Abstract
Echinocandins have become a first-line therapy for invasive candidiasis (IC). Using phase 3 trial data for patients with IC, pharmacokinetic-pharmacodynamic (PK-PD) relationships for efficacy for micafungin were examined. Micafungin exposures were estimated using a population pharmacokinetic model, and univariable and multivariable logistic regressions were used to identify factors associated with outcome, including the micafungin area under the concentration-time curve (AUC)/MIC ratio. Monte Carlo simulation was used to evaluate the probability of achieving AUC/MIC ratios associated with efficacy. Mycological and clinical success rates for evaluable cases were 89.4 and 90.9, respectively. MIC50s and MIC90s for Candida species inhibition were 0.008 and 0.5 mg/liter, respectively. The median AUC/MIC ratio was 15,511 (range, 41.28 to 98,716). Univariable analyses revealed a significant relationship between the AUC/MIC ratio and mycological response, with the worst response being among patients with lower (≤3,000) AUC/MIC ratios (P = 0.005). For patients with Candida parapsilosis, AUC/MIC ratios of ≥285 were predictive of a higher mycological response (P = 0.11). Multivariable logistic regression demonstrated the AUC/MIC ratio, APACHE II score, and history of corticosteroid use to be significant independent predictors of a favorable response. PK-PD target attainment analyses suggested that 76.7% and 100% of patients would achieve an AUC/MIC ratio of ≥3,000 for an MIC of 0.03 mg/liter and an AUC/MIC ratio of ≥285 for an MIC of <0.5 mg/liter, respectively. The identification of a lower AUC/MIC ratio target for C. parapsilosis than other Candida species suggests consideration of species-specific echinocandin susceptibility breakpoints and values that are lower than those currently approved by regulatory agencies.
INTRODUCTION
Candidemia ranks as the third or fourth most common cause of hospital-acquired bloodstream infection and is associated with the highest mortality among infections caused by nosocomial pathogens (32, 33, 40). Numerous host and iatrogenic factors impact the outcome of this complex disease state. Two of the few variables under the control of the clinician are the drug choice and dosing regimen design of the antifungal therapy.
Anti-infective pharmacodynamic (PD) investigation is a critical tool for development of optimal dosing strategies and for establishment of definitions of clinically relevant drug resistance (2, 14). Pharmacokinetic (PK)-PD analyses of clinical data for anti-infective agents provide the opportunity to evaluate the relationships between efficacy and drug exposure relative to drug potency (MIC). Numerous studies have demonstrated the ability to characterize these relationships for antibacterial, antifungal, and antiviral drugs (2, 11, 15). The likelihood of detecting PK-PD relationships for efficacy is increased when individual PK data for patients with the disease process are collected, data from a sufficient number of patients are available, and variation in the drug susceptibility profile of the infecting pathogens is evident.
Advances in antifungal drug development over the past decade provide several treatment options for invasive candidiasis. Drugs from the echinocandin class represent the most recent addition and have become a first-line (12, 27, 44) option for this disease (32). One major advantage of these compounds is enhanced activity against the majority of emerging non-Candida albicans Candida species, especially Candida glabrata (36, 37, 38, 39). These agents are, however, not equipotent against all Candida species. Candida parapsilosis represents the species least susceptible to the action of the echinocandins, with in vitro susceptibility that is 1 to 2 orders of magnitude less than that of other common Candida pathogens (30, 31, 37, 39, 41, 42). Despite this in vitro potency difference, clinical trials have not identified species-specific differences in treatment outcome. In fact, analyses from these trials have failed to report a relationship between drug potency (MIC) and efficacy (22, 42). However, accumulating case reports have illustrated the potential for on-therapy resistance development (17, 18, 20, 22, 23, 25, 28, 35). Thus, it is clear that there are Candida infections for which echinocandin exposure associated with standard dosing regimens is insufficient for effective therapy. The exploration of PK-PD relationships for the efficacy of micafungin in patients with invasive candidiasis or candidemia using data from two phase 3 clinical trials (24, 34) and a previously developed population PK model (53) is described herein. Using Monte Carlo simulation, resulting PK-PD targets were used to evaluate potential susceptibility breakpoints for micafungin against Candida species.
MATERIALS AND METHODS
Data set.
Data were gathered from two randomized, controlled phase 3 clinical studies with patients with invasive candidiasis or candidemia (24, 34). Micafungin treatment in the trials consisted of regimens of 100 or 150 mg once daily for 14 to 56 days. Patients considered for the current analysis were those in the per protocol set, which included patients with a confirmed diagnosis of invasive candidiasis or candidemia who received micafungin for at least 5 days and who had a baseline Candida pathogen with a corresponding MIC value (n = 493). Given the age range for patients from whom data were collected for a previously conducted population PK analysis for micafungin (53), patients who were <13 years of age were excluded. Complete details of the design and conduct of each of these studies have been described elsewhere (24, 34). The analysis data set was assembled using SAS (version 9.1.3) software (48).
Estimation of drug exposure.
The PK-PD index of interest in this evaluation was the ratio of the area under the plasma micafungin (total drug) concentration-time curve over 24 h (AUC) to the MIC (AUC/MIC ratio). This PK-PD measure has been demonstrated to be descriptive of the efficacy of echinocandins, including micafungin, in animal model candidiasis studies (3, 6, 7, 8, 26, 54).
Among patients from whom PK data were collected, steady-state AUC/MIC ratios were based on individual predicted AUC values determined using a previously developed population PK model (53). A brief summary of the population PK model employed, which was developed in part using data from the patients described herein from whom PK data were collected and which was used for the determination of micafungin exposure, is provided below. In the case of patients from whom PK data were not collected, population mean predicted AUC values were determined using a covariate relationship between body weight and clearance (CL). A brief summary of the population PK model employed and the determination of micafungin exposure is provided below.
