Abstract
Current therapies for human African trypanosomiasis (HAT) are unsatisfactory and under threat from emerging drug resistance linked to the loss of transporters, e.g., the P2 aminopurine transporter (TbAT1). Here we compare the uptake and trypanocidal properties of furamidine (DB75), recently evaluated in clinical trials against stage 1 (haemolymphatic) HAT, and two aza analogues, DB820 and CPD0801 (DB829), which are candidate compounds for treatment of stage 2 (neurological) disease. Values of 50% inhibitory concentrations (IC50s) determined in vitro against both wild-type and transporter mutant parasites were submicromolar, with DB75 trypanotoxicity shown to be better than and DB820 trypanotoxicity similar to that of the widely used veterinary trypanocide diminazene, while CPD0801 was less active. Activity correlated with uptake and with the minimum drug exposure time necessary to kill trypanosomes: DB75 accumulated at double and 10-fold the rates of DB820 and CPD0801, respectively. All three compounds inhibited P2-mediated adenosine transport with similar Ki values, indicating affinity values for this permease in the low to submicromolar range. Uptake of DB75, DB820, and CPD0801 was significantly reduced in tbat1−/− parasites and was sensitive to inhibition by adenine, showing that all three compounds are substrates for the P2 transporter. Uptake in vitro was significantly less than that seen with parasites freshly isolated from infected rats, correlating with a downregulation of P2 activity in vitro. We conclude that DB75, DB820, and CPD0801 are actively accumulated by Trypanosoma brucei brucei, with P2 as the main transport route. The aza analogues of DB75 accumulate more slowly than furamidine itself and reveal less trypanocidal activity in standard in vitro drug sensitivity assays.
INTRODUCTION
Human African trypanosomiasis (HAT), also known as sleeping sickness, occurs in 36 countries across equatorial Africa, where millions of people are at risk of infection (46). The disease is caused by two subspecies of Trypanosoma brucei. T. b. rhodesiense is transmitted by tsetse flies in Eastern and Southern Africa, which are areas of trypanosomiasis endemicity, and is associated with a rapidly progressing form of the disease; T. b. gambiense, which circulates in Western and Central Africa, causes a more chronic condition (5). Both forms of the disease are believed to be universally fatal in the absence of drug intervention. There are two distinct stages in the progression of HAT: in stage 1 disease, trypanosomes are restricted to the blood and lymphatic systems; eventually, however, parasites penetrate the blood-brain barrier (BBB) to infect the central nervous system (CNS), causing the neurological symptoms associated with stage 2 disease (41).
Existing drugs for HAT are highly unsatisfactory, requiring parenteral administration and causing toxic effects in patients (9). Early-stage infection is treated with pentamidine (T. b. gambiense) or suramin (T. b. rhodesiense). Pafuramidine (DB289), which is the oral prodrug of furamidine (DB75), a pentamidine analogue, was recently evaluated in phase III clinical trials. Unfortunately, trials had to be discontinued due to the delayed manifestation of liver toxicity and renal insufficiency in a number of recipients from a retrospective phase I safety trial (37).
Treatment for stage 2 disease is performed with eflornithine (T. b. gambiense only) or melarsoprol. Melarsoprol is highly toxic (27), and the rate of treatment failure with melarsoprol has reached over 30% in some areas (8). Administration of eflornithine, particularly in the form of nifurtimox and eflornithine combination therapy (NECT) (38), has become the treatment of choice since the 1990s in many areas where T. b. gambiense infection is endemic (2). NECT, however, is not proposed for treatment of T. b. rhodesiense infection and may not halt the spread of eflornithine resistance indefinitely. There is, therefore, an urgent need for new therapies against HAT, particularly for second-stage disease.
Two aza analogues of furamidine, DB820 and CPD0801 (previously known as DB829) (Fig. 1), are promising drug candidates for treatment of second-stage HAT. Both are potently trypanocidal in vitro and, unusually for diamidines, have been shown to be curative in a T. b. brucei GVR35 mouse model of second-stage trypanosomiasis (47).
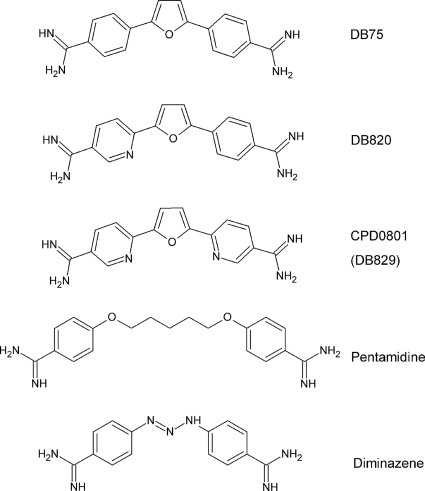
Fig. 1.
Structure of DB75 (furamidine) and its aza analogues DB820 and CPD0801 (DB829) compared to those of pentamidine and diminazene.
Consideration of the likelihood of emergence of resistance to new drugs is critical. Melarsoprol resistance in field isolates has been linked to the loss of activity of the P2 aminopurine transporter (23, 30, 33) encoded by the tbat1 gene (30), which is the principal carrier mediating uptake of the drug (7, 10, 16, 33, 34). P2 is also responsible for transport of the diamidines pentamidine (11, 18), diminazene (an essential veterinary trypanocide) (3, 20), and furamidine (29), and loss of P2 is associated with some level of resistance to these compounds in vitro. Resistance or sensitivity to diamidines and melaminophenyl arsenicals is thus primarily linked to the presence of transporters responsible for their uptake (22). However, the P2 transporter is not the only carrier for these drugs. Pentamidine, for example, also depends to a large extent on the high-affinity pentamidine transporter HAPT1 (18). Hence, for pentamidine, loss of P2 alone does not lead to significant resistance. Melarsoprol (or melarsen oxide) also uses HAPT1 as a secondary transporter, and it is only when P2 and HAPT1 are both lost that significant levels of resistance are observed (7). Diminazene (3, 20) and DB75 (29), however, appear to have a more dramatic overall dependence on P2, with secondary routes playing a lesser role and loss of P2 alone provoking higher levels of in vitro resistance. Enhanced understanding of the transport of DB820 and CPD0801 into T. brucei is therefore a key component in determining their potential as therapeutic agents against second-stage HAT and would give insight into potential cross-resistance patterns.
Given the observation that the aza analogues of furamidine show activity against stage 2 HAT (47), where drugs need to reach trypanosomes in the brain, it can be concluded that these compounds have clear differences with regard to membrane permeability in mammals in that they must be able to cross the blood-brain barrier. It is therefore important also to understand whether different transport capabilities exist in the parasites as well.
The fluorescent properties of DB75 and CPD0801 have previously been exploited to study the subcellular localization of the drugs (32); staining of the nucleus and kinetoplast by fluorescent diamidines is delayed in P2-deficient parasites, forming the basis of a proposed diagnostic test for resistance in field isolates (42). We have further utilized the fluorescent properties of these diamidines to develop a method for the quantitative determination of their uptake (45). Here we present details of transport kinetics, definitively identifying the P2 adenosine transporter as the main entry route for this class of furamidine analogues while still showing the existence of minor routes of uptake in addition to P2.
