Abstract
Echinocandins are highly bound to serum proteins, altering their antifungal properties. The addition of 50% human serum to the MIC assay improves the identification of echinocandin-resistant Candida spp. harboring fks hot spot mutations. However, this modification cannot readily be applied to the method of the CLSI M27-A3 document due to safety and standardization difficulties. The aim of this study was to evaluate commercial bovine serum albumin (BSA) as a safe and standardized alternative to human serum. A collection of 28 echinocandin-susceptible strains, 10 Candida parapsilosis sensu lato strains (with naturally reduced echinocandin susceptibility), and 40 FKS hot spot mutants was used in this work. When RPMI 1640 was used for susceptibility testing, wild-type strains and fks mutants showed MIC range overlaps (−2, −1, and −3 2-fold-dilution steps separated these populations for anidulafungin, caspofungin, and micafungin, respectively). On the other hand, the addition of BSA to RPMI 1640 differentially increased echinocandin MIC values for these groups of strains, allowing better separation between populations, with no MIC range overlaps for any of the echinocandin drugs tested. Moreover, the use of RPMI-BSA reduced the number of fks hot spot mutant isolates for which MIC values were less than or equal to the upper limit for the wild type (very major errors) from 9, 2, and 7 with RPMI alone to 3, 0, and 3 for anidulafungin, caspofungin, and micafungin, respectively. When RPMI-BSA was used to study the susceptibility of C. parapsilosis sensu lato species to echinocandins, the strains behaved as anidulafungin- and micafungin-resistant isolates (MIC, ≥8 μg/ml). These data support the need for a revision of the CLSI protocol for in vitro testing of echinocandin susceptibility in order to identify all or most of the fks hot spot mutants. Also, caspofungin could be used as a surrogate marker of reduced susceptibility to echinocandins.
INTRODUCTION
Echinocandin drugs inhibit the 1,3-β-d-glucan synthase complex (EC 2.4.1.34) (GS), which catalyzes the biosynthesis of 1,3-β-d-glucan, the major glucan component of fungal cell walls. GS is an enzyme complex with at least two subunits, Fksp and Rho1p. Fksp, encoded by three related genes, FKS1, FKS2, and FKS3, is the catalytic subunit, and it is the target of the echinocandin drugs. Echinocandin resistance resulting in clinical failure has been linked to dominant mutations in the Fksp subunit of GS (27). These amino acid substitutions have been mapped onto two conserved regions of Fks1p (Candida spp.) and Fks2p (in Candida glabrata only) (13–16, 19, 27) named hot spot regions (e.g., C. albicans Fks1p hot spot 1 [Phe-641 to Pro-649] and hot spot 2 [Asp-1357 to Leu-1364]) (26). Recently, the CLSI Antifungal Subcommittee established a MIC of ≤2 μg/ml as an interpretative MIC breakpoint for the susceptibility of Candida spp. to the three echinocandin drugs (7, 33). However, it has been demonstrated that the CLSI breakpoints are not able to distinguish all the echinocandin-resistant fks mutants from wild-type (WT) isolates (2, 15, 16), which led us to propose new interpretative MIC breakpoints for the echinocandins against Candida species (for echinocandins against C. albicans, C. tropicalis, and C. krusei, ≤0.25 μg/ml for susceptibility, 0.5 μg/ml for intermediacy, and ≥1.00 μg/ml for resistance; for anidulafungin [ANF] and caspofungin [CSF] against C. glabrata, ≤0.25 μg/ml for susceptibility, 0.50 μg/ml for intermediacy, and ≥1.00 μg/ml for resistance; for micafungin [MCF] against C. glabrata, ≤0.06 μg/ml for susceptibility, 0.12 μg/ml for intermediacy, and ≥0.25 μg/ml for resistance; and for echinocandins against C. parapsilosis and C. guilliermondii, ≤2.00 μg/ml for susceptibility, 4.00 μg/ml for intermediacy, and ≥8.00 μg/ml for resistance) (27). In order to improve the detection of fks hot spot mutants with the CLSI susceptibility breakpoint, different options have been proposed: (i) reduce the ANF and MCF susceptibility breakpoints for C. albicans and C. glabrata (15, 16), (ii) use CSF as a surrogate marker for echinocandin cross-resistance and as the agent for the detection of fks hot spot mutations (5, 15, 16), (iii) identify fks hot spot mutants by testing for susceptibility to ANF with a susceptibility breakpoint defined as two 2-fold dilutions higher than the MIC50 (MIC at which 50% of isolates are inhibited) for the wild-type population of each Candida species (2), or (iv) add 50% human serum to the MIC assay medium in order to improve the identification of echinocandin-resistant Candida spp. harboring FKS mutations (15, 16). The last proposal more closely simulates the physiochemical properties of the active drug following intravenous administration, but it is difficult to apply in a clinical assay due to safety and standardization difficulties associated with human serum. The aim of this study was to overcome this issue by evaluating commercial fatty-acid-free bovine serum albumin (BSA) as a safe and standardized alternative to human serum for better discrimination between susceptible strains and resistant clinical isolates harboring fks mutations.
