Abstract
Constitutive AmpC hyperproduction is the most frequent mechanism of resistance to the weak AmpC inducers antipseudomonal penicillins and cephalosporins. Previously, we demonstrated that inhibition of the β-N-acetylglucosaminidase NagZ prevents and reverts this mechanism of resistance, which is caused by ampD and/or dacB (PBP4) mutations in Pseudomonas aeruginosa. In this work, we compared NagZ with a second candidate target, the AmpG permease for GlcNAc-1,6-anhydromuropeptides, for their ability to block AmpC expression pathways. Inactivation of nagZ or ampG fully restored the susceptibility and basal ampC expression of ampD or dacB laboratory mutants and impaired the emergence of one-step ceftazidime-resistant mutants in population analysis experiments. Nevertheless, only ampG inactivation fully blocked ampC induction, reducing the MICs of the potent AmpC inducer imipenem from 2 to 0.38 μg/ml. Moreover, through population analysis and characterization of laboratory mutants, we showed that ampG inactivation minimized the impact on resistance of the carbapenem porin OprD, reducing the MIC of imipenem for a PAO1 OprD mutant from >32 to 0.5 μg/ml. AmpG and NagZ targets were additionally evaluated in three clinical isolates that are pan-β-lactam resistant due to AmpC hyperproduction, OprD inactivation, and overexpression of several efflux pumps. A marked increase in susceptibility to ceftazidime and piperacillin-tazobactam was observed in both cases, while only ampG inactivation fully restored wild-type imipenem susceptibility. Susceptibility to meropenem, cefepime, and aztreonam was also enhanced, although to a lower extent due to the high impact of efflux pumps on the activity of these antibiotics. Thus, our results suggest that development of small-molecule inhibitors of AmpG could provide an excellent strategy to overcome the relevant mechanisms of resistance (OprD inactivation plus AmpC induction) to imipenem, the only currently available β-lactam not significantly affected by P. aeruginosa major efflux pumps.
INTRODUCTION
The increasing prevalence of nosocomial infections produced by multidrug-resistant (MDR) Pseudomonas aeruginosa strains severely compromises the selection of appropriate treatments and is therefore associated with significant morbidity and mortality (21, 34). Indeed, one of the most striking features of P. aeruginosa is its extraordinary capacity for developing resistance to almost any available antibiotic by the selection of mutations in chromosomal genes (24, 28). Among the mutation-mediated β-lactam resistance mechanisms, particularly noteworthy are those leading to the constitutive overexpression of the inducible chromosomal cephalosporinase AmpC, which confers resistance to penicillins, cephalosporins, and monobactams (7, 14). Additionally, mutations that lead to the repression or inactivation of the porin OprD, acting synergistically with inducible or constitutively overexpressed AmpC, confer resistance to carbapenems (8, 26, 37).
AmpC is a chromosomally encoded group I, class C cephalosporinase produced by P. aeruginosa, as well as many other nonfermenting Gram-negative bacilli and most Enterobacteriaceae (3). Although AmpC is produced at very low basal levels in wild-type strains, its expression is inducible in the presence of certain β-lactams (β-lactamase inducers), such as cefoxitin and imipenem (27). In fact, the activity of the antipseudomonal penicillins (such as ticarcillin and piperacillin), cephalosporins (such as ceftazidime and cefepime), and monobactams (such as aztreonam) relies on the fact that they are very weak AmpC inducers, since they too are hydrolytically inactivated by this enzyme (27). For this reason, during treatment with these weak inducers, mutants showing constitutive high-level AmpC production (AmpC derepressed mutants) are frequently selected, leading to the failure of antimicrobial therapy (7, 13, 14, 24, 25).
There are several genes involved in the regulation of ampC expression, a process that was first investigated in the Enterobacteriaceae and found to be intimately linked to peptidoglycan recycling (33, 36). ampG encodes an inner membrane permease for GlcNAc-1,6-anhydromuropeptides, which are peptidoglycan catabolites that, upon entry into the cytosol, are processed by the β-N-acetylglucosaminidase, known as NagZ, to generate 1,6-anhydromuropeptides (4, 18, 40). The 1,6-anhydromuropeptide products of NagZ are thought to induce AmpC production by interacting with the LysR-type transcriptional regulator AmpR (2, 6, 11, 12, 22). During regular bacterial growth, 1,6-anhydromuropeptides are processed by the N-acetyl-anhydromuramyl-l-alanine amidase AmpD, avoiding ampC induction (10, 20, 36). On the other hand, during growth in the presence of strong β-lactamase inducers, large amounts of muropeptides are generated and accumulate in the cytoplasm, which leads to the AmpR-mediated induction of ampC expression (6, 11, 12, 22). It is also well-known that the mutational inactivation of AmpD leads to the accumulation of 1,6-anhydromuropeptides and high-level ampC expression, even in the absence of β-lactamase inducers, producing the classical constitutively derepressed phenotype of AmpC production (23).
