Abstract
Trioxaquine PA1259 is an efficient drug on larval- and adult-stage schistosomes, able to alkylate heme inside worms treated with it, leading to the formation of covalent heme-drug adducts. Such a mechanism, similar to one reported for other trioxaquines in Plasmodium, indicates that heme may be a common target of these trioxane-based drugs in different blood-feeding parasites.
INTRODUCTION
Schistosoma mansoni is a flatworm responsible for a chronic parasitic disease called schistosomiasis (or bilharziasis) (7). Vaccines are not yet available (10), and chemotherapy is the only way to control schistosomiasis. Chemotherapy consists of a single drug, praziquantel (PZQ). Praziquantel has been effectively used for about 40 years, and resistance to it is currently emerging (6, 11). Consequently, the development of new drugs is an urgent need for a highly neglected disease (8, 15, 18, 19).
Hemoglobin metabolism is a common feature of Schistosoma and Plasmodium. Host hemoglobin is ingested by schistosomes and degraded to amino acids in the ceca of the parasites. The free heme released by this metabolism is polymerized as hemozoin and regurgitated. Hemozoin is a disposal product generated by both Plasmodium and Schistosoma (14). Targeting the free heme of hematophagous parasites via an alkylation mechanism has been the rationale for the design of hybrid molecules named trioxaquines, containing a 1,2,4-trioxane linked to an aminoquinoline (2, 13, 17).
Since the activities of praziquantel and artemether have already been reported to be complementary on Schistosoma (20), our first attempt with hybrid antischistosomal molecules was based on a 1,2,4-trioxane linked to praziquantel. These compounds did not reach the expected level of activity (9). We then decided to evaluate a series of trioxaquines, molecules active against chloroquine-resistant Plasmodium falciparum strains, on Schistosoma mansoni (1, 5, 12, 13, 16, 17), and several of these molecules were highly active on both larval and mature stages of S. mansoni (2). On Plasmodium, trioxaquines exhibit a dual mode of action: (i) alkylation of heme via the trioxane entity, and (ii) stacking with heme via the aminoquinoline moiety, leading to the inhibition of hemozoin formation (1, 5, 12). As reported for artemisinin derivatives (16), trioxaquines are efficiently activated by heme, leading to the formation of covalent heme-drug adducts detected in mice infected with Plasmodium (malaria) (4).
For a better understanding of the molecular bases of the antischistosomal activity of trioxaquines, we investigated their reactivity toward heme and hemozoin in S. mansoni. Mature S. mansoni worms were treated with trioxaquine PA1259 (Fig. 1), and we report the characterization of heme-drug adducts generated inside the worms.
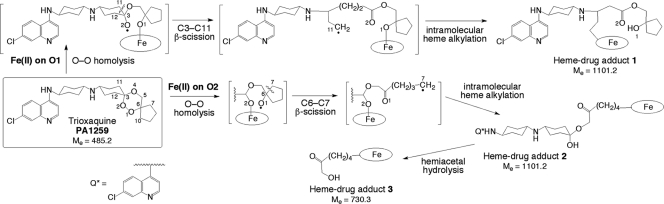
Fig. 1.
Reductive activation of trioxaquine PA1259 by iron(II)-heme, leading to the covalent heme-drug adducts 1, 2, and 3.
