 sexual transmission. Because of the multiple modes of transmission and susceptible cell types in the cervicovaginal compartment, it is likely that multiple compounds used in combination will be required to provide full protection from infection. Potential combination strategies for preventing HIV-1 sexual transmission may involve the following: enhancement of innate defenses (e.g., maintaining the low pH within the vaginal environment, and the enhancement/enrichment of cervicovaginal secretions that provide barrier and antimicrobial activities) within the cervicovaginal environments (1), inactivation of cell-free and cell-associated viruses (2), prevention of infection by inhibiting viral binding and entry either through the blocking of viral envelope proteins or the blocking of host cellular receptors (e.g., CD4, CCR5, and CXCR4) (3), inhibition of mechanisms that facilitate viral dissemination (e.g., DC-SIGN on dendritic cells which acts to disperse HIV-1 virions throughout the body undetected) (4), and inhibition of critical replicative events (e.g., reverse transcription and integration) in the HIV-1-infected cell (5). To date, the majority of microbicides in development for use as either single agents or as a partner in a combination act to inhibit binding and entry or act to inhibit reverse transcription. However, the current development of microbicidal agents that exploit other mechanisms of inhibition will add to the repertoire of possible combination partners, potentially increasing the likelihood that a combination with efficacy against HIV-1 transmission and infection during sexual intercourse will be developed.
PRECLINICAL MICROBICIDE COMBINATION STUDIES
One of the first compounds to be evaluated as a combination microbicide partner was cellulose acetate phthalate (CAP). CAP blocks HIV-1 infection by blocking the coreceptor binding site on HIV-1 gp120 without interfering with viral binding to CD4 (72). Initial investigations combined CAP with soluble CD4 (sCD4), which has also been shown to block HIV-1 infection (87). It has been hypothesized that CAP and sCD4 bind to distinct sites on HIV-1 gp120 and that simultaneous binding of these two agents would synergistically block infection. CI values were calculated for inhibition of HIV-1 IIIB (CXCR4-utilizing) and HIV-1 BaL (CCR5-utilizing); values ranged from 0.29 to 0.76 and from 0.40 to 0.52 for IIIB and BaL, respectively (70). These results, which indicated moderately to highly synergistic activity against HIV-1 and independence from coreceptor usage, served to confirm the original hypothesis (Table 3).
Table 3.
Summary of combination microbicide studies and their results
| Combination |
Study type | CI range (HIV tropism)a | Effectb | Reference | |
|---|---|---|---|---|---|
| Compound A | Compound B | ||||
| CAP | sCD4 | In vitro | 0.29–0.79 (X4) | + | 87 |
| 0.40–0.52 (R5) | + | ||||
| CAP | UC781 | In vitro | 0.12–0.23 (X4)c | + | 7 |
| 0.39–0.52 (X4)d | + | ||||
| CAP | EFV | In vitro | 0.14–0.30 (X4) | + | 7 |
| CAP | AZT | In vitro | 0.22–0.30 (X4) | + | 7 |
| PRO 2000 | IgGb12 | In vitro | 0.69–1.38 (X4) | +/− | 40 |
| 0.50–0.59 (R5)c | + | ||||
| 0.61–1.58 (R5)d | +/− | ||||
| PRO 2000 | T-20 | In vitro | 0.47–0.98 (X4) | + | 40 |
| 0.46–1.26 (R5) | +/∼ | ||||
| PRO 2000 | TAK779 | In vitro | 0.26–0.52 (R5)c | + | 40 |
| 0.20–0.69 (R5)d | + | ||||
| PRO 2000 | Cyanovirin-N | In vitro | 1.56–2.00 (X4) | − | 40 |
| 0.10–1.11 (R5) | +/∼ | ||||
| Carrageenan | MIV-150 | In vitro | 0.90–1.16 (X4) | ∼ | 36 |
| alt-PSMA | PSS | In vitro | 0.20–1.12 (X4) | +/∼ | Pirrone et al., unpublished data |
| alt-PSMA | Cyanovirin-N | In vitro | 0.17–0.34 (X4) | + | Pirrone et al., unpublished data |
| alt-PSMA | EFV | In vitro | 0.90–1.30 (X4) | ∼ | Pirrone et al., unpublished data |
CI calculated using CalcuSyn. NA, CI not available.