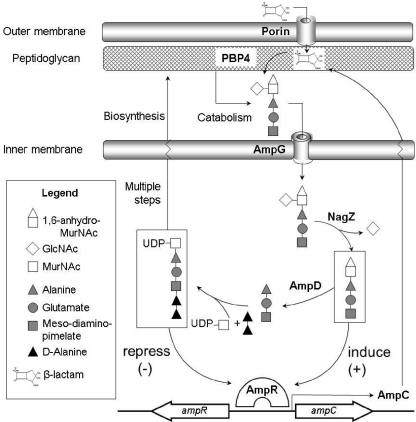
Pseudomonas aeruginosa AmpG (17, 43), AmpR (16), NagZ (1), and AmpD (19) homologues have been identified. Further studies showed that P. aeruginosa has 3 ampD genes (ampD, ampDh2, and ampDh3) and that their sequential inactivation leads to a stepwise upregulation of ampC expression, reaching full derepression with very high level basal ampC expression in the triple mutant (15). Recent work showed, however, that one-step high-level resistance in P. aeruginosa frequently results, in clinical strains, from the inactivation of dacB, encoding the nonessential penicillin-binding protein 4 (PBP4) (31). The inactivation of PBP4 was shown to give rise to a complex β-lactam resistance response, triggering overproduction of the chromosomal β-lactamase AmpC and the specific activation of the CreBC (BlrAB) two-component regulator (31). A schematic representation of the interplay between peptidoglycan recycling, ampC regulation, and β-lactam resistance is shown in Fig. 1.
Fig. 1.
Schematic representation of the interplay between peptidoglycan recycling, ampC regulation, and β-lactam resistance.
Development of strategies for combating these resistance mechanisms is crucial for preserving the activity of needed β-lactam antibiotics (29). Given that NagZ removes GlcNAc to produce the 1,6-anhydromuropeptides (4, 40), inhibitors of this enzyme have been shown to mitigate AmpC-driven resistance (39). In previous studies, we have demonstrated that inactivation or direct inhibition of NagZ in P. aeruginosa prevents and reverts resistance to the weak AmpC inducers antipseudomonal penicillins and cephalosporins driven by constitutive overexpression of AmpC caused by either AmpD or PBP4 mutations (1, 42). NagZ inactivation also attenuated the high-level resistance of the AmpD-PBP4 double mutant, although wild-type susceptibility was not fully restored (42). Additionally, NagZ inactivation did not block ampC inducibility in the presence of the strong inducer cefoxitin (42). The molecular basis for this incomplete inhibition of the AmpC regulatory pathway remains unclear, although the possibility that it could arise from the interaction of the accumulated NagZ substrates (GlcNAc-1,6-anhydromuropeptides) with AmpR cannot be ruled out. In any event, we speculate that blocking peptidoglycan recycling earlier in the pathway could be more efficient. Thus, in this work we comparatively evaluated NagZ with a second candidate target, AmpG, in a collection of P. aeruginosa laboratory mutants and well-characterized pan-β-lactam-resistant clinical strains.
MATERIALS AND METHODS
Strains, plasmids, and susceptibility testing.
The bacterial strains and plasmids used or constructed in this study are listed in Table 1. MICs of ceftazidime, cefepime, aztreonam, piperacillin-tazobactam, imipenem, meropenem, and ciprofloxacin were determined using Etest strips (AB Biodisk, Solna, Sweden) on Mueller-Hinton (MH) agar, according to the manufacturer's recommendations. Additionally, MICs of cefoxitin were determined by microdilution in 100 μl of cation-adjusted MH broth following CLSI guidelines (5). The phenotypic determination of AmpC inducibility was performed by assessing MH agar plates for the presence of antagonism between imipenem and ceftazidime disks (separated 5 to 30 mm) as previously described (15).
Table 1.