MATERIALS AND METHODS
Materials.
DB75 [2,5-Bis(4-amidinophenyl)furan], DB820 [2-(4-amidinophenyl)-5-(5-amidinopyidyl-2-yl)furan], and CPD0801 (previously designated DB829) [2,5-Bis(5-amidino-2-pyridyl)furan] were obtained from the laboratory of David Boykin (Georgia State University). Pentamidine isethionate, diminazene aceturate, and all other chemicals, including nucleosides and nucleobases, were of the highest quality available from Sigma. Diamidine stocks were prepared in dimethyl sulfoxide (DMSO) and diluted prior to use in complete HMI-9 media (i.e., HMI-9 media [BioSera Ltd., East Sussex, United Kingdom] supplemented with 10% fetal calf serum [PAA Laboratories, Pasching, Austria] and 2 mM β-mercaptoethanol [25]), ensuring that the final concentration of DMSO was always less than 2%. [3H]adenosine (20 to 30 Ci/mmol) was obtained from Moravek Biochemicals Inc.
Trypanosomes.
Bloodstream-stage T. b. brucei clone s427 (MiTat 1.2/221) and its derivative cell lines tbat1−/− (34) and B48 (7) were cultured, using complete HMI-9 media at 37°C in 5% CO2. Ex vivo cells were obtained from adult female Wistar rats. Blood was collected via cardiac puncture at peak parasitemia into tubes containing heparin (CP Pharmaceuticals Ltd., Wrexham, United Kingdom), and trypanosomes were isolated on a DEAE cellulose (DE52) anion exchange column (Whatman, Maidstone, United Kingdom) primed with phosphate-buffered saline with glucose (PSG) buffer as described by Lanham (28).
alamarBlue assay.
Values of 50% inhibitory concentrations (IC50s) for diamidines were determined using an adaptation of the alamarBlue method (39). A 100-μl volume of bloodstream-stage trypanosomes containing 4 × 104 cells per ml was added to 96-well plates containing a 100-μl doubling-dilution series of test diamidines and processed as previously described (29). IC50s were calculated using the sigmoidal algorithm of Prism 5.0 (GraphPad). Experiments were performed in duplicate on three independent occasions.
Time-dose response.
The in vitro diamidine exposure required to kill trypanosomes was studied using an adaptation of the procedure described by Kaminsky et al. (26). Briefly, 1 to 2 × 105 trypanosomes in 1 ml of complete HMI-9 media were exposed to a range of concentrations of DB75, DB820, CPD0801, and diminazene for various periods of time under standard culture conditions. Drug concentrations were based on the measured IC50s against wild-type s427 and set at IC50 × 1, × 5, × 20, and × 100 for each drug; in addition, fixed concentrations of 3.2 μM and 32 μM were used. Drug-free controls were tested in parallel. Exposure was stopped after 0 min (pulse), 5 min, 15 min, 1 h, 3 h, 6 h, 24 h, 48 h, and 96 h, when cells were washed twice in 10 ml of ice-cold complete HMI-9 media to remove the test compound. Cells were then resuspended in 1 ml of the same medium and cultivated in 24-well plates under standard culture conditions. Drug exposures of 1 h or less were performed in 15-ml plastic centrifugation tubes (Greiner); longer exposure times were performed in 24-well plates. All sets of conditions were tested in triplicate. Cultures were examined daily over 10 days for the presence of live motile trypanosomes and subcultured as required.
Determination of diamidine uptake.
Uptake of DB75, DB820, and CPD0801 was measured at room temperature (RT) using the recently developed fluorescent uptake method (45). Kinetic parameters were established using trypanosomes isolated from infected rats prepared as described above. A 100-μl volume of the cell suspension was added to microcentrifuge tubes containing an equal volume of permeant solution in complete HMI-9 media at double the final concentration. Transport was stopped after 10 min, when uptake for all three diamidines had reached a concentration that could be accurately determined; this time point was considered to represent the linear phase of uptake. For longer-term uptake assays, bloodstream-stage s427, Tbat1−/−, and B48 cultures at a starting density of 1 × 106 cells per ml were exposed to 7.5 μM DB75, DB820, or CPD0801 under standard tissue culture conditions. Samples (10 ml) of each culture were taken after 0, 1, 4, 8, and 24 h. The cells were pelleted by centrifugation at 2,500 × g for 10 min and washed twice in drug-free medium. Cell pellets were lysed in 0.1 M HCl (8:1 vol/vol) methanol overnight before transfer to a 96-well plate. Fluorescence was measured as described previously (45). At the end of each experiment, trypanosomes were inspected for motility under the light microscope and found to be viable. All experiments were performed in at least triplicate on at least three independent occasions.
Uptake of radiolabeled adenosine.
The uptake of [3H]adenosine by the P2 transporter was studied over 30 s using the rapid oil-stop method as previously described (10, 29, 44) and bloodstream forms of T. b. brucei (strain 427 [wild type]) isolated from rat blood. P1 activity was inhibited by the presence of 1 mM inosine. Aliquots of freshly isolated ex vivo cells (1 × 108 cells ml−1) were also incubated in complete HMI-9 media supplemented with penicillin-streptomycin (Invitrogen) (100 units ml−1 to 0.1 mg ml−1) and kanamycin (Sigma) (30 μg ml−1) under standard culture conditions; the cell cultures are referred to here as cultured ex vivo cells. These cultured ex vivo cells were washed in assay buffer at predetermined time points (1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, and 24 h) and resuspended to achieve a concentration of 1 × 108 cells ml−1 followed by an assay to determine [3H]adenosine uptake rates as described above. All experiments were performed in triplicate, with trypanosomes inspected for viability under the light microscope at the conclusion of the procedure.
RESULTS
In vitro trypanocidal activity and in vitro time-dose response of diamidines.
DB75, DB820, and CPD0801 were tested against various T. brucei strains in vitro and their levels of efficacy compared to those of the registered diamidine drugs pentamidine and diminazene. Activity was tested against T. b. brucei s427 and two derived cell lines deficient in known diamidine transporters: a genetic knockout cell line for the P2 aminopurine transporter, tbat1−/− (34), and B48, derived from tbat1−/− by selection for high-level pentamidine resistance in vitro, resulting in the loss of high-affinity pentamidine transporter (HAPT) activity (7). IC50s and resistance factors (RFs) for the transporter-deficient cell lines are shown in Table 1.
Table 1.
