Abstract
Acinetobacter baumannii isolate AP2 was recovered from a bronchial lavage sample of a patient hospitalized in Paris, France. A. baumannii AP2 was resistant to all β-lactams, including carbapenems, and expressed the extended-spectrum β-lactamase (ESBL) PER-7, which differs from PER-1 by 4 amino acid substitutions. Compared to PER-1, PER-7 possessed higher-level hydrolytic activities against cephalosporins and aztreonam. The blaPER-7 gene was chromosomally located and associated with a mosaic class 1 integron structure. Additionally, isolate AP2 expressed the carbapenem-hydrolyzing oxacillinase OXA-23 and the 16S RNA methylase ArmA, conferring high-level resistance to aminoglycosides.
INTRODUCTION
Acinetobacter baumannii is an opportunistic pathogen that is an important source of nosocomial infections such as pneumonia and urinary tract and wound infections (2). Treatment of infections due to this microorganism is becoming a serious clinical concern since A. baumannii is frequently resistant to multiple antibiotics (21, 22). The main mechanism of resistance to β-lactams in A. baumannii corresponds to the production of β-lactamases. In A. baumannii, resistance to carbapenems is mostly related to production of metallo-β-lactamases or carbapenem-hydrolyzing oxacillinases (CHDL) (33), while resistance to broad-spectrum cephalosporins mostly results from overexpression of the natural AmpC-type enzyme (4) or from acquisition of extended-spectrum β-lactamases (ESBLs). The ESBL genes that have been identified in A. baumannii are blaPER-1 and blaPER-2, blaGES-11 and blaGES-14, blaVEB-1 and blaVEB-1a, and blaTEM-92 and blaCTX-M-2 (3, 7, 14–16, 20, 27, 28, 30). One study reported the occurrence of a PER-like ESBL in A. baumannii in Korea, but nothing is known about its precise hydrolytic properties (19). The blaPER-1 gene was first identified in a Pseudomonas aeruginosa isolate (17), and then this gene was identified worldwide in many species, including Alcaligenes faecalis (13), Salmonella enterica (25), Proteus mirabilis (18), Providencia stuartii (25), Aeromonas media (24), Aeromonas punctata (39), and A. baumannii (27, 30). Several PER-like variants have been identified, corresponding to two subgroups, one consisting of PER-1-like point mutant derivatives (namely, PER-3, PER-4, and PER-5) and one consisting of PER-2 and PER-6, differing by 22 amino acids from each other and showing 85% amino acid identity with PER-1 (9, 20, 35). The blaPER-1 gene was found to be part of a composite transposon named Tn1213 (25). This transposon is made of one copy of ISPa12 and one copy of the unrelated ISPa13 element (25). The blaPER-2 gene was identified in association with one copy of ISPa12 upstream of the β-lactamase gene but no copy of ISPa13 (25, 35). The blaPER-6 gene identified in Aeromonas allosaccharophila was located inside a mosaic structure composed of truncated genes encoding mostly proteins of unknown functions (9).