Using data pooled from four phase 2/3 clinical trials in which micafungin was evaluated in adult patients (n = 364) who received micafungin at doses ranging from 12.5 to 200 mg infused over approximately 1 h each day (or 300 mg over 1 h administered every other day) for up to 7 days, a two-compartment model with zero-order input and first-order elimination was found to best describe the PKs of micafungin. Samples for PK analysis were typically collected on day 1 and at steady state. The population PK analysis was performed using the first-order conditional estimation method with η-ε interaction in the NONMEM (version VI) program. Pharmacokinetic covariates were assessed using stepwise forward selection (α = 0.05) and backward elimination (α = 0.001) procedures. Population mean estimates of CL, the central and peripheral volumes of distribution (Vc and Vp, respectively), and distribution clearance (CLd) were 1.05 liters/h, 10.2 liters, 10.3 liters, and 6.59 liters/h, respectively. Alpha- and beta-phase half-lives were estimated to be approximately 0.5 and 14 h, respectively. Interpatient variability for CL, Vc, CLd, and Vp were modeled using an exponential model (assuming that these parameters are log-normally distributed) and were estimated to have coefficients of variation (CV) of 36.0, 28.3, 84.5, and 50.5%, respectively. Residual variability was estimated using a proportional error model and was estimated to have a CV of 19.3%. The covariate assessment revealed a statistically significant relationship between CL and body weight (P < 0.0001). Patients weighing at least 100 kg had CL values that were approximately 30% higher than the CL for the overall population, while patients weighing <45 kg had CL values that were approximately 30% lower than the mean CL for the overall population. No statistically significant relationships in micafungin PKs and any clinical laboratory parameter or disease-related characteristics were detected (53).
Using the Bayesian PK parameter estimates obtained from the above-described population PK model, individual predicted total micafungin concentrations were generated every 6 min (0.1 h) during the 24-hour dosing interval at steady state for each patient. The steady-state 24-hour AUC was calculated by the linear trapezoidal rule using the individual predicted total micafungin concentration-time data.
The relationship between body weight and CL described above was also used to generate a population mean predicted steady-state AUC value (i.e., including additional intersubject variability not described by body weight) for all patients using Equation 1.
| (1) |
where dose is in mg and weight is in kg.
The use of population mean predicted steady-state AUC values as a surrogate measure of Bayesian steady-state AUC values in the PK-PD analyses described below was evaluated for those patients from whom PK data were not collected. Using the previously described population PK analysis data set containing data for 364 patients (53), measures of bias and precision were used to assess the appropriateness of the population PK model and covariate relationship to predict steady-state AUC. This was accomplished by examining the distribution of the percent prediction errors (PEs) and |PEs|, respectively (49). The % PE was calculated as the population mean predicted AUC minus the individual predicted AUC multiplied by 100 and then divided by the individual predicted AUC, while |% PE| was calculated as the absolute value of % PE.
PK-PD analyses.
The dependent variables considered for univariable and multivariable PK-PD analyses for efficacy included the clinical and mycological responses at the end of treatment. The independent variables considered included the following: AUC/MIC ratio and MIC for baseline pathogens and patient demographics, such as age, body weight, APACHE II score, baseline Candida organism, and other available variables of clinical importance, including baseline neutropenia, history of corticosteroid use, history of a hematopoietic stem cell transplant, history of a solid organ transplant, renal failure, malignancy, HIV infection, diabetes, and presence of a central venous catheter at baseline. Definitions for these variables have been previously reported (24, 34). Since nonclinical findings suggest that PK-PD targets are variable for different Candida spp. (9), analyses were also conducted by species.
PK-PD analyses were performed using SYSTAT software, version 11.0 (Richmond, CA) (51), and R software, version 2.4.1 (45). Initial exploratory analyses for clinical and mycological responses were conducted to identify relationships between response and independent variables. For continuous independent variables such as AUC/MIC ratio and MIC, exploratory analyses included the examination of the relationship between a successful response and each dependent variable among patients categorized by increasing ranges of AUC/MIC ratio or MIC value (e.g., quartiles) and using classification and regression tree (CART) analysis to identify thresholds for values that distinguish cohorts with the most impressive differences in response with respect to minimized residual deviance. In addition, breakpoint pairs that split continuous independent variables into three groups were assessed to investigate potential nonlinearity, with an optimal pair chosen on the basis of that which achieved the greatest statistical significance in the likelihood ratio P value when logistic regression was used to compare three groups of at least 10 patients in each group.
Univariable analyses, which consisted of the chi-square or Fisher's exact test for categorical independent variables and logistic regression for continuous independent variables, were used to identify factors associated with clinical and mycological responses. Continuous independent variables were evaluated as such and also as categorical variables, as identified on the basis of exploratory analyses.
Multivariable logistic regression models were constructed with forward inclusion of independent variables (α = 0.1) for each dependent variable. If the degree of concordance between dependent variables was high, multivariable analyses were limited to the dependent variable manifesting the strongest univariable relationship with the AUC/MIC ratio. Models based on forward inclusion instead of those based on backward elimination were constructed, since parameter overfitting among starting and interim models was anticipated during the backward elimination process. In order to reduce overparameterization and construct parsimonious multivariable models that contained the number of independent variables recommended by Hosmer and Lemeshow (21), a stringent exclusion and inclusion criterion (α = 0.05) was used in the forward stepwise procedure. Candidate independent variables for model inclusion were chosen on the basis of significance of univariable associations with response (P ≤ 0.20). Separate sets of models, in which a given independent variable was considered continuously or categorically, were evaluated. In addition to the above-described considerations, discrimination among candidate final multivariable models was also based on clinical judgment, including the utility of certain independent variables.