In vitro trypanocidal activity of diamidines against T. b. brucei s427 and derivative cell lines (n = 3)a
| Compound | IC50 [nM] (SD) for s427 | IC50 [nM] (SD) for TbAT1−/− | RF | IC50 [nM] (SD) for B48 | RF |
|---|---|---|---|---|---|
| Pentamidine | 0.08 (± 0.01) | 0.35 (± 0.02) | 4 | 299 (± 17.0) | 3,738 |
| Diminazene | 13 (± 1.28) | 77 (± 13.1) | 6 | 43 (± 2.58) | 3 |
| DB75 | 1.5 (± 0.24) | 37 (± 7.23) | 25 | 32 (± 2.92) | 21 |
| DB820 | 15 (± 0.33) | 156 (± 9.38) | 10 | 86 (± 2.41) | 6 |
| CPD0801 | 136 (± 6.00) | 609 (± 103) | 4 | 380 (± 22.9) | 3 |
RF, resistance factor (i.e., the ratio of the IC50 value for a given compound for the derived cell line to its IC50 value for wild-type s427); S.D., standard deviation.
All compounds tested exhibited submicromolar IC50s for the cell lines studied. Pentamidine was highly active against wild-type s427 and TbAT1−/− (IC50 < 1 nM) but, as expected, showed significantly less activity against B48 (resistance factor [RF] > 3,000). Diminazene was active against wild-type s427, with an IC50 of 13 nM, and showed a moderate loss of activity against tbat1−/− and B48 (RFs of 6 and 3, respectively). Of the test diamidines, DB75 proved the most potent, with IC50s lower than that of diminazene against all three cell lines. The loss of the P2 transporter apparently brings the IC50s for the test diamidines closer together, indicating that the interaction with P2 plays an important role in determining the differential activities of these compounds. The IC50 for DB820 was comparable to that of diminazene for wild-type s427, but DB820 showed a greater loss of activity against the transporter-deficient cell lines. CPD0801 was considerably less potent than the other diamidines, with an IC50 of 136 nM against wild-type s427. Resistance factors for CPD0801, however, were the lowest noted in this study, indicating a relatively small loss of efficacy against the derived (resistant) cell lines.
The minimum drug exposure time and concentration necessary to kill trypanosomes were investigated by incubating trypanosome cultures with a range of concentrations of each test diamidine for various lengths of time. Cultures were monitored for 10 days for the presence of live motile parasites. Table 2 shows the minimum exposure times necessary for trypanocidal effect presented as survival rates (n/3 of growing cultures) at day 10 for each drug concentration. Trypanosomes began to die on day 3 following initial exposure. However, many cultures that were apparently sterile on days 3 to 5 grew back by day 10 (not shown). Results correlated well with those of the alamarBlue assay, with the transporter-deficient cell lines less susceptible to all four diamidines than the parental s427 line. DB75 was able to sterilize cultures of all cell lines at lower concentrations and shorter exposure times than the other test compounds, requiring only a “pulse” exposure before washing in order to kill 3/3 s427 cultures at 32 μM and sterilizing 3/3 cultures after 96 h of exposure to 30 nM. Diminazene and DB820 gave very similar results: 24 h at 260 nM and 300 nM, respectively, or 5 min at 32 μM to kill 3/3 cultures of s427. CPD0801 required longer exposure periods and the highest concentrations to kill trypanosomes, needing 3 h of exposure at 32 μM to sterilize s427 cultures.
Table 2.
Survival of cultures at 10 days after initiation of treatmenta
| Drug | Concn | s427 |
tbat1−/− |
B48 |
|||
|---|---|---|---|---|---|---|---|
| Exposure time(s) | Survival ratio(s) | Exposure time(s) | Survival ratio(s) | Exposure time(s) | Survival ratio(s) | ||
| Diminazene | 32 μM | 5 min | 0/3 | 3 h | 0/3 | NT | NT |
| Pulse | 1/3 | ||||||
| 3.2 μM | 3 h | 0/3 | 6 h | 2/3 | NT | NT | |
| 1.3 μMb | 24 h | 0/3 | 24 h | 0/3 | 24 h | 0/3 | |
| 6 h | 1/3 | ||||||
| 260 nMc | 24 h | 0/3 | 96 h | 0/3 | 48 h | 0/3 | |
| 48 h | 1/3 | ||||||
| 65 nMd | 48 h | 0/3 | 96 h | 1/3 | 96 h | 0/3 | |
| 24 h | 1/3 | ||||||
| 13 nMe | 96 h | 3/3 | 96 h | 3/3 | 96 h | 3/3 | |
| DB75 | 32 μM | Pulse | 0/3 | 3 h | 0/3 | NT | NT |
| 3.2 μM | 1 h | 0/3 | 6 h | 0/3 | NT | NT | |
| 150 nMb | 24 h | 0/3 | 48 h | 0/3 | 48 h | 0/3 | |
| 30 nMc | 96 h | 0/3 | 96 h | 3/3 | 96 h | 3/3 | |
| 7.5 nMd | 96 h | 3/3 | 96 h | 3/3 | 96 h | 3/3 | |
| 1.5 nMe | 96 h | 3/3 | 96 h | 3/3 | 96 h | 3/3 | |
| DB820 | 32 μM | 5 min | 0/3 | 6 h | 0/3 | NT | NT |
| 3 h | 2/3 | ||||||
| 3.2 μM | 6 h | 0/3 | 6 h | 3/3 | NT | NT | |
| 1.5 μMb | 24 h | 0/3 | 24 h | 0/3 | 24 h | 0/3 | |
| 6 h | 2/3 | ||||||
| 300 nMc | 24 h | 0/3 | 96 h | 0/3 | 96 h | 0/3 | |
| 75 nMd | 48 h | 0/3 | 96 h | 3/3 | 96 h | 3/3 | |
| 15 nMe | 96 h | 3/3 | 96 h | 3/3 | 96 h | 3/3 | |
| CPD0801 | 32 μM | 3 h | 0/3 | 6 h | 3/3 | NT | NT |
| 3.2 μM | 6 h | 3/3 | 6 h | 3/3 | NT | NT | |
| 13.6 μMb | 24 h | 0/3 | 24 h | 0/3 | 24 h | 0/3 | |
| 2.72 μMc | 24 h | 0/3 | 24 h | 0/3 | 24 h | 0/3 | |
| 6 h | 2/3 | ||||||
| 680 nMd | 48 h | 0/3 | 96 h | 0/3 | 96 h | 0/3 | |
| 24 h | 2/3 | 48 h | 2/3 | ||||
| 136 nMe | 96 h | 0/3 | 96 h | 3/3 | 96 h | 3/3 | |
Survival ratios represent surviving cultures of three replicate determinations at the end of the experiment. Exposures ranged from a “pulse” to 96 h for each drug concentration. The exposure values shown indicate the minimum incubation time necessary for trypanocidal effect. NT, not tested.
100 × IC50 against wild-type s427 (alamarBlue assay).
20 × IC50 against wild-type s427 (alamarBlue assay).
5 × IC50 against wild-type s427 (alamarBlue assay).
1 × IC50 against wild-type s427 (alamarBlue assay).
Uptake of diamidines by ex vivo s427 T. brucei.