A. baumannii AP2 was isolated from a bronchial sample of a 30-year-old patient hospitalized at the intensive care unit of the Necker-Enfants Malades University hospital (Paris, France) in April 2010. Identification of A. baumannii AP2 was performed by using the API32GN system (bioMérieux, Marcy l'Etoile, France) and was confirmed by 16S rRNA gene sequencing as described previously (6). A. baumannii AP2 was resistant to penicillins, β-lactamase inhibitor-penicillin combinations, broad-spectrum cephalosporins, aztreonam, and carbapenems (Table 1) (5). Synergy between ceftazidime and clavulanic acid suggested the production of an ESBL. In addition, A. baumannii AP2 was resistant to aminoglycosides, fluoroquinolones, sulfonamides, and tetracycline and showed susceptibility only to colistin (MIC of 0.5 μg/ml). Whole-cell DNA of A. baumannii isolate AP2 was extracted as described previously (23). This DNA was used as a template under standard PCR conditions (37) with a series of primers designed for the detection of class A β-lactamase genes (blaTEM, blaSHV, blaPER-1, blaVEB-1, blaGES-1, and blaCTX-M) (3, 26, 30, 31, 34), of the intrinsic blaampC gene (10), of class B β-lactamase genes (blaIMP, blaVIM, blaSIM, and blaNDM), and of class D β-lactamase genes (blaOXA-23, blaOXA-40, blaOXA-51, blaOXA-58, and blaOXA-143) (11, 27, 28, 32). Detection of the ISAba1 element upstream of the blaampC and blaOXA-51 genes was performed as described previously (10). A. baumannii AP2 harbored a blaOXA-51-like naturally occurring gene, and further sequencing revealed that it corresponded to blaOXA-64, also known as blaOXA-Ab-2 (32). The ISAba1 element was not identified upstream of the intrinsic blaOXA-Ab-2 and blaampC genes in A. baumannii AP2, suggesting that both genes were not overexpressed. A. baumannii isolate AP2 harbored the blaOXA-23 CHDL gene, encoding resistance to carbapenems, and possessed a blaPER-like gene that corresponded to the novel blaPER-7 gene variant (see below). In addition, A. baumannii AP2 harbored the 16S RNA methylase armA gene, conferring high-level resistance to all aminoglycosides.
Table 1.
MICs of β-lactams for A. baumannii strain AP2, E. coli pTOPO-PER-1, E. coli pTOPO-PER-6, and E. coli pTOPO-PER-7 in E. coli TOP10 or E. coli HB4 reference strainsa
| β-Lactams | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A. baumannii isolate AP2 | E. coli TOP10 (pTOPO-PER-1) | E. coli TOP10 (pTOPO-PER-6) | E. coli TOP10 (pTOPO-PER-7) | E. coli TOP10 | E. coli HB4 (pTOPO-PER-1) | E. coli HB4 (pTOPO-PER-6) | E. coli HB4 (pTOPO-PER-7) | E. coli HB4 | |
| Amoxicillin | >256 | >256 | >256 | >256 | 2 | >256 | >256 | >256 | 8 |
| Amoxicillin-CLA | >256 | 4 | 4 | 8 | 2 | 32 | 64 | 64 | 8 |
| Ticarcillin | >256 | >256 | >256 | >256 | 2 | >256 | >256 | >256 | 4 |
| Ticarcillin-CLA | >256 | 16 | 16 | 16 | 2 | 32 | 32 | 32 | 4 |
| Piperacillin | >256 | 32 | 256 | 256 | 1 | 128 | 256 | 256 | 4 |
| Piperacillin-TZP | 128 | 8 | 16 | 16 | 1 | 16 | 32 | 32 | 4 |
| Cephalothin | >256 | >256 | >256 | >256 | 4 | >256 | >256 | >256 | 64 |
| Cefuroxime | >256 | >256 | >256 | >256 | 2 | >256 | >256 | >256 | 16 |
| Cefoxitin | 64 | 2 | 2 | 2 | 2 | >256 | >256 | >256 | >256 |
| Cefotaxime | >256 | 8 | 16 | 32 | 0.06 | 64 | 64 | 64 | 0.38 |
| Cefotaxime-CLA | 0.5 | 0.03 | 0.06 | 0.12 | 0.06 | 0.5 | 0.5 | 0.5 | 0.38 |
| Ceftazidime | >256 | >256 | >256 | >256 | 0.12 | >256 | >256 | >256 | 4 |
| Cefepime | >256 | 4 | 4 | 4 | 0.06 | 32 | 32 | 32 | 0.75 |
| Cefepime-CLA | 1 | 0.06 | 0.06 | 0.12 | 0.06 | 0.5 | 0.5 | 1 | 0.75 |
| Cefpirome | >256 | 16 | 8 | 16 | 0.06 | >32 | >32 | >32 | 0.5 |
| Aztreonam | >256 | 128 | 128 | 256 | 0.03 | >256 | >256 | >256 | 4 |
| Meropenem | >32 | 0.03 | 0.03 | 0.03 | 0.03 | 0.5 | 1 | 0.5 | 0.25 |
| Ertapenem | ND | 0.03 | 0.06 | 0.03 | 0.03 | 1 | 4 | 1.5 | 1 |
| Imipenem | >32 | 0.12 | 0.12 | 0.12 | 0.12 | 0.5 | 4 | 1.5 | 0.25 |
| Imipenem-CLA | >32 | 0.12 | 0.12 | 0.12 | 0.12 | 0.5 | 1 | 0.5 | 0.25 |
CLA, clavulanic acid (4 μg/ml); TZB, tazobactam (4 μg/ml); ND, not determined.