Model-predicted probabilities of successful response relative to observed proportions of successful response were assessed for cohorts of patients described by the independent variables included in the final multivariable logistic regression models. Continuous independent variables were categorized using the univariable CART-derived breakpoints for the purpose of computing observed proportions of successful response for combinations of categories. Model-predicted probabilities within cohorts were averaged among the patients in each cohort.
Prediction of PK-PD target attainment.
Using AUC/MIC ratio targets derived from the PK-PD analyses, Monte Carlo simulation was conducted to evaluate the probability of PK-PD target attainment by fixed MIC values for simulated patients receiving micafungin at 100 mg every day. PK-PD target attainment analyses, which were conducted using a 10,000-patient population simulation, were performed using the R program, version 2.4.1 (45). Simulated patient body weights were selected from the observed weight distribution for all evaluable patients. Population mean predicted steady-state AUC was calculated using the covariate relationship between body weight and CL (53).
RESULTS
Data set.
The final data set for the per protocol population contained 493 evaluable patients with 515 Candida organisms isolated at baseline. The demographic and clinical characteristics of these patients are provided in Table 1.
Table 1.
Demographic and clinical characteristics of patients at baseline in the per protocol population
| Characteristic | % (no. with the characteristic/total no. tested) | Median (minimum, maximum) |
|---|---|---|
| Age (yr) | 55 (13, 89) | |
| Sex (% male) | 58.8 (290/493) | |
| Race category | ||
| Caucasian | 65.9 (325/393) | |
| Black | 12.2 (60/493) | |
| Other | 21.9 (108/493) | |
| Body wt (kg) | 68 (28, 155) | |
| Baseline APACHE II score | 14 (0, 38) | |
| Primary diagnosis disease category at baseline | ||
| None | 7.3 (36/493) | |
| Hematologic malignancy | 13.0 (64/493) | |
| Solid tumor | 5.9 (29/493) | |
| HIV | 1.6 (8/493) | |
| Solid organ transplant | 1.0 (5/493) | |
| Other | 71.2 (351/393) | |
| Presence of central catheter at baseline | 69.4 (342/393) | |
| Hemodialysis at baseline | 11.7 (35/300) | |
| History of corticosteroid use | 22.1 (109/493) | |
| History of hematopoietic stem cell transplant | 2.4 (12/493) | |
| History of solid organ transplant | 7.9 (39/493) | |
| Neutropenia at baseline | 10.1 (50/493) |
Species and MIC distribution.
MIC distributions by Candida species are shown in Table 2. When the MIC distribution for C. parapsilosis was compared to that for C. albicans, one can see that the MIC50 value was 7- to 8-fold higher in doubling dilution steps. The differences in the MIC distributions for C. parapsilosis and those for C. tropicalis and C. glabrata were similarly marked.
Table 2.
Distribution of MICs and AUC/MIC ratios by Candida species
| Candida species | No. of isolates | MIC distribution (mg/liter) |
AUC/MIC ratioa |
|||||
|---|---|---|---|---|---|---|---|---|
| 50% | 90% | Minimum | Maximum | Median | Minimum | Maximum | ||
| C. albicans | 218 | ≤0.004 | 0.008 | ≤0.002 | 0.016 | 24,306 | 5,851 | 98,716 |
| C. tropicalis | 99 | 0.008 | 0.016 | ≤0.002 | 1 | 11,962 | 150.4 | 50,954 |
| C. parapsilosis | 77 | 0.5 | 1.0 | 0.12 | 2.0 | 196.2 | 41.28 | 2,534 |
| C. glabrata | 62 | 0.008 | 0.016 | ≤0.002 | 0.016 | 12,495 | 4,917 | 38,476 |
| C. krusei | 17 | 0.12 | 0.125 | 0.03 | 0.25 | 1,165 | 564.8 | 2,896 |
| Other Candida species | 20 | 0.12 | 0.5 | <0.004 | 1.0 | 960.6 | 98.08 | 38,620 |
| Total | 493 | 0.008 | 0.5 | ≤0.002 | 2.0 | 15,511 | 41.28 | 98,716 |
AUC/MIC ratios were based on AUC values that were calculated using individual predicted steady-state AUC values for patients for whom PK data were collected and population mean predicted steady-state AUC values for patients for whom PK data were not collected.
Estimation of drug exposure.
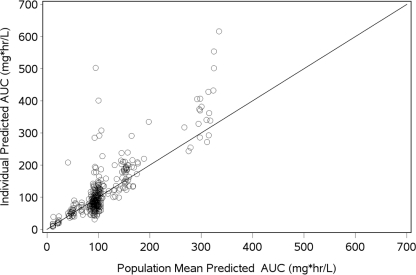
As shown by Fig. 1, there was good agreement between the individual and population mean predicted steady-state AUC values based on the data from the 364 patients in the population PK analysis data set; the coefficient of determination for this relationship was 0.71. Given the strength of the relationship between individual and population mean predicted values and reasonable measures of median bias (PE = −1.49%) and precision (|PE| = 18.1%), the use of population mean predicted steady-state AUC values for patients from whom no PK data were available was considered a reasonable approach for the PK-PD analyses.
Fig. 1.
Relationship between individual predicted and population-predicted micafungin AUC values. Median bias (PE) and precision (|PE| were −1.49% and 18.1%, respectively. Solid line, line of identity. Regression fit is individual AUC = −19.9 + 1.278 · population AUC (r2 = 0.710).
