Abstract
INX-08189 is an aryl-phosphoramidate of 6-O-methyl-2′-C-methyl guanosine. INX-08189 was highly potent in replicon assays, with a 50% effective concentration of 10 ± 6 nM against hepatitis C genotype 1b at 72 h. The inhibitory effect on viral replication was rapid, with a 50% effective concentration (EC50) of 35 ± 8 nM at 24 h. An intracellular 2′-C-methyl guanosine triphosphate (2′-C-MeGTP) concentration of 2.43 ± 0.42 pmol/106 cells was sufficient to achieve 90% inhibition of viral replication. In vitro resistance studies confirmed that the S282T mutation in the NS5b gene conferred an approximately 10-fold reduction in sensitivity to INX-08189. However, the complete inhibition of S282T mutant replicons still could be achieved with an EC90 of 344 ± 170 nM. Drug combination studies of INX-08189 and ribavirin indicated significant synergy in antiviral potency both in wild-type and S282T-expressing replicons. Genotype 1b replicons could be cleared after 14 days of culture when exposed to as little as 20 nM INX-08189. No evidence of mitochondrial toxicity was observed after 14 days of INX-08189 exposure in both HepG2 and CEM human cell lines. In vivo studies of rats and cynomolgus monkeys demonstrated that 2′-C-MeGTP concentrations in liver equivalent to the EC90 could be attained after a single oral dose of INX-08189. Rat liver 2′-C-MeGTP concentrations were proportional to dose, sustained for greater than 24 h, and correlated with plasma concentrations of the nucleoside metabolite 2′-C-methyl guanosine. The characteristics displayed by INX-08189 support its continued development as a clinical candidate for the treatment of chronic HCV infection.
INTRODUCTION
Hepatitis C virus (HCV) is one of the most important causes of chronic liver disease worldwide (36). In the United States alone, an estimated 3.9 million people are infected with HCV (16), and an estimated 10,000 to 12,000 HCV-related deaths occur annually (http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc/).
The HCV inhibitory activity of 2′-C-modified nucleosides has been well studied and has been shown to specifically inhibit HCV RNA replication both in biochemical assays and in cell-based replicon assays (6). The corresponding intracellular triphosphates of these 2′-substituted nucleosides were potent, competitive inhibitors of NS5B-catalyzed reactions in vitro. The incorporation of the 2′-modified monophosphates onto the 3′ end of the RNA strand resulted in the efficient termination of the elongation of the growing RNA chain. Accordingly, 2′-substituted nucleosides have been shown to function as nonobligate chain terminators (5). Despite the potential of 2′-C-modified nucleosides in the inhibition of RNA-dependent RNA polymerase (RdRp) activity, they have failed to progress as drug candidates due to one or more of the following: lack of oral bioavailability, poor pharmacokinetic characteristics, lack of cell penetration, and inefficient intracellular conversion to the active triphosphate (6, 12, 28). For example, the triphosphate of 2′-C-methyl guanosine (2′-C-MeGTP) has been shown to be a highly potent inhibitor of RdRp activity in biochemical assays, and the nucleoside analog 2′-C-methyl-guanosine (2′-C-MeG) had high oral bioavailability in rats, but it lacked potency in cell-based subgenomic replicon assays (12, 28).
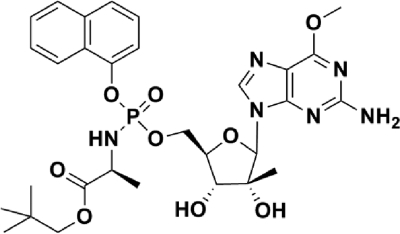
In an effort to unlock the potential of nucleoside NS5B inhibitors, we have employed a phosphoramidate prodrug approach to improve upon the characteristics of cellular uptake and intracellular activation. This approach is designed to bypass the rate-limiting initial phosphorylation step of activation by delivering the monophosphate form of the nucleoside analog to the liver, where it can be efficiently converted to the active triphosphate (24). INX-08189 is a phosphoramidate of O-6-methyl-2′-C-methyl guanosine (Fig. 1) (26). This compound was selected from a number of phosphoramidate candidates because of its significant potency in replicon assays and its ability to efficiently generate intracellular triphosphate in primary human hepatocytes (26).
Fig. 1.
Structure of INX-08189: (2S)-neopentyl 2-(2R,3R,4R)-5-(2-amino-6-methoxy-9H-purin-9-yl)-3,4-dihydroxy-4-methyltetrahydrofuran-2-yl)(methoxy)(naphthalen-1-yloxy)(phosphorylamino) propanoate.
The current study reports a detailed characterization of INX-08189, including a description of its potency against multiple HCV genotypes, the relationship between potency and intracellular 2′-C-MeGTP production, the resistance genotypes selected by the compound, and its pharmacokinetic and pharmacodynamic properties in rats and primates.
MATERIALS AND METHODS
INX-08189.
INX-08189 was synthesized in the laboratory of Christopher McGuigan at the Welsh School of Pharmacy, Cardiff University, as described previously (26).
Replicon assays.
The HCV inhibitory activity of INX-08189 was evaluated in replicon cell culture systems (Apath, LLC, Brooklyn, NY). For HCV genotype 1b (Con1), a Huh-7 cell line (29) expressing a stable, bicistronic subgenomic replicon encoding the Renilla luciferase reporter gene was utilized (2). For HCV genotype 2a (J6/JFH), Huh-7 cells were transfected transiently with a full-length, monocistronic HCV genome construct expressing Renilla luciferase. The inhibitory effect on HCV RNA replication using the luciferase assay and the cytotoxic effect of compounds were performed as previously described (24). For HCV genotype 1a (H77), a stable, full-length, bicistronic replicon cell line was used (3). Genotype 1a HCV RNA replication was monitored in this cell line using a quantitative reverse transcriptase PCR assay (qRT-PCR; TaqMan) as follows. Total RNA was isolated using the RNeasy 96 kit (Qiagen, Valencia, CA). The RNA preparations were quantified using the RiboGreen RNA quantitation kit (Invitrogen) and were used as the template in a qRT-PCR mixture containing 1× EZ buffer, 1× Mn acetate, 300 μM deoxynucleoside triphosphates (dNTPs), 1 μl (2.5 U) rTh, 300 nM primers specific for the HCV 3′-untranslated region (UTR) (forward, 5′-GGCTCCATCTTAGCCCTAGTC-3′; HCV-R, reverse, 5′-AGTATCGGCACTCTCTGCAGT-3′) and 150 nM TaqMan probe (6-carboxyfluorescein [FAM]-ATGCGGCTCACGGACC) (MGB probe). Amplification was performed using an ABI 7500 real-time PCR system and the following thermal cycling program: 95°C for 60 s and 60°C for 35 min, followed by 40 cycles of 95°C for 45 s and 60°C for 45 s. The HCV RNA copy number was calculated from a standard curve generated with synthetic HCV RNA standards of known concentrations.
Generation of NS5B mutant replicons.