Shotgun cloning using HindIII-restricted genomic DNA and HindIII-restricted pBK-CMV plasmid was performed as described previously (17). Recombinant plasmids were selected onto Trypticase soy (TS) agar plates containing ceftazidime (2 μg/ml) and kanamycin (30 μg/ml). The resulting recombinant Escherichia coli strain (pBK-PER-7) displayed an ESBL phenotype with high-level resistance to broad-spectrum cephalosporins and aztreonam and remained susceptible to cefoxitin and carbapenems. In addition, it was resistant to sulfonamides and chloramphenicol and showed reduced susceptibility to rifampin, but it remained susceptible to fluoroquinolones, aminoglycosides, and tetracycline. Sequence analysis of the cloned DNA fragment identified the blaPER-7 gene. Compared to PER-1, PER-7 exhibited four amino acid substitutions, Q119E, V245I, R246K, and A294T (1). The last three substitutions had previously been identified in PER-6.
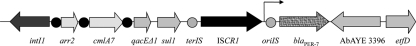
Sequencing of the insert of recombinant plasmid pBK-PER-7 revealed that an ISCR1 element (38) was present immediately upstream of the blaPER-7 gene, but no additional ISCR1 copy was identified downstream of blaPER-7. ISCR1 possessed, at its right extremity, promoter sequences named PCR1-1 (made of the −35 [TAAACG] and −10 [TAAGAT] regions) that were previously shown to be responsible for the expression of blaCTX-M and qnrA genes (36). The similar genetic context (the distance separating the oriIS extremity of ISCR1 from the blaPER-7 start codon being only 74 bp) that we identified in A. baumannii AP strongly suggests that blaPER-7 expression might be also under the control of PCR1-1.
Further analysis of the ISCR1 left extremity (according to Fig. 1) identified a class 1 integron that contained two gene cassettes, namely, arr-2 and cmlA7, that encode resistance to rifampin and chloramphenicol, respectively. The 3′ conserved sequence (3′CS) extremity was made of a fusion of qacEΔ1 and sul1, but the orf5 gene, usually described as being associated with a class 1 integron, was absent, as previously described, with the complex class 1 integron associated with ISCR1 (38).
Fig. 1.
Schematic map representing the genetic structure surrounding the blaPER-7 gene. The genes and their corresponding transcriptional orientations are indicated by horizontal arrows. The 59-base elements are indicated by a black circle. The replication origin oriIS and terminus terIS of ISCR1 are indicated by a gray circle. AbAYE, A. baumannii AYE.
Downstream of the blaPER-7 gene, a gene encoding a hypothetical protein followed by a gene encoding a putative electron transfer flavoprotein-ubiquinone oxidoreductase previously found on the chromosome of A. baumannii AYE (8) were identified, further reinforcing the hypothesis of a chromosomal location for the ISCR1-blaPER-7-associated complex class 1 integron (Fig. 1).