The distribution of micafungin AUC/MIC ratio by Candida species is shown in Table 2. Just as with the MIC distribution differences noted among Candida species, the median AUC/MIC ratio for C. parapsilosis was lower than the ratios for C. albicans, C. tropicalis, and C. glabrata.
PK-PD analyses.
Mycological and clinical success rates were 89.4% (439/491 patients) and 90.9% (447/492 patients), respectively.
Selected results for the univariable evaluation of factors associated with mycological response based on data for all evaluable patients with Candida species and the non-C. parapsilosis and C. parapsilosis populations are provided in Table 3. Univariable evaluation for factors associated with mycological response revealed a significant relationship between treatment response and AUC/MIC ratio when they were evaluated categorically as a three-level group variable (P = 0.005). The impact of MIC on mycological response also appeared to be important, with the univariable relationship nearly reaching statistical significance (P = 0.07). Despite the significant and borderline-significant univariable relationships observed for both AUC/MIC ratio and MIC and mycological response, respectively, the evaluation of AUC alone failed to reveal similar such relationships. However, as reflected by the P values for each of these variables, the association with mycological response was the strongest for the AUC/MIC ratio which considered MIC in the context of AUC.
Table 3.
Univariable evaluation for factors associated with mycological response based on data for all evaluable patients with Candida species and the non-C. parapsilosis and C. parapsilosis populations
| Patient population and independent variable | % (no. of patients with characteristic/total no. tested) by mycological response |
P value | |
|---|---|---|---|
| Success | Failure | ||
| All evaluable patients with Candida species | |||
| APACHE II score (continuous) | 0.06 | ||
| APACHE II score | 0.012 | ||
| <12 | 94.2 (162/172) | 5.8 (10/172) | |
| ≥12 | 86.5 (257/297) | 13.5 (40/297) | |
| AUC/MIC ratio | 0.005 | ||
| ≤3,000 | 85.1 (97/114) | 14.9 (17/114) | |
| >3,000 to 12,000 | 98.0 (98/100) | 2.0 (2/100) | |
| >12,000 | 88.1 (244/277) | 11.9 (33/277) | |
| MIC | 0.07 | ||
| <0.5 mg/liter | 90.5 (380/420) | 9.5 (40/420) | |
| ≥0.5 mg/liter | 83.1 (59/71) | 16.9 (12/71) | |
| History of corticosteroid use | 0.025 | ||
| Yes | 83.5 (91/109) | 16.5 (18/109) | |
| No | 91.1 (348/382) | 8.9 (34/382) | |
| Non-C. parapsilosis population | |||
| APACHE II score | 0.024 | ||
| <12 | 94.8 (128/135) | 5.2 (7/135) | |
| ≥12 | 87.4 (228/261) | 12.6 (33/261) | |
| AUC/MIC ratio | 0.007 | ||
| ≤5,000 | 86.7 (39/45) | 13.3 (6/45) | |
| >5,000 to 12,000 | 97.8 (90/92) | 2.2 (2/92) | |
| >12,000 | 88.1 (244/277) | 11.9 (33/277) | |
| History of corticosteroid use | 0.032 | ||
| Yes | 84.2 (80/95) | 15.8 (15/95) | |
| No | 91.8 (293/319) | 8.2 (26/319) | |
| C. parapsilosis population | |||
| Age (yr) | 0.002 | ||
| <52 | 100 (31/31) | 0 (0/31) | |
| ≥52 | 76.1 (35/46) | 23.9 (11/46) | |
| APACHE II score | 0.044 | ||
| <11 | 96.6 (28/29) | 3.4 (1/29) | |
| ≥11 | 79.5 (35/44) | 20.5 (9/44) | |
| AUC/MIC ratio | 0.11 | ||
| <285 | 82.0 (50/61) | 18 (11/61) | |
| ≥285 | 100 (16/16) | 0 (0/16) | |
The percentage of mycological success was higher for patients with AUC/MIC ratios of >3,000 to ≤12,000 than those with AUC/MIC ratios of ≤3,000 (98.0% versus 85.1%). However, patients with AUC/MIC ratios of >12,000 had a lower percentage of mycological success (88.1%). A similar relationship between AUC/MIC ratio and clinical response was evident (P = 0.09). The percentage of clinical success was also higher for patients with AUC/MIC ratios of >3,000 to ≤12,000 than for patients with AUC/MIC ratios of ≤3,000 (96.1% versus 91.2%). Just as with the mycological response, the percentage of clinical success was lower for patients with AUC/MIC ratios of >12,000 (88.8%). Given the concordance between clinical and mycological responses (see the information in the supplemental material) and the stronger PK-PD relationship for mycological response (P = 0.005), multivariable analyses were limited to this dependent variable. In the same population, the influence of MIC on mycological response using CART analysis demonstrated a breakpoint of 0.5 mg/liter. The percentage of mycological success was higher for patients with Candida species with MIC values of <0.5 mg/liter than for those with MIC values of ≥0.5 mg/liter (90.5% versus 83.1%, P = 0.07).
In addition to above-described univariable relationships for mycological response, significant relationships were also evident for APACHE II score and history of corticosteroid use. While the presence of a central venous catheter at baseline was expected to be an important predictor of response, reliable catheter management data were not available to assess for the presence of such a relationship. Results of univariable evaluation for the group with C. parapsilosis isolated at baseline also demonstrated the influence of the AUC/MIC ratio on mycological response. The percentage of mycological success was higher for patients with AUC/MIC ratios of ≥285 than those with AUC/MIC ratios of <285 (100% versus 82.0%, P = 0.11). Given the borderline significance of this relationship and the limited sample size of this group, multivariable analyses were not conducted. Results of univariable analysis excluding the C. parapsilosis group were similar to those for the entire Candida population (Table 3) and were subjected to multivariable analyses. The similarity in the thresholds for the AUC/MIC ratios defining subgroups of each of the two three-group variables suggested a lack of influence of data from patients with C. parapsilosis at baseline.