Strains and plasmids used or constructed
| Strain or plasmid | Genotype/relevant characteristicsa | Reference or source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Reference strain completely sequenced | Laboratory collection |
| PAΔD | PAO1 ΔampD::lox | 15 |
| PAΔdB | PAO1 ΔdacB::lox | 31 |
| PAdacB | 1A1 spontaneous dacB mutant (W273X) of PAO1 | 31 |
| PAdacBΔD | PadacB ΔampD::lox | 31 |
| PAΔR | PAO1 ΔampR::lox | 31 |
| PAΔC | PAO1 ΔampC::lox | 30 |
| PAΔnZ | PAO1 ΔnagZ::lox | 42 |
| PAΔDnZ | PAO1 ΔampD::lox ΔnagZ::lox | 42 |
| PAΔdBnZ | PAO1 ΔdacB::lox ΔnagZ::lox | 42 |
| PAdacBΔDnZ | PadacB ΔampD::lox ΔnagZ::lox | 42 |
| PAΔG | PAO1 ΔampG::lox | This work |
| PAΔDG | PAO1 ΔampD::lox ΔampG::lox | This work |
| PAΔdBG | PAO1 ΔdacB::lox ΔampG::lox | This work |
| PAdacBΔDG | PadacB ΔampD::lox ΔampG::lox | This work |
| PAOD1 | Spontaneous oprD null mutant (W65X) of PAO1 | 32 |
| PAOD1ΔnZ | PAOD1 ΔnagZ::Gm lox | This work |
| PAOD1ΔG | PAOD1 ΔampG::Gm lox | This work |
| JSG2A1 | Pan-β-lactam resistant P. aeruginosa clinical strain | 14 |
| JSG2A1ΔnZ | JSG2A1 ΔnagZ::Gm lox | This work |
| JSG2A1ΔG | JSG2A1 ΔampG::Gm lox | This work |
| MQB1C5 | Pan-β-lactam-resistant P. aeruginosa clinical strain | 14 |
| MQB1C5ΔnZ | MQB1C5 ΔnagZ::Gm lox | This work |
| MQB1C5ΔG | MQB1C5 ΔampG::Gm lox | This work |
| OFC2I4 | Pan-β-lactam-resistant P. aeruginosa clinical strain | 14 |
| OFC2I4ΔnZ | OFC2I4 ΔnagZ::Gm lox | This work |
| OFC2I4ΔG | OFC2I4 ΔampG::Gm lox | This work |
| E. coli | ||
| XL1-Blue | F′::Tn10 proA+proB+lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rk− mk−) mcrB1 | Laboratory collection |
| S17.1 | recA pro (RP4-2Tet::Mu Kan::Tn7) | Laboratory collection |
| Plasmids | ||
| pUCP26 | Tcr, pUC-18-based Escherichia-Pseudomonas shuttle vector | 41 |
| pUCP26ampG | pUC26 containing PAO1 ampG gene | This work |
| pEX100Tlink | AprsacB, pUC19-based gene replacement vector with an MCS | 38 |
| pEX18Tc | TcrsacB, pUC18-based gene replacement vector with an MCS | 9 |
| pUCGmlox | Apr Gmr, pUC18-based vector containing the lox-flanked aacC1 gene | 38 |
| pCM157 | Tcr, cre expression vector | 38 |
| pEXnZ | pEX100Tlink containing 5′and 3′ flanking sequence of nagZ | 42 |
| pEXnZGm | pEX100Tlink containing 5′and 3′ flanking sequence of nagZ::Gm lox | 42 |
| pEXTcΔampG::Gm | pEX18Tc containing 5′and 3′ flanking sequence of ampG::Gm lox | This work |
MCS, multiple cloning site; Apr, ampicillin resistant; Gmr, gentamicin resistant; Tcr, tetracycline resistant.
Construction of nagZ- and ampG-knockout mutants.
The nagZ- and ampG-knockout mutants generated in this study from several PAO1 derivative mutants and pan-β-lactam-resistant clinical strains are shown in Table 1. Knockout mutants were constructed following well-established procedures (15, 31) based on the cre-lox system for gene deletion and antibiotic resistance marker recycling in P. aeruginosa (38). The previously constructed plasmid pEXnZGm (ΔnagZ::Gm) (42) was used as the donor for the generation of nagZ-knockout mutants. For the construction of ampG-knockout mutants, upstream and downstream PCR products (Table 2) of ampG (using PAO1 DNA as the template) were digested with either EcoRI or HindIII and XbaI and cloned by a three-way ligation into pEX18Tc (9). The resulting plasmid (pEXTcΔampG) was transformed into Escherichia coli NM522, and transformants were selected on LB agar plates supplemented with 5 μg/ml tetracycline. The lox-flanked gentamicin resistance cassette (aac1) obtained by XbaI restriction of plasmid pUCGmlox was cloned into the single site for this enzyme formed by the ligation of the two flanking fragments. The resulting plasmid (pEXTcΔampG::Gm) was again transformed into E. coli NM522, and transformants were selected on LB agar plates supplemented with 5 μg/ml tetracycline and 5 μg/ml gentamicin. The plasmid was then transformed into the E. coli S17.1 helper strain. The different nagZ- or ampG-knockout mutants were then generated by conjugational transfer of pEXnZGm or pEXTcΔampG::Gm from E. coli S17.1 to the corresponding P. aeruginosa strains, followed by selection of double recombinants on LB agar containing 10% sucrose and 30 μg/ml gentamicin. Double recombinants were checked first by screening for carbenicillin (for pEXnZGm) or tetracycline (for pEXTcΔampG::Gm) susceptibility and then by PCR amplification and sequencing. For the recycling of the gentamicin resistance cassettes, plasmid pCM157 was electroporated into the different mutants. Transformants were selected on LB agar plates supplemented with 250 μg/ml tetracycline. One transformant for each mutant was grown overnight in LB broth with 250 μg/ml tetracycline in order to allow the expression of the cre recombinase. Plasmid pCM157 was then cured from the strains by successive passages on LB broth. Selected colonies were then screened for tetracycline (250 μg/ml) and gentamicin (30 μg/ml) susceptibility and checked by PCR amplification and DNA sequencing.