MATERIALS AND METHODS
Organisms.
A collection of 78 Candida sp. strains was used in this study, including: 28 echinocandin-susceptible strains (14 C. albicans, 6 C. glabrata, 4 C. tropicalis, and 4 C. krusei strains), 10 isolates showing a naturally occurring amino acid substitution in Fks1p linked with reduced echinocandin susceptibility (RES) (13) (6 C. parapsilosis, 2 C. orthopsilosis, and 2 C. metapsilosis isolates), and 40 FKS hot spot mutants isolated after or during echinocandin therapy (20 C. albicans, 14 C. glabrata, 4 C. tropicalis, and 2 C. krusei isolates). The echinocandin-susceptible and RES strains used in this work were control strains (n = 7) (C. albicans ATCC 90028, ATCC 36082, and SC5314; C. glabrata ATCC 90030; C. tropicalis ATCC 750; C. krusei ATCC 6258; and C. parapsilosis ATCC 22019) or clinical isolates used previously by our group (n = 21) (2, 6, 12–16). The FKS hot spot mutants included in this work have been studied in several previous reports (2, 6, 13–16, 35, 41).
The isogenicity of the C. albicans and C. glabrata strains was determined using the multilocus sequence typing (MLST) methods described by Robles et al. (37), Tavanti et al. (39), and Dodgson et al. (10).
FKS gene sequence analysis.
Candida sp. genomic DNA was extracted from yeast cells grown overnight in YPD (2% yeast extract, 4% Bacto peptone, 4% dextrose) broth with a Qbiogene (Irvine, CA) FastDNA kit. PCR and sequencing primers were designed based on the sequences of the following genes: C. albicans FKS1 (GenBank accession no. XM_716336), C. glabrata FKS1 and FKS2 (GenBank accession no. XM_446406 and XM_448401, respectively), C. krusei FKS1 (accession no. EF426563), C. metapsilosis FKS1 (accession no. EU350514), C. orthopsilosis FKS1 (accession no. EU350513), C. parapsilosis FKS1 (accession no. EU221325), and C. tropicalis FKS1 (accession no. EU676168). Primer sequences are listed in Table S1 in the supplemental material. DNA sequencing was performed with a CEQ Dye Terminator Cycle Sequencing Quick Start kit (Beckman Coulter, Fullerton, CA) according to the manufacturer's recommendations. Sequence analysis was performed with CEQ 8000 Genetic Analysis system software (Beckman Coulter, Fullerton, CA) and the BioEdit sequence alignment editor (Ibis Therapeutics, Carlsbad, CA).
Echinocandin susceptibility testing and compounds.
Echinocandin susceptibility testing was performed in triplicate using the broth microdilution method of CLSI document M27-A3 (7) with or without 50% human serum (from human male blood, type AB; Sigma-Aldrich) or 50 mg/ml BSA. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as control strains for antifungal susceptibility testing. The drugs used were CSF (Merck & Co. Inc., Rahway, NJ), ANF (Pfizer, New York, NY), and MCF (Astellas Pharma USA, Inc., Deerfield, IL). The drugs were obtained as standard powders from their manufacturers. CSF and MCF were dissolved in sterile distilled water, and ANF was dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich). Stock solutions of each drug were kept at −86°C.
Standardization of RPMI 1640 plus BSA.
In order to establish the BSA concentration needed to mimic the effect of 50% serum on echinocandin MIC values, MIC testing was carried out according to the method of the CLSI M27-A3 document (7) with 50% (wt/vol) serum or different BSA concentrations (from 2.5 to 100 mg/ml). Once the BSA concentration was established, the effects of three fatty-acid-free BSA preparations obtained from three different companies (Affymetrix/USB, Sigma Aldrich, and Equitech Bio, Inc.) on echinocandin MIC values were assessed. Thirteen strains were used in the standardization experiments, including 5 echinocandin-susceptible strains (2 C. albicans, 1 C. glabrata, 1 C. krusei, and 1 C. tropicalis strain), 3 isolates showing a naturally occurring amino acid substitution in Fks1p with a RES phenotype (1 C. parapsilosis, 1 C. metapsilosis, and 1 C. orthopsilosis strain), and 5 echinocandin-resistant strains (1 C. albicans fks1-S645P, 1 C. glabrata fks1-S629P, 1 C. glabrata fks2-S663P, 1 C. krusei fks1-F655F/C, and 1 C. tropicalis fks1-F76S strain), representing all the species used in this study.
Isolation of the 1,3-β-d-glucan synthase complex, characterization of the 1,3-β-d-glucan product, and assay.