Table 4 shows factors associated with mycological response for three multivariable logistic regression models. Models 1 and 2 were based on data for all evaluable patients and model 3 was based on data for patients with non-C. parapsilosis infections. In addition to the AUC/MIC ratio three-group variable, two host factors were retained in each of the models presented. However, the AUC/MIC ratio categorized variable was the most predictive of mycological response for each model. As shown by model 2, when micafungin exposure (AUC) was excluded from the evaluation, MIC was retained in the model with the same independent variables as in model 1: APACHE II score and history of corticosteroid. The odds of mycological success were greater than 2-fold higher for patients with MIC values of <0.5 mg/liter than for patients with MIC values of ≥0.5 mg/liter. The statistical significance of all three independent variables in models 1 and 2 was similar.
Table 4.
Summary of multivariable logistic regression models for factors associated with mycological success
| Model description | Independent variable | Estimated | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Model 1, with AUC/MIC ratio evaluated as | APACHE II score ≤ 11a | 0.883 | 2.42 (1.16, 5.05) | 0.019 |
| an independent variable | AUC/MIC ratio ≤ 3,000b | −0.353 | 0.702 (0.362, 1.36) | 0.30 |
| 3,000 < AUC/MIC ratio ≤ 12,000b | 1.89 | 6.60 (1.54, 28.3) | 0.011 | |
| History of corticosteroid use | −0.720 | 0.487 (0.256, 0.925) | 0.028 | |
| Model 2, with MIC evaluated as an | APACHE II score ≤ 11a | 0.945 | 2.57 (1.23, 5.38) | 0.012 |
| independent variable | History of corticosteroid use | −0.686 | 0.504 (0.267, 0.951) | 0.034 |
| MIC < 0.5 mg/literc | 0.819 | 2.27 (1.07, 4.81) | 0.033 | |
| Model 3, non-C. parapsilosis population with | APACHE II score ≤ 11a | 0.853 | 2.35 (0.993, 5.54) | 0.052 |
| AUC/MIC ratio evaluated as an | AUC/MIC ratio < 5,000b | −0.210 | 0.811 (0.312, 2.11) | 0.67 |
| independent variable | 5,000 < AUC/MIC ratio ≤ 12,000b | 1.84 | 6.27 (1.46, 27.0) | 0.014 |
| History of corticosteroid use | −0.712 | 0.491 (0.241, 0.998) | 0.05 |
Reference group for APACHE II score is >11.
Reference group for AUC/MIC ratio is >12,000.
Reference group for MIC is ≥0.5 mg/liter.
The intercepts for the logistic regression function describing the multivariable models 1, 2, and 3 are 1.97, 1.37, and 1.97, respectively.
As with the multivariable logistic regression models based on data for all evaluable patients with Candida species, an AUC/MIC ratio three-group variable was found to be predictive of mycological response on the basis of data from patients with non-C. parapsilosis infection (model 3). In addition to AUC/MIC ratio, APACHE II score and history of corticosteroid use were retained in the model.
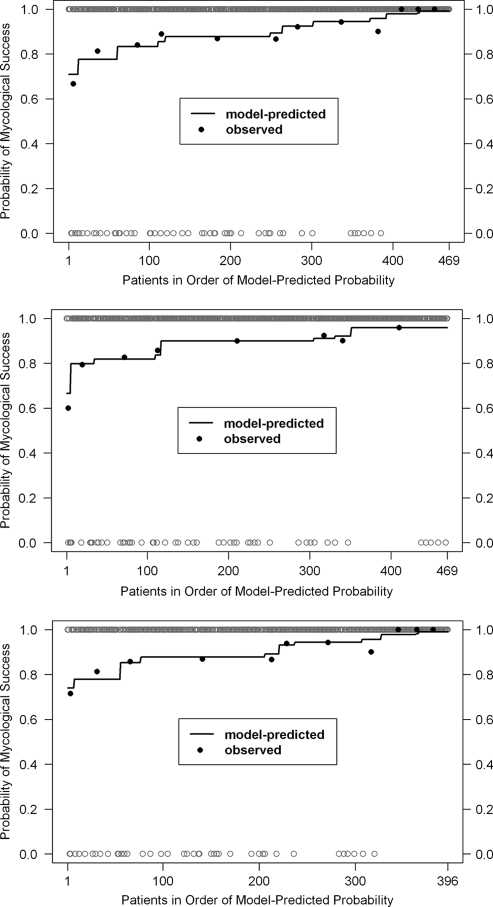
Figure 2 shows the assessment of model performance for the multivariable logistic regression models by comparing model-predicted probabilities and observed proportions of mycological success as a function of individual cohorts of patients described by the independent variables included in each of the models. As can be seen by this graphical comparison, the agreement between model-predicted probabilities and observed proportions of mycological success was strong, especially for cohorts of limited sample size, in which observed proportions have inherently large variability.
Fig. 2.
Assessment of multivariable logistic regression model performance for factors associated with mycological success. Model 1, top panel; model 2, middle panel; model 3, bottom panel.
Prediction of PK-PD target attainment.