Combined activities were assessed as follows: +, synergistic; ∼, additive; −, antagonistic.
In a cell line.
In PBMCs.
CAP was also partnered with several antiretroviral drugs, including UC781 (7, 11), EFV, or AZT. Studies began by examining inhibition of HIV-1 strain IIIB in the MT-2 cell line (54). Using a CAP/UC781 ratio of 2,000:1, analyses using CalcuSyn established CI values for this combination that ranged from 0.12 to 0.23, indicating a high level of synergy between the two compounds. When a 40,000:1 CAP/EFV combination was examined, the CI values ranged from 0.14 to 0.30, again indicating a high level of synergy. Finally, when CAP was combined with AZT (1,000:1, CAP/AZT), the CI values ranged from 0.22 to 0.30. These results indicated that the combinations that include CAP and an RTI provide synergistic antiviral activity, at least in the context of HIV-1 IIIB infection. To address the effect of coreceptor usage, a CAP-UC781 combination was evaluated for activity against a clinical CCR5-utilizing strain of HIV-1 in peripheral blood mononuclear cells (PBMCs). The combination was highly effective and was characterized by CI values ranging from 0.39 to 0.52 (Table 3). These results not only demonstrated synergy against an R5 HIV-1 strain but also validated the previous findings in primary cell populations susceptible to HIV-1 infection (54).
Recent preclinical development efforts and clinical trials have focused on microbicides containing polyanionic inhibitors of HIV-1. PRO 2000 is one of three polyanionic inhibitors that were advanced through preclinical and clinical development. Results of a recent phase III trial of 0.5% PRO 2000 showed that although it is safe for application, PRO 2000 does not prevent HIV-1 infection in women (27, 105). Prior to the release of these results, however, in vitro studies were completed to examine the combination of PRO 2000 with the following agents: IgGb12, an HIV-1 neutralizing antibody (16, 17, 100); T-20, a gp41-derived peptide fusion inhibitor (39); TAK779, a CCR5 antagonist (6, 30); and cyanovirin-N, a bacterial lectin that binds to the high-mannose oligosaccharides located predominately in the C2-C4 region of HIV-1 gp120 (14, 15, 33, 65, 94). Activities were demonstrated against X4 (HXB2) and R5 (JR-FL) HIV-1 pseudoviruses in cell lines and PBMCs, and analyses were performed using CalcuSyn. Although most combinations provided synergistic activity in the cell line experiments, there were some exceptions: PRO 2000-IgG1b12 was antagonistic against HXB2 at the 50% inhibitory concentration (IC50) of PRO 2000, PRO 2000–T-20 and PRO 2000-cyanovirin were weakly antagonistic or additive for JR-FL at the IC50 of PRO 2000 for both combinations, and PRO 2000-cyanovirin was antagonistic at all concentrations. In experiments with all of the combinations except PRO 2000-cyanovirin, there was a trend toward increasing synergy with increasing concentration. Because the combinations consisting of PRO 2000 and TAK779 or IgG1b12 appeared to be the most promising in the cell line experiments, both pairs were also evaluated in PBMCs. For the PRO 2000-TAK779 combination, synergy was demonstrated with a trend toward increasing synergy as the concentrations of compounds were increased, mirroring the results obtained in the cell line experiments. For the combination of PRO 2000-IgG1b12 against HIV-1 JR-FL, moderate synergy was observed at low concentrations. However, unlike the results obtained in cell lines, decreasing levels of synergy were associated with increasing compound concentrations, with antagonism apparent at the highest concentrations (Table 3). These results suggested that further studies of combinations containing polyanionic compounds were warranted, especially combinations containing TAK779 (40).
Carrageenan (Carraguard in its formulated form) is another polyanionic compound developed as a candidate microbicide. To evaluate carrageenan as a combination partner, studies were conducted to explore the combination of carrageenan with the NNRTI MIV-150. This combination is referred to as PC-815 (36). In assays of in vitro activity, PC-815 appeared more efficacious than either of the single components. However, CI values calculated using CalcuSyn ranged between 0.90 and 1.16, indicating an additive relationship and suggesting that the increased efficacy of the combination is based on the additive nature of the two compounds (Table 3). In assays using clinical HIV-1 isolates, PC-815 inhibited infection with 10-fold more potency than carrageenan alone. Lastly, previous studies demonstrated that certain polyanionic compounds were inactivated in seminal plasma (71). Interestingly, however, similar studies of PC-815 demonstrated that neither component of PC-815 was inactivated in the presence of seminal plasma (36). These studies indicated that combinations containing carrageenan appear to be more efficacious than carrageenan alone. Furthermore, since neither component individually was inactivated by seminal plasma, it is possible that this combination will remain active in the vaginal environment during sexual intercourse and in the presence of semen.