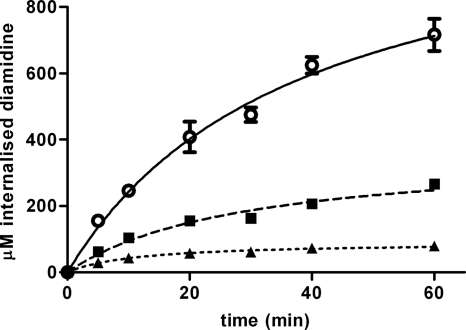
Uptake of DB75, DB820, and CPD0801 by T. brucei strain s427 ex vivo (i.e., cells grown in vivo and purified from rodent blood immediately prior to initiating uptake) was measured using an assay that determined the intrinsic fluorescence of these diamidines (45). Trypanosomes were exposed to 10 μM diamidine in complete HMI-9 media at room temperature and monitored for 1 h. In all cases, trypanosomes were still alive and apparently healthy at the end of this period as judged by microscopy. All three diamidines continued to be accumulated in trypanosomes throughout the time course, with DB75 concentrations being the highest at each time point (Fig. 2). After 1 h, the mean intracellular concentrations of DB75, DB820, and CPD0801 measured over three independent experiments were 596 ± 203 μM, 264 ± 22 μM, and 64 ± 13 μM, respectively (i.e., 60-fold, 26-fold, and 6.4-fold, respectively, above the initial extracellular concentrations) (see Table 6). Relative uptake rates for the three compounds correlated with trypanocidal efficacy as measured by the alamarBlue assay and the time-dose response experiment. Intracellular concentrations of DB820 and CPD0801 were approximately 44% and 11% that of DB75, respectively.
Fig. 2.
Uptake of diamidines by T. b. brucei strain s427. Trypanosomes isolated from infected female Wistar rats were immediately exposed to 10 μM permeant in complete HMI-9 media for up to 1 h at RT. Intracellular diamidine concentrations were measured using a fluorescence-based assay. The data are representative of the results of at least three similar experiments, each performed in at least triplicate. ○, DB75; ■, DB820; ▴, CPD0801. Bars represent standard errors of the means (SEM) of quadruplicate determinations of this particular experiment. When not shown, error bars fall within the symbol.
Table 6.
Intracellular diamidine concentration after 1 h of exposure to 10 μM permeant
| Cell line | Source condition | Diamidine concn (μM ± SEM) |
||
|---|---|---|---|---|
| DB75 | DB820 | CPD0801 | ||
| s427 | Ex vivo | 596 ± 203 | 264 ± 22 | 64.0 ± 13 |
| In vitro | 12.6 ± 2.2 | 0.43 ± 0.12 | 0.15 ± 0.03 | |
| tbat1−/− | Ex vivo | 21.3 ± 1.7 | 5.70 ± 0.22 | 1.12 ± 0.42 |
| In vitro | 12.2 ± 4.0 | 0.24 ± 0.05 | 0.15 ± 0.03 | |
Inhibition of P2 adenosine uptake activity by the test diamidines.
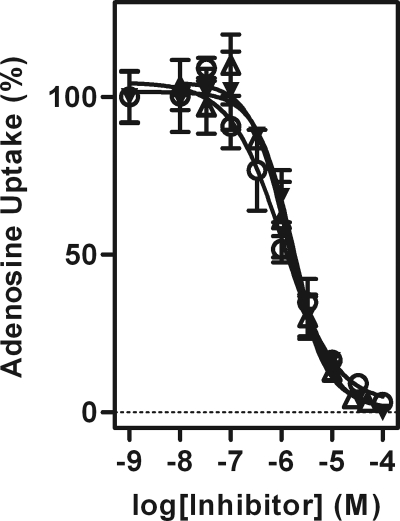
Pentamidine, diminazene, and DB75 have previously been shown to be substrates for the P2 aminopurine transporter (3, 11, 20, 29). We therefore decided to test the ability of DB820 and CPD0801 to inhibit P2-mediated transport of radiolabeled adenosine. DB75 was also tested as a control (Fig. 3). During this experiment, P1-mediated transport of adenosine was inhibited by addition of 1 mM inosine, allowing P2 activity to be studied in isolation (10, 15).
Fig. 3.
Inhibition of P2-mediated adenosine transport by furamidine analogues. Transport of 0.050 μM [3H]adenosine was assessed in the presence and absence of various concentrations of DB075 (▾), DB820 (○), and CPD0801 (Δ) over a period of 30 s. Uptake was determined in the presence of 1 mM inosine, which does not inhibit P2-mediated [3H]adenosine transport but does competitively inhibit P1-mediated uptake (15). The data are representative of the results of at least three similar experiments, each performed in triplicate. Bars represent SEM.
DB75, DB820, and CPD0801 all displayed very similar Ki values for P2 (Table 3), indicative of a high binding affinity and suggesting that DB820 and CPD0801 may be additional substrates of P2. This indicates that the pyridine nitrogens of DB820 and CPD0801 have no effect on the affinity of each compound for the exofacial binding site, even though the rates of uptake apparently differ substantially.
Table 3.
Inhibition of P2-mediated adenosine transport by furamidine analogues
| Compound | Ki (μM ± SE) | No. of expts |
|---|---|---|
| DB75 | 1.2 ± 0.2 | 4 |
| DB820 | 2.0 ± 0.3 | 3 |
| CPD0801 | 1.4 ± 0.1 | 4 |
Inhibition of diamidine uptake by the P2 substrate adenine.
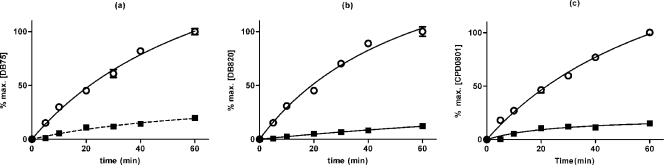
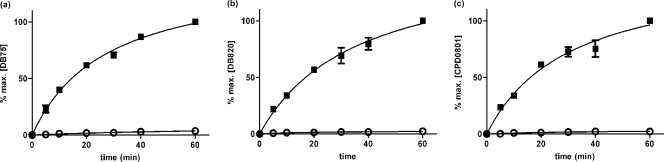
The P2 substrate adenine has been shown to inhibit uptake of DB75 over 30 s in a dose-dependent manner (29). The above experiment established that the other furamidines inhibit P2-mediated uptake. Using the requirement for reciprocal inhibition, we investigated whether P2 actually mediates the uptake of these compounds and what percentage of their uptake might be mediated by this adenosine-adenine transporter. Ex vivo trypanosomes (strain 427) were incubated with 10 μM each furamidine analog in the presence of 100 μM adenine, a high-affinity P2 substrate that does not interact with the P1 class of adenosine transporters at that concentration (1, 10, 21). Over a period of 60 min, uptake of all three diamidines was reduced by ∼80% in the presence of 100 μM adenine throughout the observation period (Fig. 4 and Table 4). DB75 was internalized at the highest and CPD0801 at the lowest rate in both the presence and the absence of adenine. These results are consistent with P2 being the primary transport route for DB820 and CPD0801. The incomplete inhibition of diamidine uptake could be ascribed to the fact that we were limited to a 10-fold excess of adenine over permeant, which was necessitated by the need for high permeant concentrations (because of detection limits using fluorescence); higher concentrations of adenine start to inhibit the P1-type transporters (14). On the basis of this experiment, the possibility cannot be excluded that a small percentage of the compounds is taken up by an adenine-insensitive transport system.