Table 2.
Primers used for cloning and construction of ampG knockout mutants
| Primer | Sequence (5′–3′)a | PCR product size (bp) | Use |
|---|---|---|---|
| AGF1-ERI | GATATAGAATTCCGGTCGCGGCGGCACCATCTG | 823 | ampG inactivation |
| AGR1-XbI | TATATCTCTAGAGCTGGCGGGAGACTTGTAGGC | ||
| AGF2-XbI | GATATATCTAGATACGTCACCGCGGTGATGGGC | 621 | |
| AGR2-HD3 | TATATCAAGCTTGTGCTGATCCTGCTGTTCCGC | ||
| AGF-ERI | GATATAGAATTCAAGAAGGAGATATACATATGACTCAGCAATCCTGG | 1,785 | ampG cloning |
| AGR-HD3 | TATATCAAGCTTTCAGTGCTGCTCGGCGTTCTGGTGTCCC |
Restriction sites for endonucleases are underlined.
Cloning of wild-type ampG gene and complementation experiments.
The wild-type PAO1 ampG gene was PCR amplified using the primers listed in Table 2. The PCR product was digested with EcoRI and HindIII and ligated into plasmid pUCP26. The ligation reaction was used to transform chemically competent E. coli NM522 cells, and transformants were selected on LB agar supplemented with 5 μg/ml tetracycline. The recombinant plasmid was isolated from a single transformant, and its presence was verified by restriction analysis and DNA sequencing. The resulting ampG expression plasmid (pUCPampG) and pUCP26 (control vector), were electroporated into the ampG-knockout mutant of PAO1 (PAΔG). Cefoxitin MICs were determined in triplicate by the broth microdilution method in 100 μl of cation-adjusted Mueller-Hinton broth. Tetracycline (50 μg/ml) was added to the broth in order to maintain the complementation plasmid.
Quantification of basal and induced ampC expression.
The relative levels of ampC mRNA were determined by real-time reverse transcription-PCR (RT-PCR) following previously described protocols (15). Briefly, total RNA from logarithmic-phase-grown cultures (with and without 50 μg/ml of cefoxitin) was obtained with an RNeasy minikit (Qiagen, Hilden, Germany). Fifty nanograms of purified RNA was then used for one-step reverse transcription and real-time PCR, using a QuantiTect SYBR green reverse transcription-PCR kit (Qiagen, Hilden, Germany) in a SmartCycler II apparatus (Cepheid, Sunnyvale, CA). Previously described conditions and primers were used (15). The rpsL housekeeping gene was used to normalize the expression levels, and results were always referred to PAO1 basal expression. All RT-PCRs were performed in duplicate, and the mean values of mRNA expression resulting from three independent experiments were considered in all cases.
Population analysis of ceftazidime and imipenem susceptibility and resistance emergence.
Serial dilutions of 10-ml overnight cultures (MH broth) of PAO1, PAΔnZ, or PAΔG were seeded on MH agar plates containing 0, 0.12, 0.25, 0.5, 1, 2, 4, 8, or 16 μg/ml of ceftazidime or imipenem. Colonies growing after 24 h of incubation were counted, to plot the numbers of CFU at each antibiotic concentration. All experiments were performed in triplicate, and the results are shown as mean values ± standard deviations.
Characterization of pan-β-lactam-resistant clinical isolates.
Three isolates resistant to all β-lactams tested (including penicillins, cephalosporins, monobactams, and carbapenems), each recovered from a different intensive care unit patient as part of a previous study (14), were used. Each isolate belonged to a different clone, and all were known to overexpress ampC due to ampD and/or dacB (PBP4) mutations (14, 31). The involvement of efflux pump overexpression in the resistance phenotype was also explored in this work. For this purpose, the expression of the genes encoding the four major P. aeruginosa efflux pumps, MexAB-OprM (mexB), MexCD-OprJ (mexD), MexEF-OprN (mexF), and MexXY-OprM (mexY), was determined by real-time RT-PCR following previously described protocols (35). Briefly, total RNA from logarithmic-phase-grown cultures was obtained with an RNeasy minikit (Qiagen, Hilden, Germany). Fifty nanograms of purified RNA was then used for one-step reverse transcription and real-time PCR, using a QuantiTect SYBR green reverse transcription-PCR kit (Qiagen, Hilden, Germany) in a SmartCycler II apparatus (Cepheid, Sunnyvale). Previously described conditions and primers were used (35). The rpsL housekeeping gene was used to normalize the expression levels, and results were always referred to PAO1 basal expression. All RT-PCRs were performed in duplicate, and the mean values of mRNA expression resulting from three independent experiments were considered in all cases. Overexpression was considered when the corresponding mRNA level was at least 3-fold (mexB) or 10-fold (mexD, mexF, mexY) higher than that for PAO1. The involvement of oprD inactivation in carbapenem resistance was explored through PCR amplification, followed by sequencing, using previously described primers and conditions (8).