Unfortunately, more recent phase III clinical trials of Carraguard demonstrated that this carrageenan-based product was not an effective inhibitor of HIV-1 transmission (86). Although carrageenan is no longer under consideration as an active microbicide, it is still being explored as an “active excipient” for future topical vaginal and rectal microbicides due to its desirable rheologic properties (55). Therefore, combination studies focused on carrageenan paired with future candidate microbicide agents may still be necessary precursors to clinical trials of safety and efficacy.
Ongoing preclinical studies have also focused on poly(styrene-alt-maleic acid) (alt-PSMA), which is a polyanionic compound characterized by antiviral activity against HIV-1 and low cytotoxicity (34, 75, 80). Combination studies with alt-PSMA included polystyrene sulfonate, cyanovirin-N, and EFV as potential combination partners (V. Pirrone and F. C. Krebs, unpublished observations). These studies incorporated both CalcuSyn and MacSynergy to provide comparative methods of analysis. However, these studies indicated that analyses provided by these two approaches are not always in agreement. Because alt-PSMA and polystyrene sulfonate are structurally similar, they are presumed to act through similar mechanisms of action. In combination experiments using these two compounds, analyses using CalcuSyn indicated interactions that were additive to antagonistic at low concentrations but synergistic at high concentrations. In contrast, MacSynergy analyses indicated that the majority of the concentrations tested were additive while some combinations were clearly characterized as antagonistic. Similarly, the alt-PSMA–cyanovirin-N combination was shown to be highly synergistic at all concentrations examined using CalcuSyn but additive at all concentrations when the analysis was performed using MacSynergy. Interestingly, the combined activity of alt-PSMA–EFV was determined to be generally additive by both CalcuSyn and MacSynergy (Table 3). However, the MacSynergy analyses also revealed instances of notable antagonism at specific compound ratios. These results indicate that the two different methods of combination analysis can provide dissimilar results and suggest that multiple methods should be used to provide more comprehensive evaluations of compound or drug combinations (V. Pirrone et al., unpublished data).
CONCLUSIONS AND PROSPECTS FOR THE FUTURE
What conclusions can be drawn from combination studies performed to date? First, combination studies require the consideration of many, potentially complex variables that may affect the safety and efficacy of combined agents. Investigations that considered mechanism of action as a variable suggested that agents with dissimilar antiviral mechanisms should be favored over compounds with similar activities. Other important determinants of combined activity include the following: (i) the choice of the combination ratio, (ii) the dilution of a given ratio, and (iii) the choice of target cells or tissues for the infection assays.
Second, computational tools used for analyses of combined activity do not necessarily provide results that are comparable. Future assessments of combined systemic and topical antiviral combinations may require multiple analytical approaches to provide a complete picture of activity and safety across a range of combination ratios and varied combination partners. Investigators involved in the development of combined agents may also need to arrive at a consensus regarding the standard analytical method (e.g., CalcuSyn versus MacSynergy) used to provide results that are comparable across the fields of therapeutic drug and microbicide development.
Third, it may be necessary to differentiate between combined activity during systemic drug administration and the activity of an agent combination in a topical microbicide. While it is tempting to use the successes of HAART to predict the efficacy of combination microbicides, there are numerous factors that may confound the ability to draw parallels between these two antiviral strategies, including (i) very large differences in combination partner concentrations administered and achieved during systemic use compared to topical application, (ii) differences in the environments in which these agents are required to function, (iii) differences in the cell types involved and the mechanisms of viral spread, and (iv) differences in the desired therapeutic outcome (i.e., treatment of an established infection versus prevention of transmission). Fundamental differences between systemic combination therapy and the use of combination microbicides may necessitate the use of dissimilar approaches to evaluating the combined activities of these agents in vitro and in vivo.