Mutations in the NS5B gene were generated in the HCV genotype 1b (Con1) subgenomic replicon. To introduce the S282T mutation in NS5B, a plasmid containing a restriction fragment incorporating the mutation (pT7GG-1b-S282T) was synthesized (Geneart Inc., Burlingame, CA), and the restriction fragment was purified and used to replace the corresponding fragment in the wild-type replicon. NS5B mutations I585T and P540T were generated by in vitro mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Briefly, an XhoI/SpeI fragment from the HCV genotype 1b replicon plasmid was cloned into pBluescript SKII and used as the template for mutagenesis using the primers 5′-TCTGTAGGGGTAGGCACCTATCTACTCCCCAAC-3′ and 5′-GTTGGGGAGTAGATAGGTGCCTACCCCTACAGA-3′ for I585T and 5′-AACTCACTCCAATCACGGCTGCGTCCCAG-3′ and 5′-CTGGGACGCAGCCGTGATTGGAGTGAGTT-3′ for P540T. The XhoI/SpeI fragments containing the mutations then were cloned into the HCV genotype 1b replicon plasmid. To generate the HCV genotype 1b replicon carrying the NS5B double mutation S282T+I585T, an SfiI restriction fragment carrying the S282T mutation was used to replace the SfiI fragment in the replicon containing the I585T mutation. The replicon carrying NS5B mutations S96T and N142T was generated as follows. The S96T mutation was generated by overlap extension PCR. In the first step, two overlapping PCR fragments were amplified from the wild-type replicon using primers XhoI-F1 (5′-GAA ATT CCC TCG AGC GAT GC-3′), S96T-R1 (5′-TTA GAT CTG GCC GtA TGT GGG GGC GT-3′), S96T-F2 (5′-ACG CCC CCA CAT aCG GCC AGA TCT AA-3′), and MfeI-R2 (5′-CAT GAT GGT GGT GTC AAT TGG T-3′) (underlining indicates the introduced restriction enzyme sites and lowercase lettering indicates point mutations introduced by the oligonucleotides). In the second step, the aforementioned overlapping PCR fragments were used as the template in a PCR mixture containing primers XhoI-F1 (5′-GAA ATT CCC TCG AGC GAT GC-3′) and MfeI-R2 (5′-CAT GAT GGT GGT GTC AAT TGG T-3′). The N142T mutation was generated by PCR using primers N142T-MfeI-F3 (5′-ACC AAT TGA CAC CAC CAT CAT GGC AAA AAcTGA GGT TTT CTG CG-3′) and SfiI-R3 (5′-TCG ACA GGC CGC AGC GGC CTT-3′). The two PCR fragments independently carrying S96T and N142T were cloned into the genotype 1b replicon. All replicons containing altered NS5B genes were confirmed by sequencing (SeqWright, Houston, TX).
Transient transfection of NS5B mutant replicons.
Replicon RNA for transfection was prepared as follows. Replicon plasmid DNA was linearized with ScaI (Fermentas, Glen Burnie, MD) and used for in vitro reverse transcription using the T7 MegaScript kit (Ambion, Austin, TX). The DNA template was removed by digestion with Turbo DNase, and the RNA was precipitated with 2.5 M LiCl. RNA was quantified using the Quant-iT RiboGreen RNA kit (Molecular Probes, Eugene, OR). In preparation for transfection, Huh-7 cells were cured of replicons by prolonged treatment with alpha interferon 2A (IFN-α-2A). Cured Huh-7 cells were treated with trypsin, washed three times with ice-cold phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA), and resuspended at 1.6 ×107 cells/ml in PBS. Ten μg of replicon RNA was combined with 0.35 ml of cell suspension and immediately pulsed three times (800 V, 100 μs) using a BTX ElectroSquare Porator ECM 830 (Harvard Apparatus, Holliston, MA). Electroporated cells were incubated at room temperature for 10 min prior to resuspension in 20 ml of Dulbecco's modified essential medium (DMEM)-high glucose medium (HyClone, Logan, UT) supplemented with 9% fetal bovine serum (FBS) (HyClone), 2 mM glutamine (Invitrogen), and 100 U/ml PenStrep (Invitrogen). Resuspended cells were plated into 96-well BioCoat collagen-treated tissue culture plates (VWR, West Chester, PA).
HCV replicon clearance studies.
HCV replicon 1b cells were seeded in 6-well plates at 1 × 105 cells/well without the selective antibiotic G418. INX-08189 was added to cell cultures 4 h after seeding at the following final concentrations: 0 nM (control), 5, 10, 20, 40, and 80 nM. The medium was changed daily, and the cells were subcultured on days 5 and 10. On days 0, 5, 8, 10, 12, and 14, the cell cultures were analyzed for HCV genome-encoded Renilla luciferase expression with the Renilla luciferase assay kit (Promega, Madison, WI) using a Veritas luminometer (Turner Biosystems, Sunnyvale, CA). On days 5, 10, and 14, a portion of the INX-08189-treated and control cell cultures were seeded into T-75 tissue culture flasks and incubated without INX-08189 but in the presence of 0.5 mg/ml of the selective antibiotic G418 (Invitrogen, Carlsbad, CA). As these secondary cultures grew, individual flasks were fixed and stained with crystal violet. For cultures where there were no visible surviving colonies, the flasks were stained after 5 weeks of G418 selection.
Measurement of intracellular 2′-C-methyl-GTP in vitro.
Genotype 1b replicon cells were seeded into six-well plates at 6 × 105 cells/well for 18 h before being cultured in the presence of INX-08189 for 6 h. Cell samples were harvested and extracted with 70% ethanol overnight at 4°C, and extracts were dried overnight under a stream of nitrogen. The dried extracts were reconstituted with 50 μl of 10 mM N,N-dimethylhexylamine, 3 mM ammonium formate in H2O. A standard curve was generated by spiking blank cell extract samples with known concentrations of 2′-C-MeGTP. Spiked samples were treated identically to the test samples, and processed samples were kept at 2 to 8°C before and during analysis. Endogenous ATP and GTP levels were monitored as internal quality controls of cell viability and extraction efficiency. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) analysis was performed with an Agilent 1100 series high-performance liquid chromatograph (HPLC) equipped with an XTerra MS C18, 3.5 μM, 2.1- by 50-mm column (Waters, Milford, MA) and an API 4000 mass spectrometer (Aplied Biosystems, Carlsbad, CA) using negative-ion polarity. The assay was linear (r2 ≥ 0.99) in the concentration range of 1 to 1,000 ng per 106 cells, with ≥90% accuracy and ≤10% coefficient of variation (CV).
Drug combination studies in genotype 1b replicon assay.
Compounds were tested both as single agents and in the following combinations: INX-08189 with IFN-α-2B (ProspecBio, Rehovot, Israel) and INX-08189 with ribavirin (Rbv; Research Products, Mt. Prospect, IL). Inhibition data from the replicon cultures was analyzed for drug interactions using a three-dimensional surface model based on the Bliss independence effects definition for additivity (MacSynergy II; obtained from M. N. Prichard, K. R. Aseltine, and C. Shipman, University of Michigan) (32). As suggested by the software authors and as utilized by others, volumes of synergy or antagonism greater than 25 μM2% were considered minor but significant, volumes greater than 50 μM2% were considered moderate and potentially important in vivo, and values greater than 100 μM2% indicate strong synergy and probable importance in vivo (10).
Pharmacokinetic studies of rats.
Rat studies were conducted at Inhibitex, Inc., in accordance with NIH guidelines and by following protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Inhibitex, Inc. INX-08189 was formulated in 95% Capmul MCM (Abitec Corp., Janesville, WI)–5% Tween 80 (Sigma, St. Louis, MO) and administered by oral gavage to male Sprague-Dawley rats (Taconic Farms, Germantown, NY). Blood and tissue collections were performed as a terminal procedure. Oral doses ranged from 3 to 25 mg/kg of body weight, and sampling times were 1, 2, 4, 8, 16, 24, 72, 96, and 168 h postadministration. Time points were selected to measure 2′-C-MeGTP concentrations at both the time to maximum concentration of drug in serum (Tmax) and at the trough (24 h). To study the relationship between liver 2′-C-MeGTP levels and plasma 2′-C-MeG concentrations, the oral dose range was expanded to 3 to 300 mg/kg, approximating human equivalent doses of 30 to 3,000 mg. Plasma samples were collected at 1, 2, 4, 8, 16, 24, 72, 96, and 168 h postadministration and correlated to trough levels of 2′-C-MeGTP in liver samples collected 24 h postadministration. Blood was collected into EDTA-containing tubes (1.6 mg EDTA/ml blood; Sarstedt, Inc., Newton, NC), and the plasma was separated by centrifugation within 30 min of collection. Liver samples were snap frozen immediately upon collection in liquid nitrogen. Plasma and liver samples were stored frozen at ≤−80°C prior to analysis.