The host-parasite system used was an albino variety of Biomphalaria glabrata and a strain of Schistosoma mansoni, both from Brazil, maintained in Swiss OF1 mice (Charles River, France). Methods for mollusc and mouse infections and for parasite recovery were previously described (3). Mice were percutaneously infected using 120 cercariae and sacrificed 49 days after infection. Groups of 25 adult schistosomes, freshly recovered, were washed, placed in a 6-well Falcon plate containing 3 ml of RPMI 1640 medium (supplemented with l-glutamine and 25 mM HEPES), and stored at 37°C. Trioxaquine PA1259 (B. Meunier, F. Coslédan, and A. Pellet, 21 December 2007, patent application WO/2007/144487) was dissolved in dimethyl sulfoxide (DMSO) (100 mg/ml), diluted in RPMI 1640 medium complemented with 2.17% Tween 80, and added to the worm cultures (final PA1259 concentration, 50 μg/ml; final solvent ratio of 1,000/0.95/3.8 [vol/vol/vol] for RPMI–Tween 80–DMSO). After 3 h, all parasites were dead (no body contractions and no movement during 30 s), whereas control worms (treated with RPMI–Tween 80–DMSO [1,000/0.95/3.8, vol/vol/vol] but without drug) exhibited normal movements. The worms were washed with water, lyophilized, and crushed with sand. The powder obtained was extracted with pyridine (500 μl). The mixture was vigorously stirred for 5 min, submitted to ultrasound for 30 min, and magnetically stirred at 37°C overnight. The pyridine supernatant was withdrawn, filtered, and evaporated to dryness. The residue was dissolved in DMSO (60 μl) and diluted 5 times in a mixture of water-methanol-formic acid (10/90/1, vol/vol/vol).
The liquid chromatography-mass spectrometry (LC-MS) analyses were performed using an Agilent 6140 machine. The following equipment and conditions were used for LC-MS analyses: high-performance liquid chromatographic (HPLC) column, 5-μm C18 X-Bridge column (150 by 4.6 mm) (Waters); linear elution gradient from water-formic acid (100/1, vol/vol) to methanol-formic acid (100/1, vol/vol) in 30 min; flow rate, 1 ml min−1; injection volume of 100 μl; UV-visible light at 398 nm; and electrospray ionization (ESI+)-MS detection with scan range of 300 to 1,200 atomic mass units (amu). The analytic conditions were previously optimized by using chemically prepared heme-PA1259 adducts. Specifically, 3.2 mg of FeIII(PPIX)Cl, 10 mole equivalents of sodium dithionite, and 1.5 mole equivalents of trioxaquine PA1259 were dissolved in 500 μl DMSO. The reaction was carried out at 37°C, under an argon atmosphere, for 2 h.
The LC-MS analyses of extracts of S. mansoni worms treated with PA1259 are reported in Fig. 2a to c. Along with the ionic current of heme [retention time (Rt) = 26.1 min; m/z = 616.2; z = 1; M+ for FeIII(PPIX); Fig. 2a], several chromatographic peaks were detected at Rts of 24.7, 25.3, 26.3, and 26.5 min, with m/z = 551.3 and z = 2, having an exact mass value of 1,101.2 amu (Fig. 2b). This mass, corresponding to the mass of heme (616.2) plus the mass of PA1259 (485.2), can be assigned to covalent adducts between heme and PA1259. The structures and mechanisms of the formation of these adducts are depicted in Fig. 1. As for artemisinin, alkylation of heme by the drug can indeed occur on the four meso positions of the porphyrin macrocycle, giving rise to regioisomeric adducts. In addition, the inner-sphere reductive activation of the peroxide bond of PA1259 can occur with coordination of iron(II)-heme either on O-1 or O-2, giving rise to the formation of alkoxy radicals either on O-2 or O-1, respectively. Subsequent β-scission of the adjacent C-3—C-11 or C-6—C-7 bond, respectively, generated C-centered radicals able to alkylate heme and provide the covalent heme-drug adducts 1 and 2. The covalent adduct 3, resulting from the hydrolysis of the hemiacetal function of adduct 2, was also detected (Rt = 25.1 and 25.7 min; m/z = 730.3; z = 1; Fig. 2c). In addition, the isotopic patterns of signals at m/z 551.3 and 730.3 clearly showed that the corresponding adducts contained one iron atom (M-1 at 550.3 and M-2 at 728.2, respectively, due to 54Fe). In contrast, one chlorine atom was detected in adducts at m/z 551.3 (M + 1 at 552.3 due to 37Cl), whereas adduct 3 contained no chlorine after release of the 7′-chloro-4′-aminoquinoline moiety (Fig. 1). The heme-trioxaquine adducts 1, 2, and 3 were undetectable in all extracts of untreated S. mansoni worms. Because the worms treated with PA1259 were carefully washed before lyophilization, the detected adducts 1 to 3 were clearly contained inside the worms and cannot be considered external contamination.