Fourth, the desired endpoints of combination studies should be considered. The mathematical approaches used to quantitatively define combined activity place the emphasis on synergistic activity, as measured by reductions in HIV-1 infection or replication, as the desired outcome. However, these analyses do not allow for the possibility that compounds may act in combination through mechanisms that are not assessed by these approaches but are nevertheless beneficial relative to the activity of each combination partner alone. For example, one compound in a combination may act to prevent the emergence of resistance to the partner compound despite its failure to achieve significant synergistic antiviral activity in the combination (84). Using the present algorithms for assessing combined activity, this potentially beneficial effect of the combined compounds would be overlooked. It may be necessary to develop new paradigms for assessing combined antiviral activity that address other beneficial endpoints (or reveal potentially detrimental effects) and provide more than a mathematical indication of combined activity. These new assessment algorithms may be particularly important as new HIV-1 inhibitors with diverse mechanisms of action are assessed for combined activity.
Finally, and perhaps most importantly, results obtained using the various cell lines, primary cells, ex vivo tissue model systems, and animal models need to be evaluated for their relevance to the clinical performance of specific drug combinations. For example, how do the exquisitely detailed and quantitative analytical methods, such as CalcuSyn and MacSynergy, predict combination efficacy in vivo? In vitro investigations using these methods have been considered to be guides for the selection of efficacious drug combinations and ratios to be used in vivo. However, the ratios of localized drug concentrations may change dramatically after in vivo administration as a consequence of differences in drug absorption, distribution, metabolism, and excretion (ADME). Therefore, drug ratios selected through in vitro experiments for their therapeutic or preventative benefits may exist only transiently in vivo as each drug is affected by specific ADME characteristics. Furthermore, drug concentration ratios that may not have been evaluated in vitro, which may be much less effective or have effects that are counter to the desired outcome, may occur following in vivo administration, again as a result of differential drug ADME. As new combination therapies and preventative approaches are explored, relationships between preclinical methods used to evaluate drug combination activities and the clinical performance of combined agents need to be firmly established.
Advances in combination therapy have provided powerful tools for the treatment of patients infected with HIV-1. However, until recently, the combinations used in patients have depended on two mechanisms of action: inhibition of HIV-1 reverse transcriptase and inhibition of protease. With the development of new antiretroviral compounds, the number of therapeutic options will continue to increase. The recent approvals of the entry inhibitor MVC and the integrase inhibitor raltegravir for clinical use will increase the number of possible drug combinations and provide more options for effective treatment. However, with each new combination developed, the possibility of adverse drug interactions that could compromise the treatment efficacy remains an important concern. Therefore, combination testing and development should be a necessary prerequisite for the development of combinatorial therapeutic strategies. Similar approaches to combination analyses should be used in the development of safe and effective microbicides used to prevent the sexual transmission of HIV-1. Studies of systemic and topical microbicide agent combinations, which will expand as more antiviral agents are developed and considered for use in microbicides, will continue to depend on the computational tools available for the analysis of combined activity.
Supplementary Material
ACKNOWLEDGMENTS
Studies of alt-PSMA were supported through a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (1 U19 AI076965; Mohamed Labib, principal investigator, and Brian Wigdahl, coprincipal Investigator) and by research development funds provided by the Department of Microbiology and Immunology and the Institute for Molecular Medicine and Infectious Disease, Drexel University College of Medicine.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Ahluwalia G., et al. 1987. Initial studies on the cellular pharmacology of 2′,3′-dideoxyinosine, an inhibitor of HIV infectivity. Biochem. Pharmacol. 36:3797–3800 [DOI] [PubMed] [Google Scholar]