Pharmacokinetic studies of cynomolgus monkeys.
Primate studies were conducted at MPI Research Inc., of Mattawan, MI, in accordance with protocols approved by the IACUC of MPI Research Inc. INX-08189 was formulated in 5% (vol/vol) dimethylacetamide–20% (vol/vol) Solutol HS 15–20% (vol/vol) polyethylene glycol 400–55% (vol/vol) 50 mM sodium acetate, pH 4.0, and was administered as a single oral gavage dose. The dose level of 25 mg/kg was chosen for comparison to the equivalent dose of 50 mg/kg in rats. Liver biopsy samples (four to five samples/animal) were collected from two animals/group/time point at 3 and 8 h postdose in an effort to measure 2′-C-MeGTP levels near the Tmax. Anesthesia was induced and maintained, and pre- and postoperative procedures were performed according to MPI Research standard operating procedures. The liver biopsy samples were pooled for each animal. Blood samples (approximately 1.2 to 2.0 ml) were collected into tubes containing sodium heparin from the femoral artery/vein or from the portal vein cannula at 0.5, 1, 2, 3, 4, 8, 12, and 24 h postadministration. Time points were selected to measure 2′-C-MeG concentrations at both the Tmax and at the trough (24 h). Plasma was separated by centrifugation and stored frozen until analysis.
Bioanalysis of pharmacokinetic samples.
The concentration of 2′-C-MeGTP in liver samples and the concentration of 2′-C-MeG in plasma samples from rats and primates was performed by LC-MS/MS as described previously (24). The assay measuring 2′-C-MeGTP in rat or primate liver was linear (r2 ≥ 0.99) in the concentration range of 100 to 4, 000 ng per g of tissue, with ≥85% accuracy and ≤2% CV. The assay measuring 2′-C-MeG in rat or primate plasma was linear (r2 ≥ 0.99) in the concentration range of 2 to 1,250 ng/ml, with ≥90% accuracy and ≤2% CV. The concentrations of INX-08189 in plasma samples collected from primates were measured by LC-MS/MS as follows. Fifty μl of each test sample was added to 200 μl of acetonitrile containing an internal standard. The samples were centrifuged at 1,300 × g at 4°C for 20 min, and 50 μl of supernatant from each sample was diluted with 50 μl H2O. Samples were covered, mixed by being vortexed, and maintained at 2 to 8°C before and during analysis. Calibration curves were generated by spiking various concentrations of INX-08189 into control plasma samples. Fifteen μl of each test sample was analyzed by LC-MS/MS. Liquid chromatography was performed with an Agilent 1100 series HPLC system equipped with a Synergi 4-μm Polar-RP 30- by 2.0-mm column (Phenomenex, Torrance, CA). The HPLC system was coupled to an API 4000 triple-quadrupole mass spectrometer (Applied Biosystems, Framingham, MA). Mass spectrometry was performed in positive-ion mode, and data were analyzed using Analyst v1.4.2 software (Applied Biosystems, Framingham, MA). The assay was linear (r2 ≥ 0.99) in the concentration range of 10 to 1,000 ng/ml, with ≥90% accuracy and ≤10% CV.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic analyses were performed on the plasma and liver concentration data for the analytes using WinNonlin v5.2 software (Pharsight, St. Louis, MO). The extravascular dosing pharmacokinetic model was used to calculate the pharmacokinetic parameters, which included maximum observed concentration (Cmax), time at maximal concentration (Tmax), terminal half-life (t1/2), and trapezoidal area determined from the plasma concentration time data from time zero to last observed concentration (AUC0-t), from time 0 to 24 h (AUC0-24), and from time 0 extrapolated to infinity (AUC0-∞). Estimates for the terminal half lives were obtained using regression analysis. Values that were below the lower limit of quantitation were assigned the value of zero for the analyses.
Mitochondrial toxicity assay.
Mitochondrial toxicity was measured by analyzing the ratio of the mitochondrial genome copy number to a nuclear gene copy number before and after drug treatment. The mitochondrial target sequence corresponded to the region between the genes TRNL1, encoding tRNA-leu, and ND1, encoding NADH dehydrogenase subunit 1, and the cellular genome comparator was the ß-globin gene (7). Total DNA from untreated CEM cells was serially diluted and used to generate a standard curve for determining the absolute copy number of the gene targets. After drug treatment, total DNA was isolated from treated cells using the DNeasy tissue kit (Qiagen, Valencia, CA). The purified DNA was quantified by spectrophotometry using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA). Duplex quantitative PCR was performed in an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA) using a 25-μl reaction volume containing 1× QuantiTect multiplex PCR (with 6-carboxy-X-rhodamine) mix; 250 nM mitochondrion-specific primers 5′-CCACCCAAGAACAGGGTTTG-3′ (forward) and 5′-TAAGAAGAGGAATTGAACCTCTGACTG-3′ (reverse); 250 nM mitochondrion-specific probe FAM-5′-TAAGATGGCAGAGCCCGGTA-3′-minor groove binding nonfluorescent quencher (MGBNFQ); 500 nM β-globin-specific primers 5′-GTGGATGAAGTTGGTGGTGAGG-3′ (forward) and 5′-CTCCACATGCCCAGTTTCTATTG-3′ (reverse); 250 nM globin-specific probe VIC-5′-CCTGGGCAGGTTGGTA-3′-MGBNFQ (Integrated DNA Technologies, Coralville, IA); and genomic DNA template. Amplification was carried out using the following thermal cycling program: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and then 60°C for 1 min. The ratios between mitochondrial and cellular genome copy numbers were derived for each sample and compared to those of control cultures. Changes in mitochondrial copy number were expressed as a percent difference from the control value.
RESULTS
Activity of INX-08189 against wild-type HCV replicons.
The HCV inhibitory activity of INX-08189 was evaluated in replicons expressing HCV genomes from genotypes 1a, 1b, and 2a. INX-08189 was found to be a highly potent inhibitor of HCV replication, with EC50s of 10 nM against genotype 1b, 12 nM against genotype 1a, and 0.9 nM against genotype 2a after 72 h of exposure (Table 1). Following 72 h of exposure, the concentration resulting in 50% cellular cytotoxicity (CC50) in cultured Huh-7 cells was 7.01 μM, resulting in therapeutic indices ranging from 584 to 7,778.
Table 1.
INX-08189 potency in wild-type repliconsa
| HCV genotype | Incubation period (h) | EC50 (μM) | EC90 (μM) | CC50 (μM) for Huh-7 |
|---|---|---|---|---|
| 1b | 24 | 0.035 ± 0.008 | 0.600 ± 0.453 | |
| 1b | 48 | 0.011 ± 0.005 | 0.061 ± 0.033 | |
| 1b | 72 | 0.010 ± 0.006 | 0.038 ± 0.020 | 7.01 ± 1.97 |
| 1a | 72 | 0.012 ± 0.004 | 0.042 ± 0.011 | 7.01 ± 1.97 |
| 2a | 72 | 0.0009 ± 0.0001 | 0.0065 ± 0.001 | 7.01 ± 1.97 |
All data are means ± standard deviations from at least three independent experiments.