Fig. 4.
Inhibition of diamidine uptake by the P2 substrate adenine. T. b. brucei s427 parasites were isolated from infected rats and immediately exposed to 10 μM permeant in complete HMI-9 media for up to 1 h at RT in the presence (■) or absence (○) of 100 μM adenine. DB75 (a), DB820 (b), and CPD0801 (c) accumulation levels are plotted as percentages of intracellular concentration at 60 min. The data shown are representative of the results of three similar experiments, each performed in at least triplicate. Bars represent SEM of triplicate determinations of the experiment displayed. When not shown, error bars fall within the symbol. Averaged over three independent experiments, permeant concentrations after 60 min ± SEM were 541 ± 169 μM (DB75), 112 ± 43 μM (DB820), and 31 ± 3.3 μM (CPD0801).
Table 4.
Average rate of diamidine uptake over 10 min in the presence and absence of 100 mM adenine
| Drug | Diamidine uptake over 10 min (pmol 107 cells−1 min−1) |
% inhibition | |
|---|---|---|---|
| Without adenine | With adenine | ||
| DB75 | 6.3 ± 1.6 | 1.4 ± 0.19 | 78 |
| DB820 | 1.4 ± 0.7 | 0.32 ± 0.14 | 73 |
| CPD0801 | 0.42 ± 0.07 | 0.15 ± 0.03 | 64 |
Uptake of diamidines by ex vivo T. brucei strains that lack a P2 transporter.
The dependence of the three test compounds on P2-mediated uptake was further investigated using a genetically modified strain from which the P2 transporter gene TbAT1was deleted. Uptake in tbat1−/− was quantified for s427 as described above. Accumulation of all three diamidines was at least 20-fold lower in tbat1−/− than in s427 (Fig. 5), which is consistent with P2 being the major, but not sole, transport route for these diamidines. Relative uptake rates for the individual diamidines were the same as those seen with wild-type s427: DB75 accumulated fastest and CPD0801 slowest. After 60 min, intracellular levels of DB75 were around twice that seen under exposure conditions (21 ± 2 μM), while intracellular DB820 and CPD0801 levels were still below exposure levels (5.7 ± 0.2 μM and 1.1 ± 0.4 μM, respectively, averaged over three independent experiments). In all cases, cells were viable and motile at the end of the experiment as judged by microscopy. The reduced uptake rate for the test diamidines was not the result of a general downregulation of transport processes in the tbat1−/− line, as glucose uptake was found to be comparable to the wild-type s427 level (data not shown).
Fig. 5.
Uptake of diamidines by T. b. brucei strain tbat1−/−. Trypanosomes isolated from infected rats were immediately exposed to 10 μM permeant in complete HMI-9 media for up to 1 h at RT. Levels of accumulation of DB75 (a), DB820 (b), and CPD0801 (c) by tbat1−/− (○) and wild-type s427 (■) are shown as percentages of the permeant concentration in s427 at 60 min. The data are representative of the results of at least three similar experiments, each performed in at least triplicate. Bars represent SEM of the triplicate determinations in this particular experiment; when not shown, error bars fall within the symbol.
Apparent kinetic parameters of diamidine uptake.
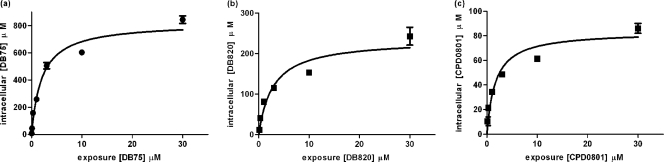
The determination of the kinetic parameters of diamidine transporters requires the measurement of true transport rates, rather than accumulation, which may be a composite process, including the intracellular binding, metabolism, or compartmentalization of the permeant. For this, uptake must be measured within the initial linear phase of transport. However, to determine the linear phase in the first place it is necessary to conduct time courses that include very short intervals. This was not possible for us, due to the sensitivity limits of the fluorescence uptake protocol (45). The best available method allowing us to arrive at an estimate of kinetic parameters was to measure uptake at 10 min, which gave a reliable level of fluorescence; also, uptake at that time point appears to be linear (Fig. 4), with r2 values of 0.91, 0.95, and 0.72 for DB75, DB820, and CPD0801, respectively (all not significantly nonlinear [runs test]).
Using a 10-min incubation thus allowed us to determine a close approximation of the Km and Vmax values for the uptake of DB75, DB820, and CPD0801. Measured Km values were very similar for all three compounds; Vmax values, however, differed considerably, with DB75 having the highest and CPD0801 the lowest Vmax (Fig. 6 and Table 5). Results for DB75 closely matched previously the published values of 3.2 μM for Km and 9.9 pmol 107 cells−1 min−1 for Vmax measured using radiolabeled permeant after 30 s (29). The Km values for all three test diamidines were statistically indistinguishable from their respective Ki values as inhibitors of P2-mediated adenosine transport (Fig. 3).
Fig. 6.
Apparent kinetic parameters of diamidine uptake by trypanosomes. DB75 (a), DB820 (b), and CPD0801 (c) uptake rates were measured by immediately incubating s427 cells isolated from female Wistar rats for 10 min with 0, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, or 30 μM permeant in complete HMI-9 media. The data are representative of the results of at least three similar experiments, each performed in at least triplicate. Bars represent SEM of the triplicate determinants in this particular experiment. When not shown, bars fall within the symbol. Apparent kinetic constants (averages ± SEM of the results of 3 or 4 independent experiments), determined by nonlinear regression using the Michaelis-Menten equation, are shown in Table 5.
Table 5.
Apparent kinetic parameters of diamidines (average ± SEM) after 15 min of incubation determined by nonlinear regression using the Michaelis-Menten equation
| Compound | Km (μM ± SEM) | Vmax (pmol 107 cells−1 min−1 ± SEM) | No. of expts |
|---|---|---|---|
| DB75 | 2.3 ± 0.2 | 15 ± 11 | 4 |
| DB820 | 1.3 ± 0.6 | 5.6 ± 1.3 | 3 |
| CPD0801 | 1.1 ± 0.5 | 1.5 ± 0. | 3 |
Diamidine uptake and P2 activity are downregulated in in vitro cultures of T. brucei s427.