The distributions for body weight and population mean predicted steady-state AUC in the evaluable and simulated patient populations based on the PK-PD target attainment analysis were comparable (see the information in the supplemental material). Table 5 shows the probability of PK-PD target attainment by MIC value for the AUC/MIC targets identified in the univariable PK-PD analyses for simulated patients receiving micafungin at 100 mg every day. PK-PD targets evaluated for all Candida species and non-C. parapsilosis were based on the lower threshold for the AUC/MIC ratio range associated with higher proportions for mycological success (the thresholds were 3,000 for all Candida species and 5,000 for Candida species excluding C. parapsilosis). In addition to these PK-PD targets, the probability of attaining an AUC/MIC ratio of 285, the CART-derived breakpoint identified for C. parapsilosis, was also evaluated. As shown in Table 5, the probability of PK-PD target attainment based on an AUC/MIC ratio target of 3,000 was 0.767 for simulated patients with isolates with MIC values of 0.03 mg/liter. For an AUC/MIC ratio target of 285, high probabilities of PK-PD target attainment were achieved for MIC values of <0.5 mg/liter.
Table 5.
Probability of PK-PD target attainment by MIC
| MIC (mg/liter) | Probability of AUC/MIC target of: |
||
|---|---|---|---|
| 285a | 3,000b | 5,000c | |
| ≤0.015 | 1 | 1 | 1 |
| 0.03 | 1 | 0.767 | 0 |
| 0.06 | 1 | 0 | 0 |
| 0.125 | 1 | 0 | 0 |
| 0.25 | 1 | 0 | 0 |
| 0.5 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
AUC/MIC target based on the univariable analysis patients with C. parapsilosis isolated at baseline.
AUC/MIC target based on the univariable analysis for patients with any Candida species isolated at baseline.
AUC/MIC target based on the univariable analysis for patients with non-C. parapsilosis isolated at baseline.
DISCUSSION
Candida species are the most common systemic fungal pathogens in humans and cause a disease spectrum ranging from mucosal to systemic infections. Invasive candidiasis has high attributable mortality, especially in immunosuppressed patients. The addition of the echinocandin class of antifungals marks a significant advance in therapy of these diseases, having enhanced safety and efficacy profiles compared to those of previously available antifungal therapies. However, as with the other available antifungal therapies, the echinocandins exhibit variable in vitro potency against different fungal species, and accumulating case reports have illustrated the potential for resistance development on therapy (17, 18, 20, 23). Thus, it is clear that there are Candida infections for which echinocandin exposure following standard dosing regimens is insufficient for effective therapy. Understanding the relationship between echinocandin exposure, measured by the AUC/MIC ratio, and response should aid in identification of susceptibility breakpoints that are more predictive of outcome and guide optimal antifungal treatment strategies.
The MIC distribution of the echinocandin class is relatively similar against most Candida species. The species for which in vitro potency is reduced is C. parapsilosis. Most isolates are 50- to 100-fold less susceptible to the echinocandins than other common Candida species. Nonetheless, clinical trials have demonstrated their effectiveness for management of both mucosal and systemic candidiasis caused by each of the common Candida species, including C. parapsilosis (24, 29, 34, 46). Furthermore, reports from these large trials have failed to demonstrate a relationship between in vitro susceptibility and treatment efficacy. However, the ability of individual trials to discern this relationship has been limited, likely due to the narrow range of MIC values for study isolates, very few isolates with higher MIC values, and frankly, the limited pharmacodynamic approaches considered in analysis.
Given the above-described limitations, consideration of additional data are required in order to be able to define susceptibility breakpoints for the echinocandins and Candida species. As advocated by the Clinical and Laboratory Standards Institute (CLSI), clinical outcome relative to MIC, the results of experimental and clinical PD analyses, and MIC distribution data should be considered parts of a “blueprint” for defining susceptibility breakpoints (42, 43, 47).
The evaluation of the clinical PDs of one echinocandin, micafungin, is the focus of the current report. The specific goal of the present study was to determine the relationship between micafungin exposure indexed to the MIC and response for patients with invasive candidiasis or candidemia. In addition, we explored these relationships in the context of other response modifiers of demonstrated importance for outcome in this disease state. While micafungin treatment outcome was influenced by MIC, the AUC/MIC ratio demonstrated a stronger observed association with mycological response. Interestingly, univariable analyses for mycological response evaluating MIC as an independent variable using data for patients with infections arising from all Candida species or the results of Monte Carlo simulations based on an AUC/MIC ratio target of 285 suggested that a MIC breakpoint of <0.5 μg/ml was predictive of response for the entire Candida population.
The AUC/MIC ratio category associated with reduced mycological efficacy in these clinical analyses for Candida species was a value of less than 3,000. Interestingly, this is very similar to the value identified for treatment effect in the neutropenic murine disseminated candidiasis model for C. albicans (8, 9). The same animal model PD studies also suggested that while the MIC was a relevant predictor of outcome, the amount of echinocandin relative to the MIC (AUC/MIC ratio) that was needed for treatment effect was not consistent among Candida species. The AUC/MIC ratio associated with efficacy was 7-fold lower for C. parapsilosis than C. albicans. This previously identified difference was in part the basis for exploration of the impact of Candida species, in addition to other host and disease variables, on the PK-PD relationships for efficacy in the current investigation. Among the factors evaluated, Candida species had a marked influence on mycological response in a manner similar to that found in the experimental models. The lower threshold of the range for the AUC/MIC ratio associated with efficacy among patients with any type of Candida species relative to that for patients with C. parapsilosis was 7.8-fold higher (3,000 versus 285).