To determine the magnitude of HCV inhibitory activity that could be achieved with less than 72 h of incubation, genotype 1b replicon cells were incubated with INX-08189 for 24 or 48 h, and the EC50s and EC90s were determined (Table 1). Similar levels of potency were observed when cells were incubated with INX-08189 for 48 or 72 h. If exposure time was reduced to only 24 h, the concentration required to achieve the EC50 level increased 3.5-fold; however, INX-08189 still was able to inhibit HCV replication by 90% (EC90 = 0.6 μM).
Intracellular metabolism in HCV replicon cells.
The intracellular conversion of INX-08189 to 2′-C-MeGTP was determined in genotype 1b replicon cells. Replicon cells were incubated with concentrations of INX-08189 that produce 50% inhibition of HCV replication (EC50 = 10 nM), 90% inhibition (EC90 = 40 nM), and twice the EC90 (2× EC90 = 80 nM). Cells were harvested after 6 h of exposure to INX-08189, and the intracellular 2′-C-MeGTP concentrations were measured (Table 2). There was a linear relationship (R2 = 0.91) between the measured intracellular 2′-C-MeGTP concentrations and the concentrations of INX-08189. At 40 nM INX-08189, the concentration of 2′-C-MeGTP in Huh-7 cells was 2.43 ± 0.42 pmol/1 × 106 cells. Given an estimated liver cellularity of 1 × 108 cells per g (37), the intracellular concentration of 2′-C-MeGTP that would be expected to result in 90% viral inhibition in liver tissue was calculated to be 243 pmol/g of tissue. This value was useful for interpreting the relevance of liver 2′-C-MeGTP levels measured in subsequent in vivo pharmacokinetic studies.
Table 2.
Intracellular 2′-C-methyl GTP in genotype 1b replicon upon incubation with INX-08189a
| INX-08189 concn (nM) | Amt of intracellular 2′-C-methyl GTP (pmol/106 cells) | Estimated equivalent liver concnb (pmol/g tissue) |
|---|---|---|
| 1× EC50 (10) | 0.84 ± 0.36 | 84 |
| 1× EC90 (40) | 2.43 ± 0.42 | 243 |
| 2× EC90 (80) | 4.97 ± 0.80 | 497 |
All values are means ± standard deviation from six determinations, all measured at 6 h of incubation with INX-08189.
Estimates are based on a liver cellularity of 1 × 108 cells per g.
To determine the intracellular half-life of 2′-C-MeGTP, actively dividing genotype 1b replicon cells were incubated with 1 μM INX-08189 for 8 h, at which time the drug was removed. Intracellular 2′-C-MeGTP levels were measured at 12, 24, 32, 48, and 56 h after the drug was initially added (Fig. 2). Based on these data, the half-life of intracellular 2′-C-MeGTP in Huh-7 cells was calculated to be approximately 24 h.
Fig. 2.
Determination of 2′-C-MeGTP intracellular half-life in genotype 1b replicon. Genotype 1b replicon cells were incubated with 1 μM INX-08189 for 8 h, at which point the drug was removed. Intracellular 2′-C-MeGTP concentrations were measured by LC-MS/MS. Symbols are means ± SD for triplicate determinations in a single experiment.
Selection and characterization of mutants resistant to INX-08189.
To determine the resistance mutations selected by INX-08189, long-term cultures of genotype 1b and 1a replicons were performed in the presence of G418 and with concentrations of INX-08189 that were increased up to eight times the EC50 (80 nM) in the 1b replicon and 4× the EC50 (40 nM) in the 1a replicon. The establishment of stable INX-08189 escape mutants was significantly delayed, requiring selection during a 6- to 9-week span in culture. In total, five single-colony-derived resistant cell lines were selected in the genotype 1b background, and one was selected in the genotype 1a background. The nucleotide sequences of the NS5B genes from these clones were determined. Two consistent NS5B mutations, a codon 282 alteration from serine to threonine (S282T) and a change in amino acid position 585 from isoleucine to threonine (I585T), were identified in drug-resistant clones from the genotype 1b background. The S282T substitution had been identified previously as a resistance mutant for 2′-C-Me-GTP (28). In the single clone derived from the genotype 1a replicon selection, the only alteration in the NS5B gene sequence was a codon change from alanine to threonine in amino acid position 540 (A540T in genotype 1a). To determine if these amino acid substitutions were sufficient to confer resistance to INX-08189, they were introduced singly or in combination into the genotype 1b replicon background, and potency was assessed in a transient replication assay. For comparative purposes, a replicon carrying the substitutions S96T and N142T that has been shown to confer resistance to 4′-azidocytidine (R1479) (20) was tested in parallel. INX-08189 potency against each of these mutant replicons and their relative replication competencies are summarized in Table 3. The presence of the S282T mutation resulted in drastically reduced replication efficiency, at approximately 4% of the rate of the wild-type replicon. Combining the I585T mutation with S282T improved replication efficiency to 8% of the wild-type level. INX-08189 potency against S282T mutant replicons was reduced approximately 10-fold, while INX-08189 potency against all other mutant replicons tested was unaffected, suggesting that S282T was sufficient to produce an INX-08189-resistant phenotype. Despite the shift in potency observed, exposure to INX-08189 still could significantly inhibit HCV replication in the S282T mutant replicons with an EC90 of 344 ± 170 nM (Fig. 3).
Table 3.
INX-08189 potency against transient replicons expressing mutant NS5Ba
| NS5B sequence | Replication efficiencyc (%) | EC50 of: |
INX-08189 + Rbv synergyd (μM2%) | ||
|---|---|---|---|---|---|
| INX-08189 (μM) | Rbv (μM) | IFN-α EC50 (IU) | |||
| Con1 WT | 100 | 0.006 ± 0.003 | 12.157 ± 1.753 | 4.974 ± 1.511 | 808 |
| S282T | 4 | 0.074 ± 0.026 | 1.728 ± 0.016 | 3.116 ± 0.439 | 117 |
| I585T | 165 | 0.005 ± 0.001 | 15.247 ± 1.639 | 3.380 ± 1.383 | |
| A540Tb | 86 | 0.004 ± 0.001 | 17.250 ± 3.235 | 3.294 ± 1.251 | |
| S282T/I585T | 8 | 0.055 ± 0.018 | 1.736 ± 0.110 | 1.937 ± 0.662 | |
| S96T/N142T | 102 | 0.006 ± 0.001 | |||
All data are averages ± standard deviations from at least three independent experiments.
The genotype 1b strain Con1 encodes a proline at position 540, as opposed to the alanine in genotype 1a strain H77.
Percent luciferase activity compared to activity of the wild-type replicon.
Bliss independence effects definition for additivity (MacSynergy II).
Fig. 3.
Inhibition of HCV replication. Representative inhibition curves are plotted for replicons expressing wild-type (•), S282T (Δ), I585T (■), A540T (▾), S282T/I585T (♢), and S96T/N142T ( ) NS5B sequences. Concentrations of INX-08189 are indicated on the x axis, and the measured luminescent signals are expressed as a percentage of signal obtained in no-treatment controls on the y axis. Symbols are means ± SD for triplicate determinations in a single experiment.
) NS5B sequences. Concentrations of INX-08189 are indicated on the x axis, and the measured luminescent signals are expressed as a percentage of signal obtained in no-treatment controls on the y axis. Symbols are means ± SD for triplicate determinations in a single experiment.
Effect of combining INX-08189 and Rbv.