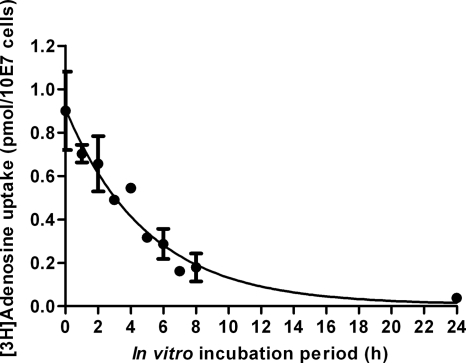
Uptake of test diamidines by in vitro cultures of s427 was measured under the same conditions as used for the ex vivo s427 experiments described above, with an exposure of 10 μM to DB75, DB820, or CPD0801 for up to 1 h at room temperature, and was found to be reduced by approximately 40- to 50-fold in vitro compared to the results seen with cells freshly isolated from infected rats. The rank order of uptake rates remained the same, with DB75 taken up quickest and CPD0801 slowest. After 1 h, the intracellular concentration of DB75 was around the level of exposure (12.6 ± 2.2 μM), while those of DB820 and CPD0801 were below the level of exposure (0.43 ± 0.12 μM and 0.15 ± 0.03 μM, respectively). Diamidine uptake was also measured for in vitro-cultured tbat1−/− cells under the same conditions and was found to be statistically identical to that exhibited by in vitro cultured wild-type s427 cells (12.2 ± 4.0 μM, 0.24 ± 0.05 μM, and 0.15 ± 0.03 μM after 1 h for DB75, DB820, and CPD0801, respectively) (Table 6). This suggests that P2 activity may be strongly downregulated in s427 cells grown in vitro compared to the same cell line when freshly isolated from infected rats. In order to investigate this hypothesis, uptake of the P2 substrate was measured in s427 cells freshly isolated from a rat and after various periods of adaptation to in vitro conditions in complete HMI-9 media. P2-mediated uptake was measured using radiolabeled adenosine in the presence of the P1 inhibitor inosine (3, 10, 15). The P2 transporter uptake rate for [3H]adenosine appeared to decrease over time upon in vitro incubation of the ex vivo cells (Fig. 7). The decrease (from 100% activity to 4.2%) occurred gradually over 24 h, confirming that a dramatic P2 downregulation occurs in T. b. brucei parasites cultivated in tissue culture. Although the rate of P2-mediated uptake in culture diminished with time, the apparent affinity of the transporter was unaltered, as the IC50 for adenosine did not change. Uptake of pentamidine (also a P2 substrate [11, 18]) was also found to be reduced in s427 after cultivation in vitro; glucose uptake, however, was unchanged, indicating that cells were healthy (data not shown).
Fig. 7.
P2-mediated [3H]adenosine uptake by s427 cells brought into culture immediately after isolation from rat blood and cultured for the indicated times. P1 transporter activity was inhibited by the presence of 1 mM inosine. Incubation time was 30 s. Error bars represent SEM of the results of triplicate determinations.
Longer-term diamidine accumulation by T. brucei in vitro.
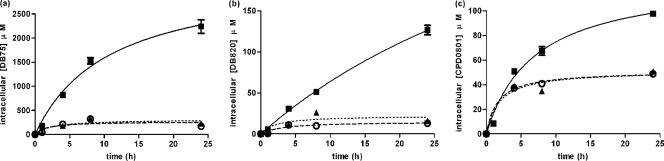
P2 activity and diamidine transport are rapidly downregulated in s427 in vitro to levels that cannot be readily distinguished from those of in vitro tbat1−/− when measured over 1 h at room temperature. However, there are significant differences in the sensitivities of s427 and tbat1−/− to diamidines in vitro as measured by the 72-h alamarBlue assay and in time-dose response experiments. In order to investigate this apparent contradiction between in vitro uptake and sensitivity results, it was decided to study diamidine uptake over a longer time course under standard in vitro culture conditions (37°C and 5% CO2). Bloodstream forms of s427, tbat1−/−, and B48 were grown in complete HMI-9 media and exposed to 7.5 μM DB75, DB820, or CPD0801. Samples were taken after 0, 4, 8, and 24 h and washed twice in drug-free medium, and the intracellular concentrations of the diamidines were established fluorimetrically. The experiment was carried out in triplicate, and each compound was tested on at least three separate occasions.
The longer incubation times did reveal faster uptake rates in cultured wild-type trypanosomes compared to tbat1−/− and B48 cell results, as was apparent for all three diamidines tested (Fig. 8). The latter two cell lines displayed very similar patterns of accumulation. For all cell types, DB75 was accumulated faster than the other test diamidines, which is consistent with its higher activity against all three cell lines compared to those compounds. Wild-type s427 cells continued to internalize all three compounds throughout the period of observation, although the rate of accumulation appeared to be slowing after 8 h. In contrast, there was very little change in intracellular levels of diamidines in tbat1−/− and B48 after 4 to 8 h of exposure. This indicates that P2 activity is retained in vitro in wild-type cells but at a level that is very significantly below that observed in ex vivo cells.
Fig. 8.
Uptake of diamidines by trypanosomes cultured in vitro. Trypanosomes of strains 427 (■), tbat1−/− (○), and B48 (▴) were exposed to 7.5 μM DB75 (a), DB820 (b), or CPD0801 (c) in complete HMI-9 media under standard culture conditions, and intracellular diamidine concentrations were measured after 1, 4, 8, and 24 h of exposure. The data are representative of the results of at least three similar experiments, each performed in at least triplicate. Bars represent SEM.
DISCUSSION
New drugs are urgently required for treatment of human African trypanosomiasis (HAT), also known as sleeping sickness, particularly for the second stage of the disease, when current drugs are unsatisfactory (6, 23).
There are a number of highly trypanocidal diamidine compounds with a surprising pharmacokinetic variability, including ring–aza-furamidines, which have activity against mouse models of stage 2 disease (31, 45) and therefore must be penetrating the central nervous system. As dications, these compounds do not diffuse across phospholipid membranes; hence, they must be substrates for transporters at the blood-brain barrier. Pentamidine and furamidine have been shown to be substrates for the human organic cation transporter 1 (hOCT1) (35); other as-yet-unspecified transporters may be selective for the aza analogues but not furamidine itself. These important differences in transporter recognition affect not only blood-brain barrier permeability but also seem likely to affect other pharmacokinetic properties of the aza analogues. For example, preliminary data indicate that the aza analogues accumulate less in kidney cells than furamidine itself (36), which could also translate into reduced risk of renal toxicity for these compounds.
The important variability in mammalian membrane transporter recognition of these compounds rendered it important also to identify, in detail, how these analogues enter trypanosomes.
Furamidine has previously been shown to enter T. brucei principally via the P2 aminopurine transporter (29), known to be a key component for the uptake of other diamidines such as pentamidine and diminazene as well as for melaminophenyl arsenicals (4, 22) and possibly isometamidium (19). The P2 transporter has a high affinity for these substrates and is likely to be energy dependent, transporting substrates against a concentration gradient, as was shown for several closely related T. brucei transporters (12, 13, 14). More recently, it was also shown that resistance to eflornithine is linked to the loss of a single amino acid transporter gene, TbAAT6 (43). The close correlations between drug efficacy and transport efficiency, as well as between drug resistance and changes in transporter function or expression (10, 17, 22, 30, 42), reinforce the importance of studying drug-transporter interactions, especially in cases of parasitic disease. In addition to TbAT1 and P2, two additional drug transporters have been described for pentamidine and melaminophenyl arsenicals in T. brucei, HAPT1 and LAPT1, explaining the absence of high levels of resistance for these compounds in tbat1−/− (7, 34). These secondary routes of uptake are thus clearly important in delineating the prospects of (cross-)resistance, and P2-defective parasites can still be treated with diamidines. Even in the case of furamidine, loss of the P2 transporter led to significant loss of in vitro sensitivity, while the susceptibility of the parasites in in vivo experiments was similar to that of the wild type (29).