RESULTS AND DISCUSSION
Comparison of AmpG and NagZ as targets to suppress AmpC-driven β-lactam resistance.
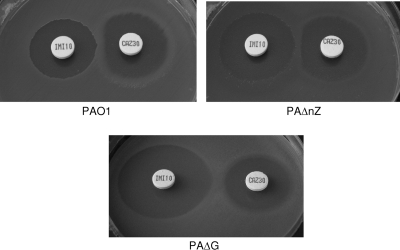
In previous work (1, 42), we showed that the inactivation of nagZ fully restored susceptibility and basal ampC expression of ampD or dacB single mutants. Major reductions of MICs and ampC expression were also observed for the ampD-dacB double mutant, although wild-type levels were not fully reached. Furthermore, we showed that nagZ inactivation had little effect on ampC inducibility. In order to identify effective targets for suppressing AmpC-driven resistance in P. aeruginosa, here we compared the effects of ampG and nagZ inactivation, and the results are shown in Table 3. As shown, ampG inactivation fully restored susceptibility and basal ampC expression in ampD and dacB single and double mutants. More importantly, in contrast to nagZ inactivation, it fully blocked AmpC inducibility, as evidenced by ampC expression data (Table 3), results of the imipenem-ceftazidime double-disk AmpC induction test (Fig. 2), or the marked hypersusceptibility of the ampG mutants to the AmpC inducers imipenem and cefoxitin.
Table 3.
MICs and basal and induced ampC expression in the studied strains
| Straina | MIC (μg/ml)b |
Relative mRNA levelc |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CAZ | FEP | IMP | MER | PTZ | ATM | FOX | Basal | Inducedd | |
| PAO1 | 1 | 1 | 2 | 0.5 | 3 | 3 | 2,048 | 1 | 78 ± 34 |
| PAΔnZ | 1 | 1 | 1 | 0.38 | 3 | 3 | 1,024 | −2.1 ± 1.5 | 469 ± 230 |
| PAΔG | 1 | 1 | 0.38 | 0.38 | 3 | 2 | 64 | −1.1 ± 0.4 | 1.9 ± 1.0 |
| PAΔD | 8 | 4 | 2 | 1.5 | 32 | 8 | 4,096 | 47 ± 9.5 | 134 ± 11 |
| PAΔDnZ | 2 | 2 | 1 | 0.75 | 6 | 3 | 1,024 | 1.0 ± 0.4 | 483 ± 26 |
| PAΔDG | 1 | 1.5 | 0.38 | 0.5 | 3 | 1.5 | 64 | −1.5 ± 0.6 | 1.6 ± 0.8 |
| PAΔdB | 24 | 12 | 2 | 0.75 | 64 | 16 | 2,048 | 51 ± 16 | 232 ± 67 |
| PAΔdBnZ | 1.5 | 1.5 | 1 | 0.5 | 4 | 3 | 1,024 | 3.0 ± 1.9 | 661 ± 374 |
| PAΔdBG | 1 | 0.75 | 0.38 | 0.38 | 2 | 1.5 | 64 | −1.1 ± 1.3 | 1.2 ± 0.9 |
| PAdacBΔD | 96 | 32 | 2 | 2 | >256 | 48 | 4,096 | 1,770 ± 414 | 1,950 ± 480 |
| PAdacBΔDnZ | 4 | 2 | 1.5 | 0.75 | 24 | 6 | 1,024 | 40 ± 17 | 906 ± 80 |
| PAdacBΔDG | 0.75 | 1 | 0.38 | 0.25 | 1.5 | 1.5 | 64 | 1.7 ± 0.6 | 1.8 ± 1.5 |
| PAΔR | 1.5 | 1.5 | 0.5 | 0.25 | 4 | 4 | 64 | 3.8 ± 0.4 | 3.3 ± 0.8 |
| PAΔC | 1 | 1 | 0.5 | 0.25 | 3 | 3 | 64 | NA | NA |
PAO1 mutants: PAΔnZ, nagZ; PAΔG, ampG; PAΔD, ampD; PAΔDnZ, ampD-nagZ; PAΔDG, ampD-ampG; PAΔdB, dacB; PAΔdBnZ, dacB-nagZ; PAΔdBG, dacB-ampG; PAdacBΔD, dacB-ampD; PAdacBΔDnZ, dacB-ampD-nagZ; PAdacBΔDG, dacB-ampD-ampG; PAΔR, ampR; PAΔC, ampC. Complementation of the PAO1 ampG mutant (PAΔG) with plasmid pUCPampG fully restored wild-type cefoxitin MICs.
CAZ, ceftazidime; FEP, cefepime; IMP, imipenem; MER, meropenem; PTZ, piperacillin-tazobactam; ATM, aztreonam, FOX, cefoxitin.