In clinical trials, INX-08189 likely will be used in combination with standard therapy, which currently consists of pegylated interferon and Rbv. We therefore characterized the INX-08189 resistance mutant S282T as well as the other mutant replicons for their sensitivity to both Rbv and alpha interferon 2b (Table 3). Replicons expressing S282T proved to be more sensitive to the inhibitory activity of ribavirin, with an approximately 6-fold improvement in the EC50, whereas the potency of IFN-α was equivalent across all mutants tested. The difference in ribavirin potency between wild-type and S282T mutants was significant (P = 0.0093, unpaired t test with Welch's correction.) To explore this effect further, drug combination studies of INX-08189 paired with Rbv were carried out in both wild-type and transiently expressed S282T replicons (Table 3). The combination of INX-08189 and Rbv was highly synergistic in the wild-type replicon, with a synergy volume of 808 μM2%. Likewise, INX-08189 combined with Rbv against replicons expressing the S282T mutation was found to be significantly synergistic (117 μM2%).
Clearance of replicons.
To assess the antiviral effect of long-term treatment with INX-08189 on HCV replication, genotype 1b replicon cells were cultured in the presence of 0 (control), 5, 10, 20, 40, or 80 nM INX-08189 for 14 days in the absence of G418 selection. At various time points during the culture period, cell samples were harvested and luciferase expression was determined as a measure of HCV replication activity. As summarized in Fig. 4A, luciferase activity in treated cultures decreased over time compared to that of the control, and inhibition was concentration dependent. Culturing in the presence of 10 nM INX-08189 resulted in a >2 log10 reduction in HCV replication activity. Culturing in the presence of ≥20 nM (∼2× EC50) resulted in a >5 log10 reduction in HCV replication activity after 12 days. After 14 days of culture at concentrations of ≥40 nM (1× EC90), HCV replication activity was reduced to background levels. Samples of the treated cells were harvested at days 5, 10, and 14 and subcultured without INX-08189 but in the presence of G418. Any cells remaining in the cultures that retained the expression of the replicon genome would be resistant to G418 selection and after sufficient time in culture would be detected as visibly growing colonies (Fig. 4B). Cultures that were incubated in the presence of INX-08189 for only 5 days retained the expression of the replicon at all concentrations tested, although the frequency of expressing colonies diminished with increasing INX-08189 concentration. After 10 days of INX-08189 treatment, replicon-expressing cells were completely eliminated at concentrations of ≥40 nM (1× EC90). After 14 days of INX-08189 treatment, replicon-expressing cells were completely eliminated from the culture at concentrations of ≥20 nM (∼2× EC50).
Fig. 4.
Clearance of wild-type replicons in vitro with INX-08189. (A) Huh-7 genotype 1b replicons cells were cultured in the presence of INX-08189 without G418 for 14 days at concentrations of 5 (•), 10 (♢), 20 (■), 40 (○), and 80 nM ( ). Luciferase expression was monitored in the cultures at the indicated time points and expressed as log10 change in luminescence. (B) Cells were subcultured under G418 selection for up to 5 weeks, and surviving colonies were fixed and stained with crystal violet.
). Luciferase expression was monitored in the cultures at the indicated time points and expressed as log10 change in luminescence. (B) Cells were subcultured under G418 selection for up to 5 weeks, and surviving colonies were fixed and stained with crystal violet.
Mitochondrial toxicity.
Mitochondrial toxicity has been described as a manifestation of an adverse effect associated with the long-term use of certain nucleoside analogs (15). To evaluate the effect of INX-08189 on the relative mitochondrial genome copy number in the human cell lines CEM and HepG2, both a 3- and 14-day study were conducted. Mitochondrial copy numbers were determined after treatment with INX-08189 in CEM, a lymphocyte cell line, and HepG2, a human hepatocyte cell line. To distinguish mitochondrion-specific toxicity from general cellular cytotoxicity, increasing concentrations of INX-08189 were evaluated in the cell lines for 3 and 14 days. INX-08189 concentrations that did not affect overall cell viability were selected for the mitochondrial toxicity assessment. The maximum INX-08189 concentrations evaluated for mitochondrion-specific toxicity were 20 and 5 μM in CEM and HepG2 cells, respectively, treated for 3 days. In the 14-day experiments, the maximum INX-08189 concentration was 1 μM in both CEM and HepG2 cells. In CEM cells treated with a 20 μM concentration of INX-08189 for 3 days, the reduction in relative mitochondrial copy number was approximately 11% (Table 4). There were no effects on mitochondrial copy number observed in CEM cells at 1 μM for 14 days. The relative mitochondrial copy number in HepG2 cells was calculated to be 129 and 115% at 5 and 1 μM drug in 3- and 14-day treatments, respectively, demonstrating that INX-08189 did not alter the relative mitochondrial copy number in HepG2 cells. In contrast, a positive-control compound, dideoxycytosine (ddC), reduced the relative mitochondrial numbers by 56 and 69% in the 3-day study in CEM and HepG2 cells, respectively. Culturing CEM and HepG2 cells for 14 days with ddC resulted in mitochondrial copy number reductions of 80 and 99%, respectively (Table 4).
Table 4.
Mitochondrial toxicity
| Cell line | Compound | Relative mitochondrial copy no.a (% of control) on treatment day: |
|
|---|---|---|---|
| 3 | 14 | ||
| CEMb | INX-08189 | 89 ± 11 | 102 ± 14 |
| 2′-C-MeG | 109 ± 43 | 133 ± 4 | |
| ddC | 31 ± 5 | 1 ± 1 | |
| HepG2c | INX-08189 | 129 ± 22 | 115 ± 23 |
| 2′-C-MeG | 103 ± 57 | 137 ± 2 | |
| ddC | 44 ± 16 | 20 ± 24 | |
Measured as the ratio between mitochondrial genome copy number and cellular genome copy number compared to values for untreated control cultures. Values are averages from at least two experiments ± standard deviations.
Concentrations used for 3-day cultures were 20μM INX08189, 100 μM 2′-C-MeG, and 5μM ddC, and those for 14-day cultures were 1 μM INX08189, 100 μM 2′-C-MeG, and 0.5 μM ddC.
Concentrations used for 3-day cultures were 5μM INX08189, 100 μM 2′-C-MeG, and 10μM ddC, and those for 14-day cultures were 1 μM INX08189, 100 μM 2′-C-MeG, and 2μM ddC.
Pharmacokinetics in rats.
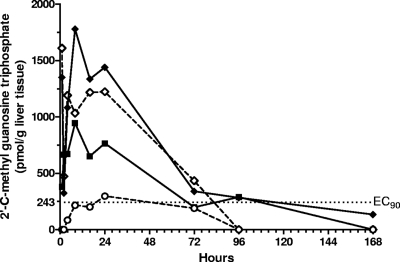
The ability of INX-08189 to deliver a monophosphate form to the liver that subsequently is converted to 2′-C-Me-GTP after oral administration was tested in Sprague-Dawley rats. 2′-C-MeGTP concentrations were measured in rat liver samples harvested during a 7-day period (Fig. 5). At doses of ≥5 mg/kg, the concentrations of 2′-C-MeGTP in the liver exceeded the EC90 soon after dosing and remained at or above this level for 72 h.
Fig. 5.
Concentrations of 2′-C-methyl GTP in rat liver following oral dosing with INX-08189. Each line represents a separate dose level: 25 mg/kg ( ), 10 mg/kg (♢), 5 mg/kg (■), and 3 mg/kg (○). Symbols represent averages from two rats with the exception of the 3-mg/kg dose group, which included data from six rats. The EC90 determined for Huh-7 cells (243 pmol/g) is indicated by the dotted line.
), 10 mg/kg (♢), 5 mg/kg (■), and 3 mg/kg (○). Symbols represent averages from two rats with the exception of the 3-mg/kg dose group, which included data from six rats. The EC90 determined for Huh-7 cells (243 pmol/g) is indicated by the dotted line.