To dissect the routes of uptake of the novel stage 2-active diamidines, we first investigated the sensitivity of wild-type and P2 knockout trypanosomes (34) along with that of a third trypanosome line, derived from a P2 knockout line exhibiting high-level pentamidine resistance, that had further lost the HAPT1 transporter (7). Loss of the P2 transporter led to reduced in vitro sensitivity to the aza-furamidines, a result similar to the loss of sensitivity demonstrated for diminazene or furamidine (29, 34).
The uptake of all of the diamidines was greatly reduced in the P2 transporter knockout line, and the P2 substrate adenine strongly inhibited uptake of all furamidines, reinforcing the conclusion of a primary role for the P2 transporter in uptake of furamidines. As with this model, all furamidines inhibited P2-mediated adenosine uptake, with Ki values identical to their estimated Km values. The Km values were similar to those measured previously using the faster transport assays that employed radiolabeled substrates for DB75 (29) and diminazene (20).
It is of interest, however, that the aza analogues showed significantly reduced rates of uptake compared to DB75. The single pyridine nitrogen in DB820 apparently slows uptake by 50% relative to that of furamidine, while CPD0801, with a pyridine nitrogen in each ring, was taken up at approximately 10% of the furamidine rate. This appears to be the result of inherently different rates of translocation across the membrane, as affinities for the transporter were identical, indicating that the pyridine nitrogens do not contribute to interactions within the P2 exofacial binding pocket. Interestingly, the rank order of the different translocation rates was preserved in tbat1−/− cells and also in mammalian cells, where the human organic cation transporter 1 (hOCT1) was previously proposed to drive uptake (with rates of 1:0.5:0.2 for furamidine, DB820, and CPD0801, respectively) (37)—rates remarkably similar to the 1:0.45:0.12 and 1:0.27:0.05 values that we observed for wild-type and tbat1−/− trypanosomes, respectively (Fig. 2 and 5). It could be speculated that the different translocation rates may have been due to the nitrogen residues forming interactions that stabilize the conformation of the different transporters, slowing their transition to a conformation that releases the substrate intracellularly. Alternatively, the pyridine nitrogens may partly restrict the diamidine conformation by limiting free rotation about the furan-pyridine ring or increase solvation of the aza analogs.
Of major significance is the fact that the aza analogues translocate more efficiently across the blood-brain barrier (BBB) (45). As dications, diamidines do not diffuse across the BBB, and the aza-furamidines are the first compounds of this class to have been shown to be active against a CNS disorder. It was recently shown that pentamidine, which is not active in the second-stage trypanosomiasis model, does cross the BBB by a transport-mediated mechanism but is quickly removed from the CNS by P glycoproteins (40). We conclude that the unknown diamidine transporter at the BBB more efficiently transports aza-furamidines because of a specific interaction lacking in other human transporters and in the T. brucei diamidine transporters or that the pyridine nitrogens of aza-furamidines render them poorer substrates for blood-brain barrier efflux systems.
The observation that the uptake of furamidines was greatly reduced in the absence of P2 has potential implications regarding possible risks for the emergence of resistance to these drugs; mutations in the TbAT1 gene have previously been found to be associated with melarsoprol resistance in the field (33). In this context, it may be significant that loss of P2 conveys a lower level of resistance to CPD0801 than to DB75. It is also noteworthy that, although DB75 was markedly less active against P2-deficient T. brucei in our in vitro assay system, our previous data from in vivo studies of mice demonstrated that there was little difference in sensitivity between the two strains (29). It may well be that the unknown secondary transporter is more highly expressed in vivo, as shown here for P2. A conclusion of this work is that treatment failure related to loss of P2 transport is not a foregone conclusion when using diamidines; instead, each member of the class should be assessed individually for this risk. Studies of several kinetoplastid parasites have shown that many nutrient transporters are highly regulated by growth conditions (17, 24, 36). We also noticed during our studies when observing the development of intracellular fluorescence that trypanosomes grown and purified from mice developed fluorescence far more quickly than did cells grown in vitro. Furthermore, the rate of uptake of each diamidine was significantly slower in in vitro- versus in vivo-grown cells. This can be at least partially explained by the downregulation of the P2 transporter in vitro; however, the unknown secondary transporter may be similarly regulated. These observations have important ramifications with regard to testing of drugs in vitro. Generally, the biochemical physiology of in vitro-grown trypanosomes has been believed to be a reflection of their physiological status in blood. However, it is clear that important differences in expression of proteins, including key transporters but possibly also other drug targets, is intensely regulated, meaning that results from in vitro testing may not necessarily be replicated in vivo.
In summary, we conclude here that novel aza analogues of furamidine that can penetrate the blood-brain barrier in mammals enter trypanosomes predominantly via the P2 aminopurine transporter in trypanosomes, although a secondary route of transport is also evident. Furthermore, uptake of the aza analogues is retarded in the trypanosome compared to that of furamidine, and this is reflected in their slower in vitro trypanocidal activity. We have also noted important differences with respect to the functional expression of the P2 transporter in trypanosomes grown in vitro compared to cells grown in vivo, which has implications in our extrapolation of in vitro drug screening data to the in vivo situation.
ACKNOWLEDGMENTS
This work was supported by the Bill and Melinda Gates Foundation through the Consortium of Parasitic Drug Development (CPDD).
We thank David Boykin for providing DB75, DB820, and CPD0801 and Mohammed Al Salabi for assistance with animal handling.