- 2. AIDS Clinical Trials Group 1993. Executive summary for ACTG 155. AIDS Clinical Trials Group, Bethesda, Maryland [Google Scholar]
- 3. Allaway G. P., et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses 11:533–539 [DOI] [PubMed] [Google Scholar]
- 4. Allendoerfer R., Marquis A. J., Rinaldi M. G., Graybill J. R. 1991. Combined therapy with fluconazole and flucytosine in murine cryptococcal meningitis. Antimicrob. Agents Chemother. 35:726–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arikan S., Lozano-Chiu M., Paetznick V., Rex J. H. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baba M., et al. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. U. S. A. 96:5698–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balzarini J., et al. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 12:1129–1138 [DOI] [PubMed] [Google Scholar]
- 8. Balzarini J., Van Damme L. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787–797 [DOI] [PubMed] [Google Scholar]
- 9. Barchiesi F., et al. 1998. In vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J. Antimicrob. Chemother. 41:59–65 [DOI] [PubMed] [Google Scholar]
- 10. Barchiesi F., et al. 1999. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J. Antimicrob. Chemother. 44:65–70 [DOI] [PubMed] [Google Scholar]
- 11. Barnard J., Borkow G., Parniak M. A. 1997. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36:7786–7792 [DOI] [PubMed] [Google Scholar]
- 12. Berger A. R., et al. 1993. 2′,3′-Dideoxycytidine (ddC) toxic neuropathy: a study of 52 patients. Neurology 43:358–362 [DOI] [PubMed] [Google Scholar]
- 13. Bliss C. I. 1939. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26:585–615 [Google Scholar]
- 14. Bolmstedt A. J., O'Keefe B. R., Shenoy S. R., McMahon J. B., Boyd M. R. 2001. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol. 59:949–954 [DOI] [PubMed] [Google Scholar]
- 15. Boyd M. R., et al. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burton D. R., Barbas C. F., 3rd 1994. Human antibodies from combinatorial libraries. Adv. Immunol. 57:191–280 [DOI] [PubMed] [Google Scholar]
- 17. Burton D. R., Barbas C. F., III 1994. Human monoclonal antibodies: recent achievements. Hosp. Pract. (Off. ed.) 29:111, 114–116, 119 passim [DOI] [PubMed] [Google Scholar]
- 18. Caliendo A. M., Hirsch M. S. 1994. Combination therapy for infection due to human immunodeficiency virus type 1. Clin. Infect. Dis. 18:516–524 [DOI] [PubMed] [Google Scholar]
- 19. Campbell T. B., et al. 1993. Inhibition of human immunodeficiency virus type 1 replication in vitro by the bisheteroarylpiperazine atevirdine (U-87201E) in combination with zidovudine or didanosine. J. Infect. Dis. 168:318–326 [DOI] [PubMed] [Google Scholar]
- 20. Cheeseman S. H., et al. 1995. Phase I/II evaluation of nevirapine alone and in combination with zidovudine for infection with human immunodeficiency virus. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:141–151 [PubMed] [Google Scholar]
- 21. Chen I. J., Neamati N., MacKerell A. D., Jr 2002. Structure-based inhibitor design targeting HIV-1 integrase. Curr. Drug Targets Infect. Disord. 2:217–234 [DOI] [PubMed] [Google Scholar]
- 22. Chou K. C. 1980. A new schematic method in enzyme kinetics. Eur. J. Biochem. 113:195–198 [DOI] [PubMed] [Google Scholar]
- 23. Chou T. C., Talalay P. 1983. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol. Sci. 4:450–454 [Google Scholar]
- 24. Chou T. C., Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55 [DOI] [PubMed] [Google Scholar]
- 25. Clay P. G. 2004. Single-class therapy for HIV is not optimal care. Ann. Pharmacother. 38:1307–1309 [DOI] [PubMed] [Google Scholar]
- 26. Coffin J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483–489 [DOI] [PubMed] [Google Scholar]
- 27. Cohen J. 14 December 2009, posting date HIV outwits yet another microbicide. Sci. Now. http://news.sciencemag.org/sciencenow/2009/12/14-01.html?ref=hp
- 28. Collier A. C., et al. 1993. Combination therapy with zidovudine and didanosine compared with zidovudine alone in HIV-1 infection. Ann. Intern. Med. 119:786–793 [DOI] [PubMed] [Google Scholar]