The measurement of 2′-C-MeGTP in liver tissue is the most direct approach for determining the bioavailability of INX-08189; however, the use of this analytical tool is limited in higher species, as it can be employed only at a few time points and is impractical for pharmacokinetic studies of humans. Another approach is to monitor the generation of a metabolite of INX-08189 in the plasma that is proportional to the production of 2′-C-MeGTP in the liver. To this end, the plasma levels of 2′-C-MeG, a major metabolite of INX-08189, were measured in the rat. The plasma AUC0-24 (in ng · h/ml) values of 2′-C-MeG were compared to the 2′-C-MeGTP C24 (ng/g) concentrations after the administration of oral doses ranging from 3 to 300 mg/kg (Table 5). At doses between 3 and 150 mg/kg, both the 2′-C-MeG plasma AUCs (ng · h/ml) and the 2′-C-MeGTP liver concentrations (ng/g) at 24 h were found to be dose proportional and linear, with R2 values equal to 0.96 and 0.99, respectively. In addition, the 2′-C-MeG plasma AUCs and the 2′-C-MeGTP liver C24 also were found to be highly correlative (R2 = 0.97) at doses between 3 and 150 mg/kg. This correlation was not maintained at the 300-mg/kg dose. These data validate the utility of measuring 2′-C-MeG exposure in the plasma as a biomarker to monitor the liver pharmacokinetics of 2′-C-MeGTP in vivo.
Table 5.
Relationship between 2′-C-MeGTP concentration in liver to 2′-C-MeG exposure in plasma following a single oral dose of INX-08189 in rats
| INX-08189 dose (mg/kg) | N | Liver 2′-C-MeGTPaC24 (ng/g) | Plasma 2′-C-MeGb AUC0-24 (ng · h/ml) |
|---|---|---|---|
| 3 | 6 | 159 | 73.4 |
| 5 | 2 | 412 | 145.6 |
| 10 | 2 | 658 | 322.5 |
| 25 | 2 | 774 | 707.9 |
| 30 | 3 | 1,263 | 1,024.3 |
| 50 | 3 | 2,880 | 2,085.3 |
| 150 | 3 | 7,130 | 3,979.2 |
| 300 | 3 | 10,610 | 4,309.3 |
Mean concentrations for 2′-C-MeGTP measured at 24 h.
Area under the curve values covering 0 to 24 h (AUC0-24) were calculated from average 2′-C-MeG plasma concentrations measured at six time points.
Pharmacokinetics in cynomolgus monkeys.
To determine the concentration of 2′-C-MeGTP in the liver upon INX-08189 oral dosing, four animals were administered 25 mg/kg, and surgical liver biopsy specimens were collected under anesthesia at 3 and 8 h postdose. The mean liver 2′-C-MeGTP concentration at 3 h was 282 ng/g and increased to 624 ng/g at 8 h (Table 6). The in vitro EC90 (130.5 ng/g) was exceeded by 2.16- and 4.78-fold at the respective sample time points.
Table 6.
Intracellular 2′-C-MeGTP concentration in liver samples from cynomolgus monkeys administered a 25-mg/kg oral dose of INX-08189
| Time (h) | 2′-C-MeGTP liver concn |
||
|---|---|---|---|
| pmol/g | ng/g | Avg ± SD (ng/g) | |
| 3 | 758 | 407 | 282 ± 177 |
| 292 | 157 | ||
| 8 | 1,377 | 740 | 624 ± 165 |
| 944 | 507 | ||
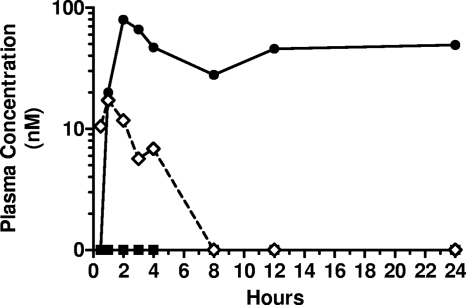
In an effort to determine the efficiency of INX-08189 extraction by the liver, cynomolgus monkeys with surgically implanted portal vein cannulas were administered 25 mg/kg INX-08189 by oral gavage. INX-08189 concentrations were measured in plasma collected from either the portal vein or the systemic circulation (femoral vein). Following a single oral dose, INX-08189 was detected in the portal circulation within 30 min of dosing and continued to be measured for up to 4 h (Fig. 6). At this dose level, INX-08189 was not detected in the systemic circulation of this animal at any of the time points tested, whereas the metabolite 2′-C-MeG was detected in the systemic circulation beginning at 1 h postdose. These data suggest that INX-08189 is efficiently extracted from the portal circulation by the liver following oral administration, which results in the formation of the active HCV polymerase inhibitor 2′-C-MeGTP in liver tissue.
Fig. 6.
Concentrations of INX-08189 and 2′-C-methyl guanosine in plasma after the oral dosing of INX-08189 in cynomolgus monkeys. Cynomolgus monkeys surgically fitted with portal vein cannulas were dosed orally with INX-08189 at 25 mg/kg. The analytes measured were 2′-C-methyl guanosine concentration in systemic plasma (•), INX-08189 in portal plasma (♢), and INX-08189 in systemic plasma (■).
DISCUSSION
The HCV RNA-dependent RNA polymerase encoded by NS5B is an attractive target for antiviral therapy. The active site of the enzyme maintains a high degree of sequence conservation across all HCV genotypes, allowing the potential for a pangenotype inhibitor with a high barrier to resistance (22, 23). Some of the most promising and widely studied NS5B inhibitors are 2′-C-methyl nucleoside analogs (5, 6, 34). Within this family of NS5B inhibitors, the triphosphate form of 2′-C-MeG has been shown to be one of the most potent inhibitors of NS5B enzyme activity, with an 50% inhibitory concentration (IC50) of 0.13 μM (28). However, the high potency of the active 2′-C-methyl GTP at the biochemical level was not reflected in the cell-based replicon assay due to poor cell penetration and a low rate of conversion of the nucleoside to the active triphosphate (13, 28).
To overcome these limitations, we have utilized an aryloxy-phosphoramidate approach to modify 2′-C-methyl guanosine analogues. This approach facilitates the direct delivery of the monophosphorylated nucleoside analogue into the cell, bypassing the first rate-limiting phosphorylation step (4). The phosphoramidate prodrug strategy has been used successfully to improve the HCV antiviral potency of several modified nucleosides (14, 19, 25, 27). In the case of 2′-C-MeG, a striking improvement in cell-based potency over that of the parent nucleoside can be achieved (>80 fold) with phosphoramidates incorporating 1-naphthyl as the aryl-leaving group (27). These prodrugs of 2′-C-MeG also have been shown to generate high concentrations of the active 2′-C-MeG triphosphate in liver tissue following oral administration in mice (24). To further improve the performance of these compounds, alterations at the C-2 and C-6 positions of the base were investigated. It was found that phosphoramidate derivates of 6-O-methyl-2′-C-methyl guanosine demonstrated improvements in cell-based potency, enhanced lipophilicity, cell permeability, and rapid intracellular conversion to the active triphosphate in human hepatocyte cultures (26). As a result of these studies, one compound that embodied all of these improvements, INX-08189, was selected for further characterization.