Eight 1,3-β-d-glucan synthase complexes were isolated from one strain each of the Candida species included in this study. Cell growth and disruption, membrane protein extraction, partial GS purification by product entrapment, and polymerization assays were performed as described previously (15, 16). The product of the reaction mixtures was characterized as 1,3-β-d-glucan using the endpoint assay Glucatell kit (13).
1,3-β-d-Glucan synthase complex inhibition curves.
Inhibition curves and 50% inhibitory concentrations (IC50s) were determined using a sigmoidal response (variable-slope) curve and a two-site competition fitting algorithm with GraphPad Prism software, version 4.0 (13). The IC50s were obtained with and without 50% serum or 50 mg/ml BSA.
Definitions.
The isolates were classified into three groups depending on the FKS hot spot sequence. The Candida strains included in the wild-type (WT) group harbor the same FKS hot spot sequence as that described in the GenBank accession number for each of the Candida species. The species showing an intrinsic RES phenotype (13) were included in the IRES group. The echinocandin-resistant (ER) group contained characteristic fks hot spot mutants isolated during or after echinocandin therapy.
To evaluate the abilities of the different medium supplements to identify fks hot spot mutants, the definitions given by Arendrup et al. (2) were utilized. Briefly, the following terms were used: (i) distance between endpoint ranges, defined as the number of 2-fold dilution steps between the MIC values of each pair of groups of strains (negative values represent overlaps); (ii) overlap, defined as the number of end points for one of the isolate groups that overlapped with the endpoint range of another group of isolates; (iii) wild-type upper-limit (WT-UL) values, defined as two 2-fold dilution steps higher than the MIC50 for the WT group; (iv) very major errors (VME), defined as fks hot spot mutant isolates with MIC values less than or equal to the WT-UL; (v) major errors (ME), defined as WT isolates with MIC values above the WT-UL; (vi) MIC50 shift, defined as the number of 2-fold dilution steps by which the MIC50 for a particular group changed when a test condition was modified (from RPMI 1640 alone to RPMI-serum or RPMI-BSA).
Data analysis.
MIC and IC50 data are the results of experiments performed in triplicate and on three separate days. Geometric means were used to compare MIC results statistically. Arithmetic means and standard deviations were used to analyze IC50s (continuous variables) statistically. The significance levels of MIC differences were determined by Student's t test (unpaired, unequal variance); a P value of 0.01 was considered significant. In order to approximate a normal distribution, the MICs were transformed to log2 values for the establishment of susceptibility differences between strains. Both on-scale and off-scale results were included in the analysis. The off-scale MICs were converted to the next concentration up or down. Statistical analyses were performed with the Statistical Package for the Social Sciences software (version 13.0) (SPSS Inc., Chicago, IL).
RESULTS
BSA modifies echinocandin MICs and IC50s, mimicking 50% serum.
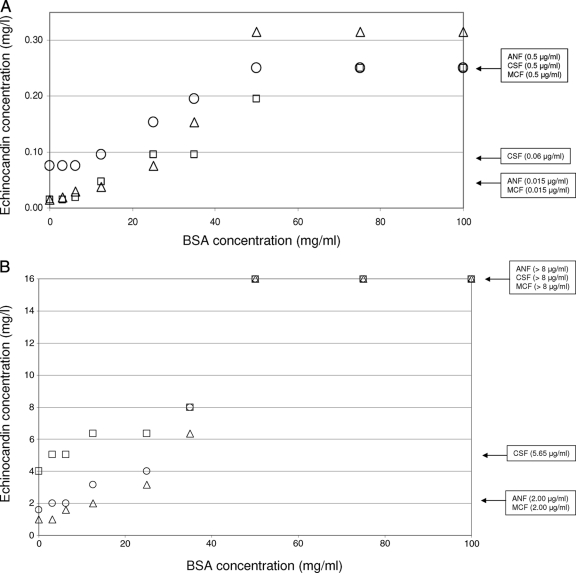
The antifungal efficacies of all the echinocandin drugs were significantly reduced in the presence of 50% human serum (P < 0.001). However, the effects of serum on the MIC50 and MIC90 were more pronounced for ANF and MCF than for CSF (Table 1). Similarly, the presence of BSA decreased the in vitro potencies of echinocandin drugs in a concentration-dependent manner, starting with 10 mg/ml BSA and saturating at 50 mg/ml. This effect was observed for the 13 strains (5 WT, 3 IRES, and 5 RES isolates) used in the standardization experiments and for all the echinocandin drugs used. (Examples of the effects of BSA on echinocandin MIC values for the C. albicans WT strain SC5314 and the C. albicans ER strain 205 are displayed in Fig. 1.) The effect of BSA at 50 mg/ml on echinocandin MIC values mimicked the behavior of 50% human serum (higher MIC values). For these reasons, 50 mg/ml of BSA was chosen as the BSA concentration to be used in this study.
Table 1.