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Al-Salabi M. I., et al. 2007. Molecular interactions underlying the unusually high affinity of a novel Trypanosoma brucei nucleoside transporter. Mol. Pharmacol. 71:921–929 [DOI] [PubMed] [Google Scholar]
- 2. Balasegaram M., et al. 2009. Effectiveness of melarsoprol and eflornithine as first-line regimens for gambiense sleeping sickness in nine Médecins Sans Frontières programmes. Trans. R. Soc. Trop. Med. Hyg. 103:280–290 [DOI] [PubMed] [Google Scholar]
- 3. Barrett M. P., Zhang Z. Q., Denise H., Giroud C., Baltz T. 1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73:223–229 [DOI] [PubMed] [Google Scholar]
- 4. Barrett M. P., Fairlamb A. H. 1999. The biochemical basis of arsenical-diamidine crossresistance in African trypanosomes. Parasitol. Today 15:136–140 [DOI] [PubMed] [Google Scholar]
- 5. Barrett M. P., et al. 2003. The trypanosomiases. Lancet 362:1469–1480 [DOI] [PubMed] [Google Scholar]
- 6. Barrett M. P., Boykin D. W., Brun R., Tidwell R. R. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 152:1155–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bridges D. J., et al. 2007. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 71:1098–1108 [DOI] [PubMed] [Google Scholar]
- 8. Brun R., Schumacher R., Schmid C., Kunz C., Burri C. 2001. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 6:906–914 [DOI] [PubMed] [Google Scholar]
- 9. Brun R., Blum J., Chappuis F., Burri C. 2010. Human African trypanosomiasis. Lancet 375:148–159 [DOI] [PubMed] [Google Scholar]
- 10. Carter N. S., Fairlamb A. H. 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361:173–176 [DOI] [PubMed] [Google Scholar]
- 11. Carter N. S., Berger B. J., Fairlamb A. H. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153–28157 [DOI] [PubMed] [Google Scholar]
- 12. de Koning H. P., Jarvis S. M. 1997. Hypoxanthine uptake through a purine-selective nucleobase transporter in Trypanosoma brucei brucei procyclics is driven by protonmotive force. Eur. J. Biochem. 247:1102–1110 [DOI] [PubMed] [Google Scholar]
- 13. de Koning H. P., Jarvis S. M. 1997. Purine nucleobase transport in bloodstream forms of Trypanosoma brucei brucei is mediated by two novel transporters. Mol. Biochem. Parasitol. 89:245–258 [DOI] [PubMed] [Google Scholar]
- 14. de Koning H. P., Watson C. J., Jarvis S. M. 1998. Characterization of a nucleoside/proton symporter in procyclic Trypanosoma brucei brucei. J. Biol. Chem. 273:9486–9494 [DOI] [PubMed] [Google Scholar]
- 15. de Koning H. P., Jarvis S. M. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162–1170 de [DOI] [PubMed] [Google Scholar]
- 16. de Koning H. P., MacLeod A., Barrett M. P., Cover B., Jarvis S. M. 2000. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol. Biochem. Parasitol. 106:181–185 [DOI] [PubMed] [Google Scholar]
- 17. de Koning H. P., Watson C. J., Sutcliffe L., Jarvis S. M. 2000. Differential regulation of nucleoside and nucleobase transport in Crithidia fasciculata and Trypanosoma brucei in response to purine stress. Mol. Biochem. Parasitol. 106:93–107 [DOI] [PubMed] [Google Scholar]
- 18. de Koning H. P. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59:586–592 [DOI] [PubMed] [Google Scholar]
- 19. de Koning H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512–522 [DOI] [PubMed] [Google Scholar]
- 20. de Koning H. P., et al. 2004. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 48:1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Koning H. P., Bridges D. J., Burchmore R. 2005. Purine transporters of protozoa: from biology to therapy. FEMS Microbiol. Rev. 29:987–1020 [DOI] [PubMed] [Google Scholar]
- 22. de Koning H. P. 2008. The ever-increasing complexities of arsenical-diamidine cross-resistance in African trypanosomes. Trends Parasitol. 24:345–349 [DOI] [PubMed] [Google Scholar]
- 23. Delespaux V., de Koning H. P. 2007. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 10:30–50 [DOI] [PubMed] [Google Scholar]
- 24. Gero A. M. 1998. Purine stress in Crithidia: adaptation of a parasite to environmental stress. Parasitol. Today 14:277–281 [DOI] [PubMed] [Google Scholar]
- 25. Hirumi H., Hirumi K. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985–989 [PubMed] [Google Scholar]
- 26. Kaminsky R., Mamman M., Chuma F., Zweygarth E. 1993. Time-dose-response of Trypanosoma brucei brucei to diminazene aceturate (Berenil®) and in vitro simulation of drug-concentration-time profiles in cattle plasma. Acta Trop. 54:13–30 [DOI] [PubMed] [Google Scholar]
- 27. Kennedy P. G. E. 2008. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann. Neurol. 64:116–126 [DOI] [PubMed] [Google Scholar]
- 28. Lanham S. M. 1968. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature 218:1273–1274 [DOI] [PubMed] [Google Scholar]
- 29. Lanteri C. A., et al. 2006. Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol. Pharmacol. 70:1585–1592 [DOI] [PubMed] [Google Scholar]
- 30. Mäser P., Sütterlin C., Kralli A., Kaminsky R. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242–244 [DOI] [PubMed] [Google Scholar]
- 31. Mathis A. M., et al. 2006. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob. Agents Chemother. 50:2185–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathis A. M., et al. 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob. Agents Chemother. 51:2801–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matovu E., et al. 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 117:73–81 [DOI] [PubMed] [Google Scholar]
- 34. Matovu E., et al. 2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2:1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ming X., et al. 2009. Transport of dicationic drugs pentamidine and furamidine by human organic cation transporters. Drug Metab. Dispos. 37:424–430 [DOI] [PubMed] [Google Scholar]
- 36. Ortiz D., et al. 2010. Purine restriction induces pronounced translational upregulation of the NT1 adenosine/pyrimidine nucleoside transporter in Leishmania major. Mol. Microbiol. 78:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paine M., et al. 2010. Diamidines for human African trypanosomiasis. Curr. Opin. Invest. Drugs 11:876–883 [PubMed] [Google Scholar]
- 38. Priotto G., et al. 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, noninferiority trial. Lancet 374:56–64 [DOI] [PubMed] [Google Scholar]
- 39. Räz B., Iten M., Grether-Bühler Y., Kaminsky R., Brun R. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (Tb rhodesiense and Tb gambiense) in vitro. Acta Trop. 68:139–147 [DOI] [PubMed] [Google Scholar]
- 40. Sanderson L., Dogruel M., Rodgers J., de Koning H. P., Thomas S. A. 2009. Pentamidine movement across the murine blood-brain and blood-cerebrospinal fluid barriers: effect of trypanosome infection, combination therapy, P-glycoprotein, and multidrug resistance-associated protein. J. Pharmacol. Exp. Ther. 329:967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simarro P. P., Jannin J., Cattand P. 2008. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 5:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stewart M. L., et al. 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366:486–487 [DOI] [PubMed] [Google Scholar]
- 43. Vincent I. M., et al. 2010. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathogens 6:e1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wallace L. J. M., Candlish D., de Koning H. P. 2002. Different substrate recognition motifs of human and trypanosome nucleobase transporters: selective uptake of purine antimetabolites. J. Biol. Chem. 277:26149–26156 [DOI] [PubMed] [Google Scholar]
- 45. Ward C. P., Burgess K. E. V., Burchmore R. J., Barrett M. P., de Koning H. P. 2010. A fluorescence based assay for the uptake of CPD0801 (DB829) by African trypanosomes. Mol. Biochem. Parasitol. 174:145–149 [DOI] [PubMed] [Google Scholar]
- 46. Welburn S. C., Maudlin I., Simarro P. P. 2009. Controlling sleeping sickness—a review. Parasitology 136:1943–1949 [DOI] [PubMed] [Google Scholar]
- 47. Wenzler T., et al. 2009. New treatment option for second-stage African sleeping sickness: in vitro and in vivo efficacy of Aza analogs of DB289. Antimicrob. Agents Chemother. 53:4185–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]