- 29. Dorr P., et al. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dragic T., et al. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. U. S. A. 97:5639–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dueweke T. J., et al. 1993. U-90152, a potent inhibitor of human immunodeficiency virus type 1 replication. Antimicrob. Agents Chemother. 37:1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eron J. J., Jr, Johnson V. A., Merrill D. P., Chou T. C., Hirsch M. S. 1992. Synergistic inhibition of replication of human immunodeficiency virus type 1, including that of a zidovudine-resistant isolate, by zidovudine and 2′,3′-dideoxycytidine in vitro. Antimicrob. Agents Chemother. 36:1559–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esser M. T., et al. 1999. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J. Virol. 73:4360–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang W., et al. 2009. Poly(styrene-alt-maleic anhydride) derivatives as potent anti-HIV microbicide candidates. Bioorg. Med. Chem. Lett. 19:1903–1907 [DOI] [PubMed] [Google Scholar]
- 35. Fatkenheuer G., et al. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11:1170–1172 [DOI] [PubMed] [Google Scholar]
- 36. Fernandez-Romero J. A., et al. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 34:9–14 [DOI] [PubMed] [Google Scholar]
- 37. Finney D. J. 1942. The analysis of toxicity tests on mixtures of poisons. Ann. Appl. Biol. 29:82–94 [Google Scholar]
- 38. Fischl M. A., et al. 1993. Zalcitabine compared with zidovudine in patients with advanced HIV-1 infection who received previous zidovudine therapy. Ann. Intern. Med. 118:762–769 [DOI] [PubMed] [Google Scholar]
- 39. Furuta R. A., Wild C. T., Weng Y., Weiss C. D. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276–279 [DOI] [PubMed] [Google Scholar]
- 40. Gantlett K. E., Weber J. N., Sattentau Q. J. 2007. Synergistic inhibition of HIV-1 infection by combinations of soluble polyanions with other potential microbicides. Antiviral Res. 75:188–197 [DOI] [PubMed] [Google Scholar]
- 41. Gerstoft J., et al. 2003. Low efficacy and high frequency of adverse events in a randomized trial of the triple nucleoside regimen abacavir, stavudine and didanosine. AIDS 17:2045–2052 [DOI] [PubMed] [Google Scholar]
- 42. Goldin A., Mantel N. 1957. The employment of combinations of drugs in the chemotherapy of neoplasia: a review. Cancer Res. 17:635–654 [PubMed] [Google Scholar]
- 43. Goldman M. E., et al. 1991. Pyridinone derivatives: specific human immunodeficiency virus type 1 reverse transcriptase inhibitors with antiviral activity. Proc. Natl. Acad. Sci. U. S. A. 88:6863–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gulick R. M., et al. 2008. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 359:1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Halpern V., et al. 2008. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a phase III trial in Nigeria. PLoS One 3:e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hicks C., Gulick R. M. 2009. Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 48:931–939 [DOI] [PubMed] [Google Scholar]
- 47. Hirsch M. S., et al. , for the International AIDS Society—USA Panel 1998. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. JAMA 279:1984–1991 [DOI] [PubMed] [Google Scholar]
- 48. Huff B. 2006. Something new under the sun. Maraviroc poised for approval. GMHC Treat. Issues 20:1–4 [PubMed] [Google Scholar]
- 49. Jacobson J. M., et al. 2000. Single-dose safety, pharmacology, and antiviral activity of the human immunodeficiency virus (HIV) type 1 entry inhibitor PRO 542 in HIV-infected adults. J. Infect. Dis. 182:326–329 [DOI] [PubMed] [Google Scholar]
- 50. Johnson V. A., et al. 1991. Two-drug combinations of zidovudine, didanosine, and recombinant interferon-alpha A inhibit replication of zidovudine-resistant human immunodeficiency virus type 1 synergistically in vitro. J. Infect. Dis. 164:646–655 [DOI] [PubMed] [Google Scholar]
- 51. Ketas T. J., et al. 2003. Entry inhibitors SCH-C, RANTES, and T-20 block HIV type 1 replication in multiple cell types. AIDS Res. Hum. Retroviruses 19:177–186 [DOI] [PubMed] [Google Scholar]
- 52. Kilby J. M., et al. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302–1307 [DOI] [PubMed] [Google Scholar]
- 53. Lederman M. M., Offord R. E., Hartley O. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371–382 [DOI] [PubMed] [Google Scholar]
- 54. Liu S., Lu H., Neurath A. R., Jiang S. 2005. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 49:1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y., Zhu Y. Y., Wei G., Lu W. Y. 2009. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 37:306–312 [DOI] [PubMed] [Google Scholar]
- 56. Loewe S. 1957. Antagonisms and antagonists. Pharmacol. Rev. 9:237–242 [PubMed] [Google Scholar]