In order to evaluate and compare the spectra of hydrolysis of PER-1, PER-6, and PER-7, the corresponding genes were amplified using the degenerated primers PERextS and specific primers PER-1extAS and PER-6extAS as described previously (9). A blaPER-7 amplicon was obtained with primers PERextS and PER-1extAS. The obtained PCR fragment was purified with a QIAquick column (Qiagen, Courtaboeuf, France) and cloned into the pTOPO vector (Qiagen, Courtaboeuf, France). The three genes were respectively cloned in the same vector and expressed in E. coli TOP10. It gave rise to the recombinant E. coli TOP10(pPER-1), E. coli TOP10(pPER-6), and E. coli TOP10(pPER-7) strains, expressing PER-1, PER-6, and PER-7, respectively. In order to compare the catalytic properties of PER-1, PER-6, and PER-7, the corresponding genes were cloned and expressed in E. coli TOP10 under the control of an identical promoter. Then, these recombinant plasmids were electroporated into E. coli HB4 in order to evaluate the impact of their production in an E. coli background that corresponds to a porin-deficient strain. As expected, expression of the blaPER-1, blaPER-6, and blaPER-7 genes in E. coli TOP10 conferred resistance to penicillins, to broad-spectrum cephalosporins, and to monobactams (Table 1). In E. coli HB4 lacking porins OmpF and OmpC (12), expression of both the blaPER-6 and the blaPER-7 genes conferred reduced susceptibility to carbapenems, whereas that of blaPER-1 did not. Noteworthy, the MICs of imipenem, meropenem, and ertapenem were higher for PER-6 than for PER-7, suggesting a weaker carbapenemase activity for PER-7. In contrast, the MICs of cefotaxime and aztreonam were higher for E. coli expressing PER-7 than for E. coli expressing either PER-6 or PER-1.
In order to characterize more precisely whether PER-7 might possess specific catalytic properties, a kinetic study was conducted as described previously (29). E. coli DH10B (pPER-7) produced a β-lactamase with a pI value of 6.1, whereas the pI values of PER-1 and PER-6 are 5.4 and 6.4, respectively (9, 17). PER-7 was purified (>90% as estimated by SDS-PAGE analysis) from E. coli TOP10 pTOPO-PER-7 crude extract by using a two-step chromatography process (a anion exchange at pH 7.5 followed by an anion exchange at pH 6.9 using a Q Sepharose column).
β-Lactamase PER-7 had a broad-spectrum hydrolysis profile, including penicillins, broad-spectrum cephalosporins, and to a lesser extent carbapenems (Table 2). PER-7 was less susceptible to inhibition by clavulanic acid and tazobactam than PER-6. The 50 percent inhibitory concentrations (IC50s) of clavulanic acid were 3 and 0.3 μM for PER-7 and PER-6, respectively, and those of tazobactam were 1 and 2 μM for PER-7 and PER-6, respectively. Since a synergy image was observed in vitro between cefoxitin and ceftazidime, IC50s were also measured using cefoxitin as an inhibitor. The IC50 for cefoxitin was at 2 μM. PER-7 showed higher catalytic efficiencies (kcat/Km) for cefotaxime, ceftazidime, cefepime, and aztreonam than PER-6 (Table 2). Overall, PER-7 showed higher Km values (lower affinity) for most substrates. The kcat/Km values for cefotaxime and ceftazidime, respectively, were slightly higher for PER-7 than for PER-6. Hydrolysis of imipenem was detected, but at lower rate than that observed for PER-6. In fact, the Km value for imipenem was 100-fold higher for PER-7 than for PER-6, evidencing the lower affinity of PER-6 for that substrate. In contrast, no significant hydrolysis was detected for meropenem.
Table 2.