Effects of 50% serum and 50 mg/ml of BSA on echinocandin MIC distributions among Candida spp.a
| Species | Groupb (no. of isolates) | Antifungal | MIC (μg/ml)c with the following additive: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None |
50% Serum |
50 mg/ml BSA |
|||||||||
| GM | 50% | 90% | GM | 50% | 90% | GM | 50% | 90% | |||
| C. albicans | WT (14) | ANF | 0.02 | 0.015 | 0.03 | 0.16 | 0.12 | 0.25 | 0.37 | 0.25 | 0.50 |
| CSF | 0.04 | 0.06 | 0.12 | 0.16 | 0.12 | 0.25 | 0.17 | 0.12 | 0.25 | ||
| MCF | 0.03 | 0.03 | 0.06 | 0.21 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | ||
| ER (20) | ANF | 0.53 | 0.50 | 2.00 | 5.86 | 2.00 | >8.00 | 9.85 | >8.00 | >8.00 | |
| CSF | 2.83 | 2.00 | 8.00 | 5.10 | 4.00 | >8.00 | 5.28 | 4.00 | >8.00 | ||
| MCF | 0.42 | 0.50 | 2.00 | 5.46 | 4.00 | >8.00 | 6.50 | 4.00 | >8.00 | ||
| C. glabrata | WT (6)d | ANF | 0.02 | 0.015 | 0.06 | 0.25 | 0.25 | 0.50 | 0.50 | 0.50 | 0.50 |
| CSF | 0.05 | 0.06 | 0.12 | 0.50 | 0.50 | 1.00 | 0.03 | 0.50 | 1.00 | ||
| MCF | 0.03 | 0.03 | 0.03 | 0.28 | 0.25 | 0.50 | 0.63 | 0.50 | 0.50 | ||
| ER (14) | ANF | 0.52 | 0.50 | 8.00 | NDA | NDA | NDA | 12.93 | >8.00 | >8.00 | |
| CSF | 4.00 | 4.00 | >8.00 | NDA | NDA | NDA | 14.38 | >8.00 | >8.00 | ||
| MCF | 1.80 | 2.00 | >8.00 | NDA | NDA | NDA | 15.17 | >8.00 | >8.00 | ||
| C. tropicalis | WT (4) | ANF | 0.02 | 0.015 | 0.03 | 0.42 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| CSF | 0.07 | 0.06 | 0.12 | 0.17 | 0.12 | 0.25 | 0.21 | 0.25 | 0.25 | ||
| MCF | 0.03 | 0.015 | 0.06 | 0.29 | 0.25 | 0.50 | 0.42 | 0.50 | 0.50 | ||
| ER (4) | ANF | 0.35 | 0.25 | 0.50 | 11.31 | >8.00 | >8.00 | 16.00 | >8.00 | >8.00 | |
| CSF | 1.19 | 2.00 | 2.00 | 9.51 | 8.00 | 8.00 | 9.51 | >8.00 | >8.00 | ||
| MCF | 0.12 | 0.12 | 0.25 | 11.31 | >8.00 | >8.00 | 11.31 | >8.00 | >8.00 | ||
| C. krusei | WT (4) | ANF | 0.07 | 0.06 | 0.12 | 0.30 | 0.25 | 0.50 | 0.35 | 0.25 | 0.50 |
| CSF | 0.14 | 0.12 | 0.25 | 0.84 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| MCF | 0.05 | 0.06 | 0.06 | 0.84 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| ER (2) | ANF | 1.41 | ND | ND | 11.31 | ND | ND | 16.00 | ND | ND | |
| CSF | 2.83 | ND | ND | 8.00 | ND | ND | 8.00 | ND | ND | ||
| MCF | 0.69 | ND | ND | 8.00 | ND | ND | 11.31 | ND | ND | ||
| C. parapsilosis | IRES (6) | ANF | 0.89 | 0.50 | 8.00 | 8.00 | 8.00 | >8.00 | 16.00 | >8.00 | >8.00 |
| CSF | 0.44 | 0.25 | 4.00 | 2.50 | 2.00 | >8.00 | 4.00 | 2.00 | >8.00 | ||
| MCF | 1.41 | 1.00 | 4.00 | 14.25 | >8.00 | >8.00 | 16.00 | >8.00 | >8.00 | ||
| C. orthopsilosis | IRES (2) | ANF | 0.25 | ND | ND | 5.66 | ND | ND | 16.00 | ND | ND |
| CSF | 0.17 | ND | ND | 0.50 | ND | ND | 1.41 | ND | ND | ||
| MCF | 0.25 | ND | ND | 16.00 | ND | ND | 16.00 | ND | ND | ||
| C. metapsilosis | IRES (2) | ANF | 0.50 | ND | ND | 2.83 | ND | ND | 11.31 | ND | ND |
| CSF | 0.25 | ND | ND | 0.22 | ND | ND | 1.00 | ND | ND | ||
| MCF | 0.35 | ND | ND | 2.83 | ND | ND | 5.66 | ND | ND | ||
Susceptibility testing was carried out according to the guidelines of the CLSI M27A3 document.