- 57. Loewe S. 1953. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3:285–290 [PubMed] [Google Scholar]
- 58. Mansky L. M., Temin H. M. 1995. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 69:5087–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martinez-Irujo J. J., Villahermosa M. L., Alberdi E., Santiago E. 1996. A checkerboard method to evaluate interactions between drugs. Biochem. Pharmacol. 51:635–644 [DOI] [PubMed] [Google Scholar]
- 60. Meek T. D. 1992. Inhibitors of HIV-1 protease. J. Enzyme Inhib. 6:65–98 [DOI] [PubMed] [Google Scholar]
- 61. Merluzzi V. J., et al. 1990. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science 250:1411–1413 [DOI] [PubMed] [Google Scholar]
- 62. Microbicide Trials Network 2009. MTN fact sheet: HPTN 035 at a glance. Microbicide Trials Network, Pittsburgh, PA: http://www.mtnstopshiv.org/news/studies/hptn035/facts [Google Scholar]
- 63. Microbicide Trials Network. 2009. Trial finds microbicide promising as HIV prevention method for women. Microbicide Trials Network, Pittsburgh, PA: http://www.mtnstopshiv.org/node/765 [Google Scholar]
- 64. Mitsuya H., Broder S. 1986. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2′,3′-dideoxynucleosides. Proc. Natl. Acad. Sci. U. S. A. 83:1911–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mori T., Boyd M. R. 2001. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob. Agents Chemother. 45:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moyle G. J. 1997. Current knowledge of HIV-1 reverse transcriptase mutations selected during nucleoside analogue therapy: the potential to use resistance data to guide clinical decisions. J. Antimicrob. Chemother. 40:765–777 [DOI] [PubMed] [Google Scholar]
- 67. Nagashima K. A., et al. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121–1125 [DOI] [PubMed] [Google Scholar]
- 68. Najera I., et al. 1995. Pol gene quasispecies of human immunodeficiency virus: mutations associated with drug resistance in virus from patients undergoing no drug therapy. J. Virol. 69:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Najera I., et al. 1994. Natural occurrence of drug resistance mutations in the reverse transcriptase of human immunodeficiency virus type 1 isolates. AIDS Res. Hum. Retroviruses 10:1479–1488 [DOI] [PubMed] [Google Scholar]
- 70. Neurath A. R., Strick N., Jiang S., Li Y. Y., Debnath A. K. 2002. Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of “dead-end” gp41 six-helix bundles. BMC Infect. Dis. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Neurath A. R., Strick N., Li Y. Y. 2006. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect. Dis. 6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Neurath A. R., Strick N., Li Y. Y., Debnath A. K. 2001. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect. Dis. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olson W. C., et al. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73:4145–4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Panel on Antiretroviral Guidelines for Adults and Adolescents. 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://www.aidsinfo.nih.gov/ContentFiles/Adultand AdolescentsGL.pdf [Google Scholar]
- 75. Pirrone V., et al. 2010. A styrene-alt-maleic acid copolymer is an effective inhibitor of R5 and X4 human immunodeficiency virus type 1 infection. J. Biomed. Biotechnol. 2010:548749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pluymers W., De Clercq E., Debyser Z. 2001. HIV-1 integration as a target for antiretroviral therapy: a review. Curr. Drug Targets Infect. Disord. 1:133–149 [DOI] [PubMed] [Google Scholar]
- 77. Prasad V. R., Goff S. P. 1990. Structure-function studies of HIV reverse transcriptase. Ann. N. Y. Acad. Sci. 616:11–21 [DOI] [PubMed] [Google Scholar]
- 78. Prichard M. N., Prichard L. E., Shipman C., Jr 1993. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob. Agents Chemother. 37:540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Prichard M. N., Shipman C., Jr 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181–205 [DOI] [PubMed] [Google Scholar]
- 80. Qian J., et al. 2005. Use of the synthetic copolymer PSMA as a component in a combination microbicide active against HIV-1. Retrovirology 2(Suppl. 1):S96 [Google Scholar]
- 81. Richman D. D., et al. 1987. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N. Engl. J. Med. 317:192–197 [DOI] [PubMed] [Google Scholar]