Relative amount of ampC mRNA compared to PAO1 basal levels ± standard deviation. ampC expression data for nagZ mutants obtained in previous work (42) were included for comparative purposes. NA, not applicable.
Induction experiments were carried out with 50 μg/ml of cefoxitin.
Fig. 2.
Double-disk (imipenem-ceftazidime) AmpC induction test with strains PAO1, PAΔnZ, and PAΔG.
Blocking ampC induction through AmpG inactivation prevents and reverts imipenem resistance driven by lack of OprD expression.
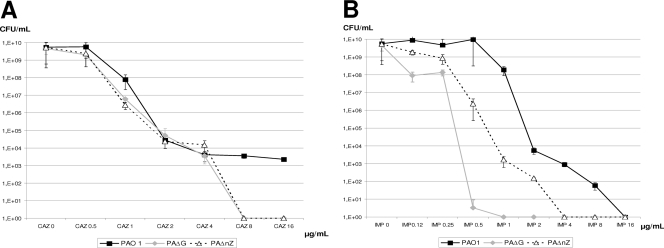
The results presented above suggested that both NagZ and AmpG are necessary for resistance to the weak inducers, including antipseudomonal penicillins and cephalosporins, acquired through AmpD or PBP4 mutations that lead to constitutive AmpC overexpression. Indeed, through a population analysis of ceftazidime susceptibility (Fig. 3A), we documented that the behaviors of nagZ and ampG mutants are essentially identical: deletion of either gene impaired the emergence of one-step ceftazidime-resistant mutants at concentrations of ≥8 μg/ml (susceptibility breakpoint), in sharp contrast to results for wild-type PAO1, in which mutants were still readily selected at concentrations of at least 16 μg/ml. On the other hand, major differences were observed between nagZ and ampG mutants in the population analysis of susceptibility to the potent AmpC inducer imipenem (Fig. 3B). While PAO1 imipenem-resistant mutants were detected at concentrations of up to 8 μg/ml (resistance breakpoint), nagZ inactivation significantly increased imipenem susceptibility and reduced the highest concentration yielding mutants by 4-fold (to 2 μg/ml). Nevertheless, remarkably, this effect was far more intense for the ampG mutant, which yielded no imipenem-resistant mutants at concentrations above 0.5 μg/ml (16-fold lower than that for PAO1).
Fig. 3.
Population analysis of PAO1, PAΔnZ, and PAΔG ceftazidime (CAZ) (A) and imipenem (IMP) (B) susceptibility. Overnight cultures of PAO1, PAΔnZ, or PAΔG were plated on MH agar containing 0, 0.12, 0.25, 0.5, 1, 2, 4, 8, and 16 μg/ml of ceftazidime or imipenem, and the CFU counts were enumerated after 24 h of incubation. The results are shown as mean values of 3 experiments ± standard deviations.
These results suggested that ampG inactivation might minimize the impact of the most relevant imipenem resistance mechanism in P. aeruginosa, the inactivation of the carbapenem porin OprD. To test this possibility, we constructed and analyzed the nagZ and ampG mutants of a previously generated oprD mutant of PAO1 (32). Indeed, as shown in Table 4, imipenem MICs of the PAOD1 oprD mutant (>32 μg/ml) were reduced to 6 μg/ml in the nagZ mutant and to 0.5 μg/ml (below wild-type PAO1 MICs) in the ampG mutant. These results clearly indicate that inducible ampC expression (which is blocked through AmpG inactivation) is necessary for OprD inactivation-driven imipenem resistance, in agreement with previous evidence (26).
Table 4.
MICs of nagZ and ampG mutants of pan-β-lactam-resistant P. aeruginosa clinical strains
| Straina | Resistance mechanism |
MIC (μg/ml)d |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AmpDb | PBP4b | OprD | Effluxc | CAZ | FEP | IMP | MER | PTZ | ATM | CIPe | |
| PAO1 | 1.5 | 1.5 | 2 | 0.38 | 2 | 4 | 0.094 | ||||
| PAOD1 | W65X | 1.5 | 1.5 | >32 | 2 | 1.5 | 4 | 0.094 | |||
| PAOD1ΔnZ | 1.5 | 1.5 | 6 | 2 | 2 | 2 | 0.094 | ||||
| PAOD1ΔG | 1.5 | 1.5 | 0.5 | 2 | 2 | 2 | 0.094 | ||||
| JSG2A1 | Ins. 1 bp (C) in nt 481 | T428P | ΔoprD | mexB (6.5-fold) | 192 | 96 | >32 | >32 | >256 | 128 | 3 |
| JSG2A1ΔnZ | 8 | 16 | >32 | >32 | 24 | 32 | 3 | ||||
| JSG2A1ΔG | 3 | 8 | 1.5 | 24 | 8 | 32 | 3 | ||||
| MQB1C5 | Q155X | W339X | mexB (14-fold) mexY (21-fold) | 24 | 24 | >32 | >32 | 96 | 64 | 1 | |
| MQB1C5ΔnZ | 3 | 16 | >32 | >32 | 12 | 32 | 1 | ||||
| MQB1C5ΔG | 3 | 8 | 2 | >32 | 12 | 32 | 0.75 | ||||
| OFC2I4 | ΔampDE | M200I, del. D201 | ΔoprD | mexY (12-fold) mexF (10-fold) | 48 | 24 | >32 | >32 | >256 | 32 | 0.38 |
| OFC2I4ΔnZ | 3 | 12 | >32 | 6 | 16 | 4 | 0.38 | ||||
| OFC2I4ΔG | 1.5 | 8 | 1.5 | 3 | 3 | 4 | 0.38 | ||||
nagZ (ΔnZ) and ampG (ΔG) mutants of the OprD mutant of PAO1 (PAOD1) and pan-β-lactam-resistant clinical strains JSG2A1, MBQ1C5, and OFC3I4.