Fig. 2.
(a to c) LC-MS analysis of the extract of Schistosoma mansoni treated with trioxaquine PA1259 (50 μg/ml). Extracted ionic current (EIC) traces for heme (heme, M+) (a), “complete” heme-PA1259 adducts 1 and/or 2 (MH+/2) (b), and heme-PA1259 adduct 3 (MH+) (c). (The insets at the right show the mass spectra of chromatographic peaks with arrows.)
These results confirm that trioxaquine PA1259 is able to alkylate heme inside Schistosoma and also strongly suggest that heme is a relevant target for antischistosomal trioxaquines.
Acknowledgments
This work was supported by Palumed, CNRS, and Agence Nationale pour la Recherche (grant ANR-08-MIEN-026-02). V.P. and J.P. are indebted to ANR for fellowships.
We thank Sonia Kitoune and Christine Salle (both from Palumed) and Bernard Dejean (from the UMR 5244) for technical assistance.
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Benoit-Vical F., et al. 2007. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob. Agents Chemother. 51:1463–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boissier J., Coslédan F., Robert A., Meunier B. 2009. Evaluation of the in vitro activity of trioxaquines against Schistosoma mansoni. Antimicrob. Agents Chemother. 53:4903–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boissier J., Mone H. 2000. Experimental observations on the sex ratio of adult Schistosoma mansoni, with comments on the natural male bias. Parasitology 121:379–383 [DOI] [PubMed] [Google Scholar]
- 4. Bousejra-El Garah F., Claparols C., Benoit-Vical F., Meunier B., Robert A. 2008. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob. Agents Chemother. 52:2966–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coslédan F., et al. 2008. Selection of a trioxaquine as a drug-candidate. Proc. Natl. Acad. Sci. U. S. A. 105:17579–17584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doenhoff M. J., Kusel J. R., Coles G. C., Cioli D. 2002. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans. R. Soc. Trop. Med. Hyg. 96:465–469 [DOI] [PubMed] [Google Scholar]
- 7. Gryseels B., Polman K., Clerinx J., Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118 [DOI] [PubMed] [Google Scholar]
- 8. Keiser J., et al. 2009. Mefloquine – an amino alcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurent S. A.-L., et al. 2008. Synthesis of trioxaquantel derivatives as potential new antischistosomal drugs. Eur. J. Org. Chem. 2008:895–913 [Google Scholar]
- 10. McManus D. P., Loukas A. 2008. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 21:225–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melman S. D., et al. 2009. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 3:e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meunier B. 2008. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 41:69–77 [DOI] [PubMed] [Google Scholar]
- 13. Meunier B., Robert A. 2010. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 43:1441–1451 [DOI] [PubMed] [Google Scholar]
- 14. Oliveira M. F., et al. 2005. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 579:6010–6016 [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro-dos-Santos G., Verjovski-Almeida S., Leite L. C. C. 2006. Schistosomiasis–a century searching for chemotherapeutic drugs. Parasitol. Res. 99:505–521 [DOI] [PubMed] [Google Scholar]
- 16. Robert A., Benoit-Vical F., Claparols C., Meunier B. 2005. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. U. S. A. 102:13676–13680 (Erratum, 103:3943, 2006.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robert A., Dechy-Cabaret O., Cazelles J., Meunier B. 2002. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc. Chem. Res. 35:167–174 [DOI] [PubMed] [Google Scholar]
- 18. Sayed A. A., et al. 2008. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 14:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao S.-H., et al. 2007. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 51:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao S.-H., et al. 2002. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop. 82:175–181 [DOI] [PubMed] [Google Scholar]