Kinetic parameter values for β-lactamase PER-7a
| Substrate |
Km (μM) |
kcat (s−1) |
kcat/Km (mM−1/s−1) |
|||
|---|---|---|---|---|---|---|
| PER-7 | PER-6 | PER-7 | PER-6 | PER-7 | PER-6 | |
| Benzypenicillin | 90 | 200 | 15 | 5 | 170 | 25 |
| Amoxicillin | 150 | NA | 5 | NA | 30 | NA |
| Ticarcillin | 25 | 9 | 1 | 0.4 | 40 | 50 |
| Piperacillin | 10 | 4 | 0.3 | 0.1 | 30 | 25 |
| Cephalothin | 100 | 55 | 20 | 8 | 200 | 145 |
| Cephaloridine | 550 | NA | 130 | NA | 240 | NA |
| Cefoxitin | 0.08b | NA | ND | ND | ND | ND |
| Cefotaxime | 1,000 | 900 | 100 | 40 | 100 | 45 |
| Ceftazidime | 3,000 | 1,000 | 120 | 10 | 40 | 10 |
| Cefepime | 2,300 | 2,000 | 70 | 10 | 30 | 5 |
| Aztreonam | 90 | 40 | 10 | 3 | 110 | 75 |
| Imipenem | 100 | 1.5 | 0.05 | 0.006 | 0.5 | 4 |
| Meropenem | 5b | 10 | ND | 0.004 | ND | 0.4 |
Data are the means of results from three independent experiments. The standard deviations were within 10% of the means. Data for PER-6 are from Girlich et al. (9). ND, not determinable; NH, no detectable hydrolysis; NA, not available.
Km was obtained as a Ki value.
This study identified a novel PER-type β-lactamase whose gene was identified inside a novel genetic structure. PER-7, as already observed for PER-6 identified in A. allosaccharophila from the aquatic environment, exhibited a weak but significant carbapenemase activity. The level of that activity remained quite low; thus, it cannot provide resistance to carbapenems by itself, but we showed that it may confer resistance or intermediate susceptibility to E. coli once associated with poor outer membrane permeability. This result may explain the high level of resistance to carbapenems of A. baumannii AP2 that coproduced PER-7 and OXA-23. This work further demonstrates that blaPER-like gene acquisition may be linked to a variety of genetic elements (Fig. 1), and the involvement of ISCR1 is especially noteworthy, since that peculiar insertion (IS) element seems to be significantly involved in the genetic plasticity of A. baumannii.
Acknowledgments
This work was funded partially by a grant from the INSERM (U914) and the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and mostly by grants from the European Community (TROCAR, HEALTH-F3-2008-223031, and TEMPOtest-QC, HEALTH-2009-241742) and from the INSERM (U914).
Footnotes
Published ahead of print on 7 March 2011.
REFERENCES
- 1. Ambler R. P., et al. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergogne-Bérézin E., Towner K. J. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnin R. A., et al. 2011. Carbapenem-hydrolyzing GES-type extended-spectrum β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonomo R. A., Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 43(Suppl. 2):S49–S56 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing. CLSI M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Dortet L., Legrand P., Soussy C.-J., Cattoir V. 2006. Bacterial identification, clinical significance, and antimicrobial susceptibilities of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J. Clin. Microbiol. 44:4471–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endimiani A., et al. 2007. Spread in an Italian hospital of a clonal Acinetobacter baumannii strain producing the TEM-92 extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 51:2211–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fournier P. E., et al. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Girlich D., Poirel L., Nordmann P. 2010. PER-6, an extended-spectrum β-lactamase from Aeromonas allosaccharophila. Antimicrob. Agents Chemother. 54:1619–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Héritier C., Poirel L., Nordmann P. 2006. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12:123–130 [DOI] [PubMed] [Google Scholar]
- 11. Higgins P. G., Poirel L., Lehmann M., Nordmann P., Seifert H. 2009. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5035–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mammeri H., Nordmann P., Berkani A., Eb F. 2008. Contribution of extended-spectrum AmpC (ESAC) β-Lactamases to carbapenem resistance in Escherichia coli. FEMS Microbiol. Lett. 282:238–240 [DOI] [PubMed] [Google Scholar]