Mutations in ampD and dacB (PBP4) were documented in previous studies (14, 31). Ins., insertion; nt, nucleotide; del., deletion.
Relative expression of efflux pump-encoding genes compared to that in wild-type PAO1. Breakpoints used for defining overexpression were ≥3-fold for mexB and ≥10-fold for mexY, mexD, and mexF.
CAZ, ceftazidime; FEP, cefepime; IMP, imipenem; MER, meropenem; PTZ, piperacillin-tazobactam; ATM, aztreonam, CIP, ciprofloxacin.
Ciprofloxacin MICs were included as a control to evaluate the specificity of the effect of nagZ and ampG inactivation on β-lactam susceptibility.
Comparison of AmpG and NagZ as targets for restoring susceptibility in pan-β-lactam-resistant P. aeruginosa clinical strains.
P. aeruginosa pan-β-lactam resistance in the clinical setting is known to frequently result from the interplay of AmpC hyperproduction, OprD inactivation, and the overexpression of efflux pumps (24, 28). Thus, we evaluated the effect of nagZ and ampG inactivation in three different clones of well-characterized pan-β-lactam-resistant P. aeruginosa clinical isolates. These strains were already known to overexpress ampC due to ampD and/or dacB (PBP4) mutations (14, 31). In this work, we documented that all three isolates additionally presented oprD-inactivating mutations and overexpressed one or several efflux pumps (Table 4). Both nagZ and ampG inactivation notably increased β-lactam susceptibility in the clinical strains, although important differences were again observed (Table 4). A marked effect on ceftazidime and piperacillin-tazobactam susceptibility was observed in both cases, although knocking out ampG had a greater effect in the two ampD-dacB double mutants. Again, the sharpest differences were observed for imipenem, with ampG inactivation fully restoring wild-type susceptibility for the three isolates. Susceptibility to meropenem, cefepime, and aztreonam was also enhanced, although to a lower extent, consistent with the higher impact of efflux pump overexpression on the activity of these antibiotics. It should thus be noted that imipenem is likely the optimal candidate for combination with future potential AmpG inhibitors, given that this strategy impairs the interplay of the most relevant mechanism of resistance (OprD inactivation and AmpC induction) to this antibiotic, which is the only currently available β-lactam not significantly impacted by any of the P. aeruginosa major efflux pumps (24).
Concluding remarks.
In summary, we show that NagZ and AmpG are excellent targets for reverting and preventing the emergence of resistance to the weak AmpC inducers antipseudomonal penicillins and cephalosporins, driven by AmpC overexpression due to an AmpD and/or DacB mutation. Indeed, we have already shown the potential utility of small-molecule NagZ inhibitors for this purpose (1, 42), and research directed to the identification of AmpG inhibitors is ongoing. The latter strategy is highly encouraging, since we show that ampG inactivation fully blocks ampC induction, additionally minimizing the impact of OprD inactivation on imipenem resistance both in laboratory mutants and in pan-β-lactam-resistant clinical strains.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministerio de Ciencia e Innovación of Spain and the Instituto de Salud Carlos III through the Spanish Network for Research in Infectious Diseases (REIPI C03/14 and RD06/0008) and grant PS09/00033, as well as the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation.
D.J.V. is a Tier II Canada Research Chair in Chemical Glycobiology and a scholar of the Michael Smith Foundation for Health Research.