Classification depends on the FKS hot spot sequence. WT isolates have the same FKS hot spot sequence as that of the GenBank accession number for the species given in Materials and Methods. IRES isolates show a phenotype of intrinsically reduced echinocandin susceptibility. ER strains show FKS hot spot mutations and were isolated during or after echinocandin therapy.
GM, geometric mean; 50% and 90%, MICs at which 50% and 90% of isolates, respectively, were inhibited; NDA, no data available (13 out of 14 C. glabrata ER strains did not grow due to the addition of 50% serum to RPMI 1640 medium); ND, not done (low number of strains).
There were 5 isolates when 50% serum was added.
Fig. 1.
Effects of different BSA concentrations on echinocandin MIC values for the C. albicans WT strain SC5314 (A) and the C. albicans ER strain 205 (Fks1p-S645P) (B). Squares, circles, and triangles represent the MIC values of ANF, CSF, and MCF, respectively (geometric means from three repetitions), obtained with BSA concentrations of 3.12, 6.25, 12.5, 25, 35, 50, 75, and 100 mg/ml. The boxes at far right indicate the echinocandin MIC values obtained with the addition of 50% serum (0.5 μg/ml and >8.00 μg/ml for all of the echinocandin drugs for the WT and ER strains, respectively) or with no additive (0.06 μg/ml and 5.65 μg/ml for CSF and 0.015 μg/ml and 2.00 μg/ml for the other echinocandins for the WT and ER strains, respectively).
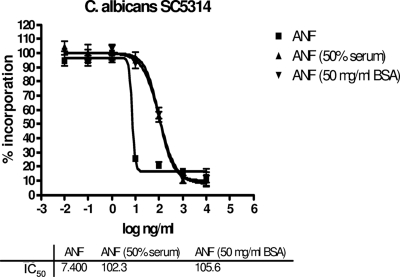
The reduced antifungal properties of the echinocandin drugs in the presence of BSA were also observed in glucan synthase (GS) inhibition assays. Again, 50 mg/ml of BSA mimicked the effect of decreased drug effectiveness observed with 50% human serum (higher IC50s) (Table 2 and Fig. 2). As reported by Paderu et al. for 50% serum (24), 50 mg/ml of BSA had a stronger effect on ANF and MCF than on CSF.
Table 2.
1,3-β-d-Glucan synthase inhibition profilesa of echinocandin drugs for the strains included in the study
| Organism | IC50 (ng/ml)b of the following drug with the indicated medium additive: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ANF |
CSF |
MCF |
|||||||
| No additive | 50% human serum | 50 mg/ml BSA | No additive | 50% human serum | 50 mg/ml BSA | No additive | 50% human serum | 50 mg/ml BSA | |
| C. albicans SC5314c | 7.0 ± 1.2 | 100.9 ± 4.6 | 104.5 ± 3.8 | 13.7 ± 1.2 | 60.5 ± 5.6 | 58.6 ± 6.1 | 26.9 ± 2.4 | 90.1 ± 4.3 | 94.3 ± 2.3 |
| C. albicans 90028c | 2.7 ± 0.5 | 60.7 ± 5.4 | 65.6 ± 4.6 | 7.3 ± 2.4 | 39.7 ± 3.5 | 35.3 ± 5.6 | 15.6 ± 1.2 | 79.0 ± 3.4 | 75.6 ± 4.5 |
| C. glabratad | 1.2 ± 0.2 | 55.2 ± 4.4 | 59.4 ± 3.3 | 3.1 ± 0.8 | 28.9 ± 1.1 | 30.2 ± 2.9 | 0.6 ± 0.2 | 71.6 ± 9.1 | 62.6 ± 1.7 |
| C. kruseic | 3.6 ± 0.1 | 88.1 ± 7.6 | 92.6 ± 5.5 | 9.2 ± 1.6 | 51.7 ± 2.4 | 48.1 ± 5.8 | 6.7 ± 1.6 | 99.1 ± 5.5 | 107.6 ± 7.0 |
| C. tropicalisc | 0.6 ± 0.1 | 44.7 ± 3.9 | 52.7 ± 6.2 | 0.4 ± 0.1 | 22.6 ± 2.0 | 26.4 ± 1.5 | 0.4 ± 0.1 | 29.5 ± 2.2 | 33.7 ± 3.1 |
| C. parapsilosise | 410.0 ± 5.5 | 1,216.1 ± 88.1 | 1,154.0 ± 121.0 | 21.2 ± 3.2 | 70.4 ± 9.0 | 65.6 ± 3.2 | 245.3 ± 4.7 | 671.8 ± 8.8 | 656.2 ± 12.4 |
| C. metapsilosise | 119.5 ± 9.0 | 421.5 ± 29.8 | 444.4 ± 57.0 | 40.3 ± 4.1 | 111.6 ± 5.9 | 120.1 ± 4.2 | 70.3 ± 3.5 | 222.6 ± 9.1 | 231.7 ± 11.6 |
| C. orthopsilosise | 133.9 ± 12.3 | 399.1 ± 38.5 | 402.0 ± 29.6 | 58.2 ± 4.5 | 142.8 ± 8.1 | 138.5 ± 10.5 | 152.7 ± 6.6 | 415.1 ± 5.0 | 401.6 ± 5.2 |
Expressed as IC50s.