In the current study, the antiviral activity of INX-08189 was evaluated in the cell-based replicon assay against three HCV genotypes. The calculated EC50s in genotype 1a, 1b, and 2a replicons were 12, 10, and 0.9 nM, respectively, making INX-08189 one of the most potent nucleoside-based NS5B inhibitors characterized to date. This magnitude of potency has been observed previously only with HCV protease inhibitors and nonnucleoside polymerase inhibitors (34). The kinetics of INX-08189 antiviral activity were observed to be rapid, with an EC50 of 35 nM after only 24 h of exposure with genotype 1b replicon-containing cells. Coupled with the rapid production of 2′-C-methyl GTP observed previously with INX-08189 in human hepatocytes (26), it is clear that cell entry and the metabolic conversion of INX-08189 is enhanced compared to that of the parent nucleoside, 2′-C-MeG. To determine the concentration of intracellular triphosphate required to achieve viral inhibition, 2′-C-MeGTP was quantified in genotype 1b replicon-expressing Huh-7 cells by LC-MS/MS analysis. After 6 h of incubation, a linear relationship was observed between INX-08189 concentrations and intracellular 2′-C-MeGTP concentrations (Table 3). Given these measurements and assuming a hepatocellularity of 1 × 108 cells in 1 g of liver (37), we calculated that 84 and 243 pmol of 2′-C-MeGTP per g of liver tissue would provide 50 and 90% levels of viral inhibition, respectively. These values were used to interpret the pharmacodynamic relevance of the liver concentrations achieved after the oral administration of INX-08189 in vivo as discussed below. The intracellular half-life of 2′-C-MeGTP also was measured in the replicon cells with a value of approximately 24 h. This finding is consistent with the half-life of 2′-C-MeGTP observed in primary human hepatocyte cultures (26). The long intracellular half-life of the active triphosphate suggests that 2′-C-MeGTP concentrations in liver sufficient for antiviral activity are achievable with once-a-day dosing of INX-08189.
The ability to reduce viral loads rapidly and prevent the emergence of resistant clones during a lengthy treatment period is critical to establishing a robust sustained viral response in patients. We used the clearance of the genotype 1b replicon from Huh-7 cells as an in vitro surrogate to measure these characteristics of INX-08189. In the absence of G418 selection, concentrations as low as 10 nM INX-08189 (∼1× EC50) resulted in a ≥2 log10 decrease in luciferase activity, and at 40 nM INX-08189 (1× EC90) a ≥7 log10 decrease was observed during the 14-day culture period. Culture in the presence of G418 demonstrated that replicon-expressing cells were completely eliminated after 14 days of treatment with as little as 20 nM INX-08189 (∼2× EC50). The ability to clear the replicon and eliminate the rebound of viral replication at such low concentrations of inhibitor are significant in light of the fact that other direct-acting antivirals, such as VX-950 (telaprevir) and HCV-796, were unable to clear the genotype 1b subgenomic replicon with up to 15 times their respective EC50s (23), and PSI-7851, a phosphoramidate nucleotide of 2′-F-2′-C-methyl uridine, required >10 times its EC50 to clear the genotype 1b replicon at day 21 (19).
The S282T substitution in the HCV NS5B gene had been identified previously as a resistance mutant for 2′-C-methyl-modified nucleosides (20, 28, 30). To determine if INX-08189 selects for this and other mutations in vitro, long-term cultures of Huh-7 cells containing genotype 1a or 1b replicons were performed. As expected, the S282T mutation was observed and confirmed as a resistance mutation leading to an approximately 10-fold change in the EC50. Despite this shift in potency, INX-08189 still was capable of the complete inhibition of HCV replication in the S282T mutant replicon, with an EC90 of 344 nM, suggesting only partial resistance to the inhibitory effect of INX-08189. Since the current standard of care for the treatment of HCV is a combination of IFN-α and Rbv, we were interested in determining what effects the S282T mutation would have on the potency of these antiviral compounds in combination. There have been previous reports of enhanced potency for Rbv against the S282T mutant both at the biochemical and cellular levels (9). Our data confirm these observations, in that we observed a 6-fold increase in potency for Rbv against HCV replicons carrying the S282T mutation. It is well documented that the S282T mutation severely limits the replication efficiency of HCV (20, 22, 28). A second mutation, I585T, was observed in the selection studies with INX-08189. As has been reported previously, the I585T mutation does not confer drug resistance; however, the presence of this amino acid change in the genotype 1b replicon improves HCV replication efficiency (35). We have shown that while compensation by I585T occurs in both wild-type and S282T-expressing replicons, it does not affect the potency of any of the treatment drugs tested. Additionally, replicon inhibition studies combining INX-08189 with Rbv demonstrated a high degree of synergy against both the wild-type and S282T mutant replicons. This is unlike some other 2′-substituted nucleosides, which are additive or antagonistic when combined with ribavirin (8). Whether the synergistic effect is due to the cooperative inhibition of RdRp enzymatic activity by metabolites of INX-08189 and Rbv or is a consequence of the downregulation of competing endogenous nucleotides by Rbv (31) remains to be determined. These data, coupled with the fact that the S282T mutation has not been detected in chronically infected HCV patients at baseline (11) or after treatment with experimental 2′-C-methyl-modified nucleoside analogues (21, 33), suggests that the combination of INX-08189 with Rbv will prove to be a highly successful strategy to minimize the generation of resistant HCV isolates clinically while at the same time optimizing viral clearance.
Mitochondrial toxicity has been observed with certain classes of nucleoside analog drugs that inhibit the activity of the mitochondrial DNA polymerase γ, and this effect has been well studied in the case of reverse transcriptase inhibitors used to treat HIV infection (1, 15). The potential liability of INX-08189 with regard to mitochondrial toxicity therefore was assessed in tissue culture studies with both a liver-derived cell line and a lymphocyte cell line. Incubation in the presence of INX-08189 for 3 or 14 days indicated no change in the ratio of mitochondrial genome copy number to cellular DNA. The results indicate a lack of mitochondrion-specific toxicity for INX-08189.
The prodrug strategy embodied by INX-08189 complicates the analysis of pharmacokinetic and pharmacodynamic properties of the molecule. In rodents, the prodrug is short lived in the plasma and cannot be measured systemically. In cynomolgus monkeys, we have shown that at a dose of 25 mg/kg, the intact prodrug was present in the portal circulation for a short time postadministration but was not detected in the systemic circulation, suggesting that at this concentration of INX-08189 the molecule was efficiently extracted by the liver. Therefore, a surrogate marker of INX-08189 bioavailability was required to study its pharmacokinetic and pharmacodynamic properties. In clinical trials using a similar prodrug strategy to deliver 2′-C-MeGTP to the liver, the nucleoside metabolite 2′-C-MeG was utilized as a surrogate analyte to monitor pharmacokinetics in HCV patients (17, 18). To validate this approach for INX-08189, we measured the generation of 2′-C-MeGTP, the active inhibitor of NS5b RdRp, which is present in the liver, and 2′-C-MeG in the plasma. In rats where both metabolites could be measured in the same animals, the data clearly indicate a linear relationship between the 2′-C-MeG AUC24 values and the concentration of 2′-C-MeGTP in the liver at 24 h. The data for cynomolgus monkeys, although more limited than those of the rodent studies, were consistent with this finding. The correlative relationship between the two metabolites suggests that 2′-C-MeG plasma AUC0-24 values can be used to assess the delivery of 2′-C-MeGTP to the liver in vivo. The rodent data also showed very good dose proportionality for INX-08189, demonstrating a linear relationship in the exposure of both metabolites across a wide range of doses. Based on the measurements of 2′-C-MeGTP concentrations required to achieve 90% viral inhibition in vitro, we evaluated if liver 2′-C-MeGTP concentrations achieved in vivo would be sufficient to inhibit viral replication. In rats, the concentration of 2′-C-MeGTP in the liver 24 h postdose sufficient for 90% viral inhibition was achieved after a single oral dose of 3 mg/kg. Since this represents a trough level of 2′-C-MeGTP, multiple dosing would be expected to further improve triphosphate liver exposures. In monkeys, concentrations greater than the triphosphate EC90 were measured 6 h after a single dose of 25 mg/kg. 2′-C-MeGTP in rodent liver was found to be long lived, with a half-life estimated to be greater than 24 h.