The reason for these species differences described herein is not entirely clear. However, previous animal model and clinical epidemiologic studies have suggested that C. parapsilosis is a less fit or virulent species than other commonly encountered Candida species (10, 33, 52). In effect, the lower fitness or virulence of the C. parapsilosis group of organisms may be associated with a reduction in the treatment hurdle (9). Perhaps these observations should not be surprising, given the demonstrated relevance of species-specific susceptibility breakpoints for antibacterial agents. For example, the free fluoroquinolone AUC/MIC ratio associated with efficacy for Streptococcus pneumoniae is near 25, while that for Gram-negative bacilli is nearly 4-fold higher (2, 4, 5). The lower micafungin AUC/MIC ratio target for C. parapsilosis than for other Candida species in experimental studies (9) and on the basis of the current clinical analysis suggests consideration of species-specific echinocandin susceptibility breakpoints and lower values than are currently approved by CLSI (42).
In addition to the difference in the magnitude of PK-PD targets between C. parapsilosis and non-C. parapsilosis species, another interesting finding of the current analysis is the identification of an inverted U-shaped relationship between micafungin exposure and response. A region of the distribution for AUC/MIC ratio which was associated with optimal efficacy was identified, with exposures above or below this region being associated with reduced rates of response. This inverted U phenomenon has been observed in numerous antimicrobial PK-PD clinical analyses and points to the complexity of these disease processes (1). The basis for this phenomenon remains unclear. For the echinocandin drug class, one hypothesis of interest is a paradoxical effect (less drug activity for very high concentrations). While this phenomenon has been observed with some fungal strains in experimental models (50), most in vivo studies have not made this observation. Furthermore, a clinical description of this effect has not been reported. Thus, this explanation appears to be unlikely. We investigated differences in host, organism, and disease factors by AUC/MIC ratio range to explain this nonintuitive finding. As would be expected, there was a higher prevalence of Candida species with lower MIC values, most of which were C. albicans, in the higher AUC/MIC ratio group. Despite the differences in prevalence of Candida species by AUC/MIC ratio group, the Candida species group failed to have a significant impact on outcome and, thus, did provide a sufficient explanation for the phenomenon. Differences in response for other factors by AUC/MIC ratio group were not observed. Further understanding of this phenomenon will be an important goal for future studies.
A second objective of this analysis was to evaluate potential susceptibility breakpoints for micafungin against Candida species using Monte Carlo simulation and PK-PD targets resulting from the PK-PD analyses for efficacy described herein. Such an approach, which accounts for the variability in PKs and which uses the best available PK-PD data (nonclinical or, when available, clinical), is advocated by groups such as the CLSI (13) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (16) and provides a basis to develop susceptibility test interpretive criteria. The results of analyses based on these methods have been the identification of PK-PD MIC cutoff values (43, 47). As described previously, such cutoff values have been used together with clinical outcome statistics by MIC value and MIC population descriptive statistics to support decisions for susceptibility breakpoints for bacterial organisms.
The approved micafungin regimen for treatment of invasive candidiasis or candidemia is 100 mg/day. If one considered the lower threshold of the range for the AUC/MIC ratio associated with efficacy for the entire population (AUC/MIC ratio > 3,000), PK-PD target attainment analyses would suggest that the majority of patients would achieve this goal with organisms for which the MIC is <0.06 mg/liter. However, if one considered the lower AUC/MIC ratio target for efficacy identified for the C. parapsilosis cohort (AUC/MIC ratio > 285), the target would be expected to be attained for organisms with MIC values as high as 0.5 mg/liter. Both of these PK-PD MIC cutoff values fall below the current CLSI breakpoint of 2 mg/liter established for all Candida species. However, it is important to note that large susceptibility surveillance studies demonstrate that micafungin MIC values for more than 90% of strains are below the species-specific PK-PD MIC cutoff values identified in these analyses. In fact, only 10% of micafungin MIC values for the wild-type C. parapsilosis population would occur above the MIC value of 0.5 mg/liter (36). Despite the low prevalence of MIC values above each of the above-described PK-PD MIC cutoff values, the relationship between micafungin clearance and weight suggests that use of higher micafungin doses in heavier patients with Candida MIC values above the PK-PD MIC cutoffs would allow a higher probability of success. Further evaluation of potential micafungin dosing strategies in this patient population may be warranted.
As with other agents within the same mechanistic class, recent investigations of nonclinical data have shown that the PK-PD target is similar among the echinocandin agents (9). PK-PD analyses for efficacy for each of the echinocandins based on clinical data would be of great value in demonstrating the applicability of nonclinical PK-PD targets. However, in the absence of such data and using the above-described clinical AUC/MIC ratio targets of 3,000 and 285 for micafungin adjusted for protein binding of 99.75% (19, 55), free-drug AUC/MIC ratio targets of approximately 7.5 and 0.7 would be derived. When one assumes protein binding estimates of 99 and 97%, respectively, for currently approved dosing regimens for anidulafungin (100 mg/day) and caspofungin (50 mg/day) for the treatment of Candida infections (27, 44) and using average 24-hour AUC values derived from healthy volunteers (112 and 98 mg · h/ml, respectively), free-drug AUC values would be 1.12 and 2.94 mg · h/ml, respectively. Without accounting for PK variability and using the free-drug AUC/MIC ratio target for C. albicans identified in the current clinical analysis (7.5), PK-PD MIC cutoff values of 0.12 and 0.25 mg/liter, respectively, are derived. The PK-PD MIC cutoffs for C. parapsilosis would be 1.0 and 2.0 mg/liter, respectively, assuming a free-drug AUC/MIC ratio target of 0.7. Thus, the PK-PD MIC cutoffs appear to be relatively similar for drugs within the class, as would be expected on the basis of the PK-PD targets identified using experimental models (9). Since species-specific differences are likely relevant for all drugs within the echinocandin class, reassessment of the current CLSI breakpoint for this class against Candida species is needed.