- 13. Mantengoli E., Rossolini G. M. 2005. Tn5393d, a complex Tn5393 derivative carrying the PER-1 extended-spectrum β-lactamase gene and other resistance determinants. Antimicrob. Agents Chemother. 49:3289–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moubareck C., Bremont S., Conroy M. C., Courvalin P., Lambert T. 2009. GES-11, a novel integron-associated GES variant in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:3579–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naas T., et al. 2006. Emergence of PER and VEB extended-spectrum β-lactamases in Acinetobacter baumannii in Belgium. J. Antimicrob. Chemother. 58:178–182 [DOI] [PubMed] [Google Scholar]
- 16. Nagano N., Nagano Y., Cordevant C., Shibata N., Arakawa Y. 2004. Nosocomial transmission of CTX-M-2 β-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J. Clin. Microbiol. 42:3978–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordmann P., et al. 1993. Characterisation of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagani L., et al. 2002. Emerging extended-spectrum β-lactamases in Proteus mirabilis. J. Clin. Microbiol. 40:1549–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park Y. K., et al. 2009. Two distinct clones of carbapenem-resistant Acinetobacter baumannii isolates from Korean hospitals. Diagn. Microbiol. Infect. Dis. 64:389–395 [DOI] [PubMed] [Google Scholar]
- 20. Pasterán F., et al. 2006. Emergence of PER-2 and VEB-1a in Acinetobacter baumannii strains in the Americas. Antimicrob. Agents Chemother. 50:3222–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paterson D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43:S43–S48 [DOI] [PubMed] [Google Scholar]
- 22. Peleg A. Y., Seifert H., Paterson D. L. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philippon L. N., Naas T., Bouthors A. T., Barakett V., Nordmann P. 1997. OXA-18, a class D clavulanic-acid inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Picão R., et al. 2008. Expanded-spectrum β-lactamase PER-1 in an environmental Aeromonas media isolate from Switzerland. Antimicrob. Agents Chemother. 52:3461–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poirel. L., Cabanne L., Vahaboglu H., Nordmann P. 2005. Genetic environment and expression of the extended-spectrum β-lactamase blaPER-1 gene in Gram-negative bacteria. Antimicrob. Agents Chemother. 49:1708–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poirel L., Girlich D., Naas T., Nordmann P. 2001. OXA-28, an extended spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poirel L., et al. 1999. Extended-spectrum β-lactamase-producing strain of Acinetobacter baumannii isolated from a patient in France. J. Antimicrob. Chemother. 43:157–158 [PubMed] [Google Scholar]
- 28. Poirel L., Lagrutta E., Taylor P., Pham J., Nordmann P. 2010. Emergence of metallo-β-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob. Agents Chemother. 54:4914–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poirel L., Le Thomas I., Naas T., Karim A., Nordmann P. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poirel L., Menuteau O., Agoli N., Cattoen C., Nordmann P. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poirel L., et al. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poirel L., Naas T., Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54:24–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poirel L., Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826–836 [DOI] [PubMed] [Google Scholar]
- 34. Poirel L., et al. 2001. GES-2, a class A β-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Power P., et al. 2007. Biochemical characterization of PER-2 and genetic environment of blaPER-2. Antimicrob. Agents Chemother. 51:2359–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodríguez-Martínez J. M., Poirel L., Canton R., Nordmann P. 2006. Common region CR1 for expression of antibiotic resistance genes. Antimicrob. Agents Chemother. 50:2544–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Toleman M. A., Bennett P. M., Walsh T. R. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xia R., Guo X., Zhang Y., Xu H. 2010. qnrVC-like gene located in a novel complex class 1 integron harboring the ISCR1 element in an Aeromonas punctata strain from an aquatic environment in Shandong Province, China. Antimicrob. Agents Chemother. 54:3471–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]