Taken together, the in vitro and in vivo data indicate that INX-08189 is a highly potent inhibitor of HCV with a high barrier for resistance and good oral pharmacokinetic properties. The data support the continued advancement of INX-08189 in clinical development for the treatment of chronic HCV infections.
ACKNOWLEDGMENTS
Work conducted at the University of Cardiff was supported by a grant from Inhibitex, Inc., to C.M.
C.M. is a board member and shareholder of Inhibitex, Inc.
We acknowledge Andrea Brancale, Nicola Zonta, and Sarah Jones for their assistance in modeling studies of the HCV RdRp.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Anderson P., Kakuda T. N., Lichtenstein K. A. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 38:743–753 [DOI] [PubMed] [Google Scholar]
- 2. Blight K. J., Kolykhalov A. A., Rice C. M. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972–1974 [DOI] [PubMed] [Google Scholar]
- 3. Blight K. J., McKeating J. A., Marcotrigiano J., Rice C. M. 2003. Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. J. Virol. 77:3181–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cahard D., McGuigan C., Balzarini J. 2004. Aryloxy phosphoramidate triesters as pro-tides. Mini-Rev. Med. Chem. 4:371–381 [DOI] [PubMed] [Google Scholar]
- 5. Carroll S. S., Olsen D. B. 2006. Nucleoside analog inhibitors of hepatitis C virus replication. Infect. Dis. Drug Targets 6:17–29 [DOI] [PubMed] [Google Scholar]
- 6. Carroll S. S., et al. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979–11984 [DOI] [PubMed] [Google Scholar]
- 7. Chiu R. W., et al. 2003. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin. Chem. 49:719–726 [DOI] [PubMed] [Google Scholar]
- 8. Coelmont L., et al. 2006. Ribavirin antagonizes the in vitro anti-hepatitis C virus activity of 2′-C-methylcytidine, the active component of valopicitabine. Antimicrob. Agents Chemother. 50:3444–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cretton-Scott E., Gupta K., Hernandez-Santiago B. I., Larsson M. 2010. Compounds and pharmaceutical compositions for the treatment of viral infections. Patent WO 2010/014134 A1
- 10. Delaney W. E. T., Yang H., Miller M. D., Gibbs C. S., Xiong S. 2004. Combinations of adefovir with nucleoside analogs produce additive antiviral effects against hepatitis B virus in vitro. Antimicrob. Agents Chemother. 48:3702–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dryer P. D., et al. 2009. Screening for hepatitis C virus non-nucleotide resistance mutations in treatment-naive women. J. Antimicrob. Chemother. 64:945–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eldrup A. B., et al. 2004. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 47:2283–2295 [DOI] [PubMed] [Google Scholar]
- 13. Eldrup A. B., et al. 2004. Structure-activity relationship of heterobase-modified 2′-C-methyl ribonucleosides as inhibitors of hepatitis C virus RNA replication. J. Med. Chem. 47:5284–5297 [DOI] [PubMed] [Google Scholar]
- 14. Gardelli C., et al. 2009. Phosphoramidate prodrugs of 2′-C-methylcytidine for therapy of hepatitis C virus infection. J. Med. Chem. 52:5394–5407 [DOI] [PubMed] [Google Scholar]
- 15. Kakuda T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22:685–708 [DOI] [PubMed] [Google Scholar]
- 16. Kim W. R. 2002. The burden of hepatitis C in the United States. Hepatology 36:S30–S34 [DOI] [PubMed] [Google Scholar]
- 17. Lalezari J., et al. 2009. Antiviral activity, safety and pharmacokinetics of IDX184, a liver-targeted nucleotide HCV polymerase inhibitor, in patients with chronic hepatitis C, abstr. LB18. Abstr. 60th Annu. Meet. Am. Assoc. Study Liver Dis. Liver Meet., Boston, MA [Google Scholar]
- 18. Lalezari J., et al. 2010. Antiviral activity, pharmacokinetics and safety of IDX184 in combination with pegylated interferon (pegIFN) and ribavirin (RBV) in treatment-naive HCV genotype 1-infected subjects. J. Hepatol. 52(Suppl.):S469 [Google Scholar]
- 19. Lam A. M., et al. 2010. PSI-7851, a pronucleotide of beta-d-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob. Agents Chemother. 54:3187–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Pogam S., et al. 2006. In vitro selected Con1 subgenomic replicons resistant to 2′-C-methyl-cytidine or to R1479 show lack of cross resistance. Virology 351:349–359 [DOI] [PubMed] [Google Scholar]
- 21. Le Pogam S., et al. 2009. No evidence of R7128 drug resistance after up to 4 weeks treatment of GT 1, 2 and 3 hepatitis C virus infected individuals. J. Hepatol. 50(Suppl.):S348 [Google Scholar]
- 22. Ludmerer S. W., et al. 2005. Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay. Antimicrob. Agents Chemother. 49:2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCown M. F., et al. 2008. The hepatitis C Virus Replicon Presents a Higher Barrier to Resistance to Nucleoside Analogs than to Nonnucleoside Polymerase or Protease Inhibitors. Anitmicrob. Agents Chemother. 52:1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGuigan C., et al. 2010. Phosphoramidate ProTides of 2′-C-methylguanosine as highly potent inhibitors of hepatitis C virus. Study of their in vitro and in vivo properties. J. Med. Chem. 53:4949–4957 [DOI] [PubMed] [Google Scholar]
- 25. McGuigan C., et al. 2009. The application of phosphoramidate ProTide technology to the potent anti-HCV compound 4′-azidocytidine (R1479). Bioorg. Med. Chem. Lett. 19:4250–4254 [DOI] [PubMed] [Google Scholar]
- 26. McGuigan C., et al. 2010. Design, synthesis and evaluation of a novel double pro-drug: INX-08189. A new clinical candidate for hepatitis C virus. Bioorg. Med. Chem. Lett. 20:4850–4854 [DOI] [PubMed] [Google Scholar]
- 27. McGuigan C., Perrone P., Madela K., Neyts J. 2009. The phosphoramidate ProTide approach greatly enhances the activity of beta-2′-C-methylguanosine against hepatitis C virus. Bioorg. Med. Chem. Lett. 19:4316–4320 [DOI] [PubMed] [Google Scholar]
- 28. Migliaccio G., et al. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164–49170 [DOI] [PubMed] [Google Scholar]
- 29. Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. 1982. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858–3863 [PubMed] [Google Scholar]
- 30. Olsen D. B., et al. 2004. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 48:3944–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker W. B. 2005. Metabolism and antiviral activity of ribavirin. Virus Res. 107:165–171 [DOI] [PubMed] [Google Scholar]
- 32. Prichard M. N., Shipman C., Jr 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181–205 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez-Torres M., et al. 2009. Antiviral activity, pharmacokinetics, safety, and tolerability of PSI-7851, a novel nucleotide polymerase inhibitor for HCV, following single and 3 day multiple ascending oral doses in healthy volunteers and patients with chronic HCV infection, abstr. LB17. Abstr. 60th Annu. Meet. Am. Assoc. Study Liver Dis. Meet., Boston, MA [Google Scholar]
- 34. Schinazi R. F., Bassit L., Gavegnano C. 2010. HCV drug discovery aimed at viral eradication. J. Viral Hepat. 17:77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomei L., et al. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225–13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams R. 2006. Global challenges in liver disease. Hepatology 44:521–526 [DOI] [PubMed] [Google Scholar]
- 37. Wilson Z. E., et al. 2003. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br. J. Clin. Pharmacol. 56:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]