- 82. Richman D. D., et al. 1994. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J. Virol. 68:1660–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Safarian D., Carnec X., Tsamis F., Kajumo F., Dragic T. 2006. An anti-CCR5 monoclonal antibody and small molecule CCR5 antagonists synergize by inhibiting different stages of human immunodeficiency virus type 1 entry. Virology 352:477–484 [DOI] [PubMed] [Google Scholar]
- 84. Schooley R. T., et al. 1996. Phase 1 study of combination therapy with L-697,661 and zidovudine. The ACTG 184 Protocol Team. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:363–370 [DOI] [PubMed] [Google Scholar]
- 85. Shiau G. T., Schinazi R. F., Chen M. S., Prusoff W. H. 1980. Synthesis and biological activities of 5-(hydroxymethyl, azidomethyl, or aminomethyl)-2′-deoxyuridine and related 5′-substituted analogues. J. Med. Chem. 23:127–133 [DOI] [PubMed] [Google Scholar]
- 86. Skoler-Karpoff S., et al. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 87. Smith D. H., et al. 1987. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science 238:1704–1707 [DOI] [PubMed] [Google Scholar]
- 88. Sondak V. K., Korn E. L., Kern D. H. 1988. In vitro testing of chemotherapeutic combinations in a rapid thymidine incorporation assay. Int. J. Cell Cloning 6:378–391 [DOI] [PubMed] [Google Scholar]
- 89. Sondak V. K., Korn E. L., Morton D. L., Kern D. H. 1988. Testing chemotherapeutic combinations in the human tumor colony–forming assay. J. Surg. Oncol. 37:156–160 [DOI] [PubMed] [Google Scholar]
- 90. Strizki J. M., et al. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 98:12718–12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Summa V., et al. 2008. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 51:5843–5855 [DOI] [PubMed] [Google Scholar]
- 92. Tremblay C., Merrill D. P., Chou T. C., Hirsch M. S. 1999. Interactions among combinations of two and three protease inhibitors against drug-susceptible and drug-resistant HIV-1 isolates. J. Acquir. Immune Defic. Syndr. 22:430–436 [DOI] [PubMed] [Google Scholar]
- 93. Tremblay C. L., et al. 2002. Anti-human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob. Agents Chemother. 46:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tsai C. C., et al. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 19:535–541 [DOI] [PubMed] [Google Scholar]
- 95. Tsamis F., et al. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 77:5201–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Van Damme L., et al. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971–977 [DOI] [PubMed] [Google Scholar]
- 97. van de Wijgert J. H., et al. 2007. Carraguard vaginal gel safety in HIV-positive women and men in South Africa. J. Acquir. Immune Defic. Syndr. 46:538–546 [DOI] [PubMed] [Google Scholar]
- 98. van Leeuwen R., et al. 2003. A randomized trial to study first-line combination therapy with or without a protease inhibitor in HIV-1-infected patients. AIDS 17:987–999 [DOI] [PubMed] [Google Scholar]
- 99. van Moorsel C. J., et al. 1999. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br. J. Cancer. 80:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Veazey R. S., et al. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 101. Veldstra H. 1956. Synergism and potentiation with special reference to the combination of structural analogues. Pharmacol. Rev. 8:339–387 [PubMed] [Google Scholar]
- 102. Vermeire K., et al. 2003. The Anti-HIV potency of cyclotriazadisulfonamide analogs is directly correlated with their ability to down-modulate the CD4 receptor. Mol. Pharmacol. 63:203–210 [DOI] [PubMed] [Google Scholar]
- 103. Vermeire K., et al. 2004. CADA, a novel CD4-targeted HIV inhibitor, is synergistic with various anti-HIV drugs in vitro. AIDS 18:2115–2125 [DOI] [PubMed] [Google Scholar]
- 104. Walker D. K., et al. 2005. Species differences in the disposition of the CCR5 antagonist, UK-427,857, a new potential treatment for HIV. Drug Metab. Dispos. 33:587–595 [DOI] [PubMed] [Google Scholar]
- 105. Warren M., Marshall K. 2009. AVAC statement on MDP 301. Press release, 14 December 2009. AIDS Vaccine Advisory Coalition, New York, NY: http://www.hivvaccineenterprise.org/sites/default/files/avac%20statement%20on%20MPD%20301%20dec%2014.pdf [Google Scholar]
- 106. Webb J. L. 1963. Enzyme and metabolic inhibitors. Academic Press, New York, NY [Google Scholar]
- 107. Woolley D. W. 1952. A study of antimetabolites. Wiley, New York, NY [Google Scholar]
- 108. Yarchoan R., et al. 1988. Phase I studies of 2′,3′-dideoxycytidine in severe human immunodeficiency virus infection as a single agent and alternating with zidovudine (AZT). Lancet 1:76–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.