In summary, these studies demonstrate a relationship between echinocandin exposures (AUC), MIC, and efficacy. More specifically, the results identify the clinical PD target required for micafungin efficacy for invasive candidiasis or candidemia and suggest differences in the AUC/MIC ratio target among Candida species. Results of exploration of the mechanistic basis for the differences in the PK-PD targets for efficacy for echinocandins against Candida species may have implications for treatment with other antifungal drugs.
Supplementary Material
ACKNOWLEDGMENT
This research was funded by a grant from Astellas.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Ambrose P. G. 2010. PK-PD in the development of antimicrobial agents for resistant pathogens: it's about the magnitude, shape and duration of drug exposure. In Proceedings from Issues in Antibacterial Resistance and Device and Drug Development Food and Drug Administration, National Institute of Allergy and Infectious Diseases, and Infectious Diseases Society of America, Washington, DC: http://www.fda.gov/Drugs/NewsEvents/ucm211146.htm [Google Scholar]
- 2. Ambrose P. G., et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 3. Andes D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andes D., Craig W. A. 2003. Pharmacodynamics of the new des-f(6)-quinolone garenoxacin in a murine thigh infection model. Antimicrob. Agents Chemother. 47:3935–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andes D., Craig W. A. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andes D., et al. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andes D., et al. 2003. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andes D. R., Diekema D. J., Pfaller M. A., Marchillo K., Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:3497–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andes D., et al. 2010. In vivo comparison of the pharmacodynamic target among echinocandin drugs and Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arendrup M., Horn T., Frimodt-Møller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286–291 [DOI] [PubMed] [Google Scholar]
- 11. Baddley J. W., Patel M., Bhavnani S. M., Moser S. A., Andes D. R. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 52:3022–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chandrasekar P. H., Sobel J. D. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 42:1171–1178 [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute. 2008. Development of in vitro susceptibility testing criteria and quality control parameters, 3rd ed. Approved guideline M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Craig W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 15. Drusano G. L. 2007. Pharmacodynamics of antivirals. In Nightingale C. H., Ambrose P. G., Drusano G. L., Murakawa T. (ed.), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed. Informa Healthcare, New York, NY [Google Scholar]
- 16. European Committee on Antimicrobial Susceptibility Testing. 2010. European Society of Clinical Microbiology and Infectious Diseases. http://www.eucast.org Accessed 18 June 2010
- 17. Garcia-Effron G., Kontoyiannis D. P., Lewis R. E., Perlin D. S. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hakki M., Staab J. F., Marr K. A. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hebert M. F., et al. 2005. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J. Clin. Pharmacol. 45:1145–1152 [DOI] [PubMed] [Google Scholar]
- 20. Hernandez S., et al. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosmer D. W., Lemeshow S. 1989. Applied logistic regression. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 22. Kartsonis N., et al. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogh-Madsen M., Arendrup M. C., Heslet L., Knudsen J. D. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 24. Kuse E. R., et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 25. Laverdière M., et al. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705–708 [DOI] [PubMed] [Google Scholar]
- 26. Louie A., et al. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merck & Co., Inc. 2009. Cancidas (caspofungin acetate) for injection, for intravenous use. Package insert. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 28. Miller C. D., Lomaestro B. W., Park S., Perlin D. S. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877–880 [DOI] [PubMed] [Google Scholar]
- 29. Mora-Duarte J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 30. Ostrosky-Zeichner L., et al. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paderu P., et al. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pappas P. G., et al. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634–643 [DOI] [PubMed] [Google Scholar]
- 34. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 35. Park S., et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfaller M. A., et al. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfaller M. A., et al. 2006. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 44:3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfaller M. A., et al. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaller M. A., et al. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfaller M. A., Diekema D. J. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pfaller M. A., et al. 2008. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 46:842–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfaller M. A., et al. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pfaller M. A., et al. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pfizer, Inc. June 2009. Eraxis™ (anidulafungin) for injection. Package insert. Pfizer, Inc., New York, NY [Google Scholar]
- 45. R Development Core Team. 2006. R: a language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna, Austria: http://www.r-project.org/ Accessed 18 June 2010 [Google Scholar]
- 46. Reboli A. C., et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472–2482 [DOI] [PubMed] [Google Scholar]
- 47. Rex J. H., et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:235–247 [DOI] [PubMed] [Google Scholar]
- 48. SAS. 2002. 9.1.3 service pack 3 for Windows. SAS, Inc., Cary, NC [Google Scholar]
- 49. Scheiner L. B., Beal S. L. 1981. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 9:503–512 [DOI] [PubMed] [Google Scholar]
- 50. Stevens D. A., Ichinomiya M., Koshi Y., Horiuchi H. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. SYSTAT Software, Inc. 2004. SYSTAT® 11. SYSTAT Software, Inc., Richmond, CA [Google Scholar]
- 52. van Asbeck E. C., Clemons K. V., Stevens D. A. 2009. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 35:283–309 [DOI] [PubMed] [Google Scholar]
- 53. Van Wart S. A., et al. 2008. Population pharmacokinetics of micafungin in patients with invasive candidiasis, candidemia and esophageal candidiasis, abstr. A-011. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 54. Wiederhold N. P., et al. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464–1471 [DOI] [PubMed] [Google Scholar]
- 55. Yamato Y., et al. 2002. Pharmacokinetics of the antifungal drug micafungin in mice, rats, and dogs and in its in vitro protein binding and distribution to blood cells. Jpn. J. Chemother. 50:74–79 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.