Arithmetic means ± standard deviations (three repetitions on three separate days). IC50s were obtained using trapped 1,3-β-d-glucan synthase enzyme. Pearson's coefficients of variation are between 1.2 and 32.9%.
Wild type at FKS1 hot spot regions.
Wild type at FKS1 and FKS2 hot spot regions.
Naturally occurring proline-to-alanine amino acid substitution at the FKS1 hot spot 1 region.
Fig. 2.
Effect of 50 mg of BSA/ml or 50% human serum on the inhibition kinetics (IC50) of ANF for the 1,3-β-d-glucan synthase complex isolated by trapping from C. albicans SC5314. IC50s were determined by monitoring the incorporation of [3H]uridine diphosphoglucose as a function of the ANF concentration.
Fifty percent human serum inhibits the growth of some C. glabrata strains.
The presence of 50% human serum in MIC plates inhibited the growth of 14 out of 20 (70%) C. glabrata strains tested in this study. Of these strains, 13 harbored mutations in the hot spot regions of FKS1 or FKS2. The only WT C. glabrata strain that did not grow in RPMI-50% serum was isogenic with an FKS2 mutant that showed the same behavior in this medium. On the other hand, the only C. glabrata mutant (Fks1p-D632E) that grew in the presence of 50% serum was isogenic with a WT strain that grew under the same conditions (6). These data indicate that the ability of C. glabrata to grow in the presence of 50% serum is independent of its FKS sequence. One C. glabrata mutant strain (Fks2p-F659S) did not grow in any of the broths used, with or without human serum or BSA. However, the same strain grew when it was cultured in a richer medium, such as YPD broth (data not included in the analysis). The inhibitory properties of serum against C. glabrata were not observed in our previous study using a different lot of serum from the same company (14). Moreover, when the same C. glabrata strains were retested using the newest serum, 62.5% (10 out of 16) failed to grow. The MIC testing was repeated with 2 other lots of serum, and the number of C. glabrata strains that did not grow differed for each lot of serum tested (data not shown). On the other hand, when different highly purified fatty-acid-free BSA products were used, growth was uninhibited, and the same results were obtained consistently for all Candida spp. and echinocandin drugs. This result suggests that the albumin fraction of serum is not responsible for the inhibitory activity of serum against C. glabrata.