We thank V. Larmour for technical assistance.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Asgarali A., Stubbs K. A., Oliver A., Vocadlo D. J., Mark B. J. 2009. Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal β-lactam resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:2274–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balcewich M. D., et al. 2010. Crystal structure of the AmpR effector binding domain provides insight into the molecular regulation of inducible AmpC β-lactamase. J. Mol. Biol. 400:998–1010 [DOI] [PubMed] [Google Scholar]
- 3. Bush K., Jacoby G. A., Medeiros A. A. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Q., Li H., Merdek K., Park J. T. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 182:4836–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CLSI/NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 6th ed. (M7-A6) CLSI/NCCLS, Wayne, PA [Google Scholar]
- 6. Dietz H., Pfeifle D., Wiedemann B. 1997. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giwercman B., Lambert P. A., Rosdahl V. T., Shand G. H., Hoiby N. 1990. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepessed β-lactamase producing strains. J. Antimicrob. Chemother. 26:247–259 [DOI] [PubMed] [Google Scholar]
- 8. Gutiérrez O., et al. 2007. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob. Agents Chemother. 51:4329–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 10. Höltje J. V., Kopp U., Ursinus A., Wiedemann B. 1994. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-l-alanine amidase. FEMS Microbiol. Lett. 122:159–164 [DOI] [PubMed] [Google Scholar]
- 11. Honore N., Nicolas M. H., Cole S. T. 1986. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 5:3709–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs C., Huang L., Bartowsky E., Normark S., Park J. T. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juan C., et al. 2005. Contribution of clonal dissemination and selection of mutants during therapy to Pseudomonas aeruginosa antimicrobial resistance in an intensive care unit setting. Clin. Microbiol. Infect. 11:887–892 [DOI] [PubMed] [Google Scholar]
- 14. Juan C., et al. 2005. Molecular mechanisms of β-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 49:4733–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juan C., Moyá B., Pérez J. L., Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high level beta-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong K. F., et al. 2005. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB beta-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob. Agents Chemother. 49:4567–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong K. F., Aguila A., Schneper L., Mathee K. 2010. Pseudomonas aeruginosa beta-lactamase induction requires two permeases, AmpG and AmpP. BMC Microbiol. 10:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korfmann G., Sanders C. C. 1989. ampG is essential for high level expression of AmpC beta-lactamase in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langaee T. Y., Cagnon L., Huletsky A. 2000. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob. Agents Chemother. 44:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee M., et al. 2009. Bacterial AmpD at the crossroads of peptidoglycan recycling and manifestation of antibiotic resistance. J. Am. Chem. Soc. 131:8742–8743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leibovici L., et al. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379–386 [DOI] [PubMed] [Google Scholar]
- 22. Lindberg F., Westman L., Normark S. 1985. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc. Natl. Acad. Sci. U. S. A. 82:4620–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindberg F., Lindquist S., Normark S. 1987. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii β-lactamase. J. Bacteriol. 169:1923–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lister P. D., Wolter D. J., Hanson N. D. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livermore D. M. 1987. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur. J. Clin. Microbiol. 6:439–445 [DOI] [PubMed] [Google Scholar]
- 26. Livermore D. M. 1992. Interplay between impermeability and chromosomal β-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:2046–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livermore D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livermore D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634–640 [DOI] [PubMed] [Google Scholar]
- 29. Llarrull L. I., Testero S. A., Fisher J. F., Mobashery S. 2010. The future of β-lactam antibiotics. Curr. Opin. Microbiol. 13:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moya B., Juan C., Alberti S., Perez J. L., Oliver A. 2008. Benefit of having multiple ampD genes for acquiring β-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3694–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moya B., et al. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PloS Pathog. 5:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moya B., et al. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against β-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Normark S. 1995. β-Lactamase induction in Gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb. Drug Resist. 1:111–114 [DOI] [PubMed] [Google Scholar]
- 34. Obritsch M. D., Fish D. N., MacLaren R., Jung R. 2004. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 48:4606–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oh H., Stenhoff S., Jalal S., Wretlind B. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 8:323–328 [DOI] [PubMed] [Google Scholar]
- 36. Park J. T., Uehara T. 2008. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72:211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quale J., Bratu S., Gupta J., Landman D. 2006. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 50:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quénée L., Lamotte D., Polack B. 2005. Combined sacB-based negative selection and cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. Biotechniques 38:63–67 [DOI] [PubMed] [Google Scholar]
- 39. Stubbs K. A., Balcewich M., Mark B. L., Vocadlo D. J. 2007. Small molecule inhibitors of a glycoside hydrolase attenuate inducible AmpC-mediated β-lactam resistance. J. Biol. Chem. 282:21382–21391 [DOI] [PubMed] [Google Scholar]
- 40. Vöstch W., Templin M. F. 2000. Characterization of a β-N-acetylglucosaminidase of Escherichia coli and elucidation of its role in muropeptide recycling and β-lactamase induction. J. Biol. Chem. 275:39032–39038 [DOI] [PubMed] [Google Scholar]
- 41. West S. E., Schweizer H. P., Dall C., Sample A. K., Runyen-Janecky L. J. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81–86 [DOI] [PubMed] [Google Scholar]
- 42. Zamorano L., et al. 2010. NagZ inactivation prevents and reverts β-lactam resistance, driven by AmpD and PBP4 mutations, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3557–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y., et al. 2010. ampG gene of Pseudomonas aeruginosa and its role in β-lactamase expression. Antimicrob. Agents Chemother. 54:4472–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]