RPMI 1640 with 50 mg/ml BSA distinguishes wild-type from fks mutant populations.
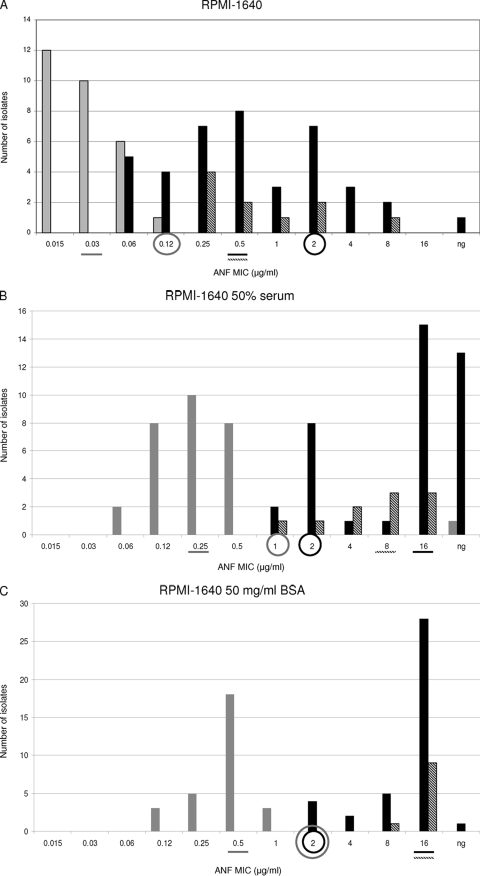
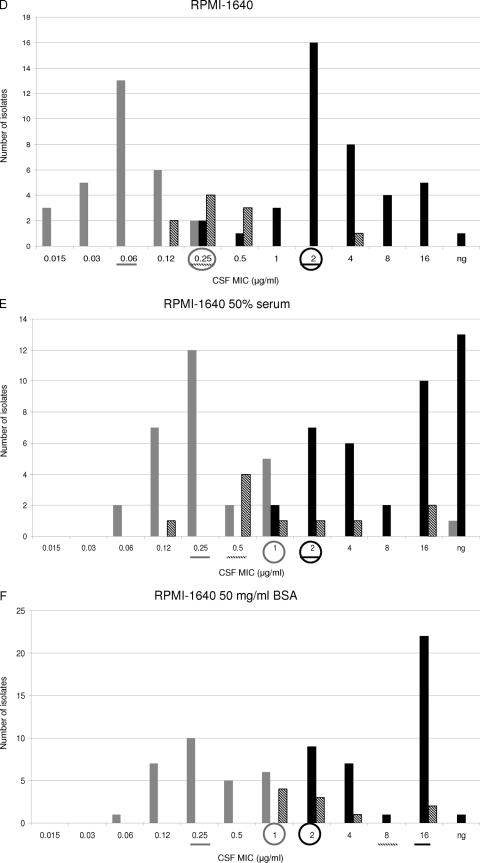
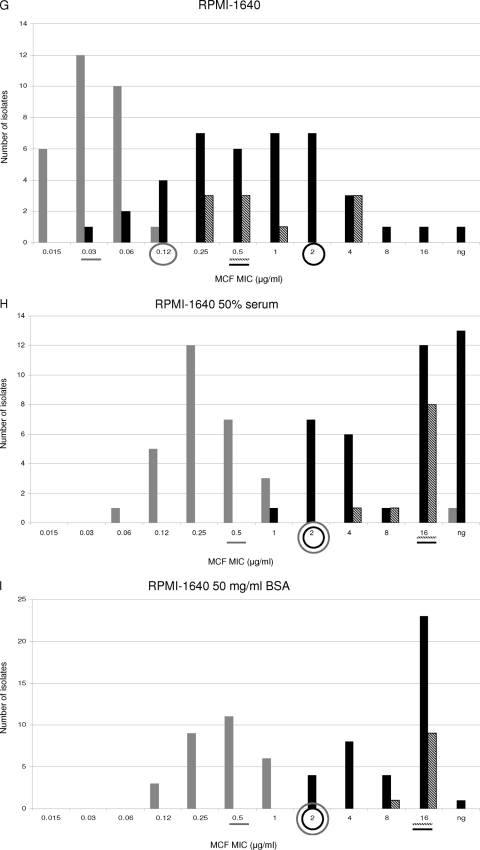
The MIC values obtained using RPMI 1640, RPMI 1640 with 50% serum, and RPMI 1640 with 50 mg/ml BSA were compared. WT and ER populations were separated by −2, −1, and −3 2-fold-dilution steps when RPMI alone was used for testing susceptibility to ANF, CSF, and MCF, respectively (Fig. 3A, D, and G). In other words, the MIC ranges of all the echinocandins overlapped for the WT and ER populations when RPMI 1640 alone was used. Moreover, when the WT-UL values were used as susceptibility breakpoints (≤0.12 μg/ml for ANF and MCF and ≤0.25 μg/ml for CSF), 9, 2, and 7 VME were found for ANF, CSF, and MCF, respectively. On the other hand, no ME were found for any of the echinocandin drugs when RPMI alone was used.
Fig. 3.
Distributions of anidulafungin (ANF), caspofungin (CSF), and micafungin (MCF) MICs for WT (shaded bars), IRES (diagonally striped bars), and ER FKS hot spot mutant (filled bars) populations. MIC susceptibility tests were performed by the method presented in the CLSI M27A3 document by using either RPMI 1640 alone (A, D, and G), RPMI plus 50% serum (B, E, and H), or RPMI plus 50 mg of BSA/ml (C, F, and I). A line under a MIC value indicates the MIC50 for the population represented by a bar with the corresponding shading. Circles outlined in gray indicate the wild-type upper-limit breakpoint. Circles outlined in black represent the CLSI echinocandin susceptibility breakpoint. ng, no growth.
When RPMI 1640 with 50% serum was used, the WT and ER groups were separated by no 2-fold dilutions with ANF, while −1 2-fold dilution step separated these populations when CSF and MCF were tested (Fig. 3B, E, and H). For RPMI-serum, when the WT-UL was used as the susceptibility breakpoint, the numbers of VME were 2, 2, and 7 for ANF, CSF, and MCF, respectively. Thus, in vitro ANF susceptibility testing using RPMI-serum showed better discriminatory power than RPMI 1640 alone in separating the WT and ER populations (P < 0.01). On the other hand, RPMI alone and RPMI-serum showed no difference in the ability to distinguish between the WT and ER groups when CSF and MCF MICs were compared (P = 0.2).
When RPMI-BSA was used, it became clear that this was the best option for differentiating between WT and ER populations. This medium showed the lowest number of VME when WT-UL values were used as the susceptibility breakpoint. RPMI-BSA showed no MIC overlap for any of the echinocandin drugs tested and showed no VME when the WT-UL value was used as the breakpoint for CSF (≤1 μg/ml) (Fig. 3C, F, and I). For the other echinocandin drugs, 3 VME were observed when WT-UL values were used as breakpoints (≤1 μg/ml for ANF and ≤2 μg/ml for MCF).
When the MIC50 values obtained with RPMI, RPMI-serum, and RPMI-BSA were compared, it was evident that the use of serum or BSA facilitated discrimination between WT and ER populations (Fig. 3). RPMI alone splits WT and ER groups by 16 to 32 2-fold dilution steps, while RPMI-serum and RPMI-BSA separated these populations by 32 and 64 2-fold dilution steps for all the echinocandin drugs (Fig. 3). These phenomena can be explained by analyzing how serum and BSA increased the echinocandin MIC50s in the different populations. When RPMI-serum or RPMI-BSA was used, the MIC50 shifts for the WT population were lower (8, 4, and 8 2-fold dilution steps for ANF, CSF, and MCF, respectively) than those for the ER group (32, 8, and 32 2-fold dilutions for ANF, CSF, and MCF, respectively) (P < 0.01), separating the populations. On the other hand, there were no differences between the MIC50 shifts obtained with RPMI-serum and RPMI-BSA (P = 0.8).
RPMI 1640 with 50 mg/ml of BSA makes ANF and MCF behave differently from CSF against the IRES population.
Overall, 9, 9, and 7 IRES strains were considered susceptible to ANF, CSF, and MCF when the CLSI susceptibility breakpoint (≤2 μg/ml) was used. With RPMI 1640 alone, the WT and IRES populations were separated by 1, −1, and 1 2-fold-dilution step for ANF, CSF, and MCF, respectively (Fig. 3A, D, and G). In addition, the IRES and ER populations were not separated when this medium was used for ANF and MCF susceptibility testing (all the IRES strains overlapped with the ER population) (P, 0.25 and 0.26, respectively). On the other hand, RPMI with no additives was the only medium able to show significant CSF MIC differences between the WT and IRES populations (P < 0.0001).
The MIC50s obtained for the IRES population with RPMI alone versus RPMI-serum were compared. Serum shifted the MIC50s for this population 16 (from 0.5 μg/ml to 8 μg/ml), 2 (from 0.25 μg/ml to 0.5 μg/ml), and 32 (from 0.5 μg/ml to 16 μg/ml) 2-fold dilution steps for ANF, CSF, and MCF, respectively (Fig. 3A, B, D, E, G, and H). The same analysis was performed to compare the MIC50s obtained for the IRES population with RPMI alone versus RPMI-BSA, and 32, 8, and 32 2-fold dilution step shifts were observed for ANF, CSF, and MCF, respectively. Interestingly, the ANF and MCF MIC ranges for the IRES population were displaced to higher values for RPMI-BSA than for RPMI-serum (ANF and MCF MICs were 8 or 16 μg/ml for all the IRES strains when RPMI-BSA was used) (P < 0.01).
DISCUSSION
Echinocandin drugs bind to the albumin fraction of serum in vitro.
It is well established that echinocandin drugs bind to serum proteins at very high levels (98% for ANF, 96.5% for CSF, and 99.8% for MCF) (17, 24, 38, 42). However, it is not clear whether echinocandins are active only as unbound free drug or whether these drugs are also active when complexed with protein. According to the free-drug hypothesis, only 2%, 3.5%, and 0.2% of the ANF, CSF, and MCF present in RPMI-serum would have pharmacological activity, respectively. Thus, the presence of serum should increase the MIC values between 200- and 500-fold. Our in vitro data suggest that the increases in echinocandin MIC50s (2 to 32 2-fold dilution steps) are not totally consistent with the free-drug hypothesis and confirm older in vitro and in vivo data (23, 24, 42, 43). In a recent report, Ishikawa et al. demonstrated that the free-drug hypothesis was unsuitable for MCF (17). Thus, we can infer that at least part of the protein-bound drug is active against Candida spp.
The data presented in this study support the contention that the addition of 50 mg/ml of BSA to the medium for the standard CLSI testing methodology mimics the in vitro activity of 50% serum with echinocandin drugs (Tables 1 and 2). The fact that BSA mimics the effect of serum is consistent with the notion that echinocandin drugs bind primarily to albumin and that the other serum fractions have minimal or no effect on the in vitro potency of these drugs. On the surface, this conclusion would appear to contradict that of Abe et al. (1), who demonstrated, using an analbuminemic rat model, that MCF pharmacodynamics were not affected by albumin. These authors concluded that MCF must be bound in vivo to other serum fractions besides albumin. However, it is likely that the echinocandin drugs bind albumin and other serum components, with the latter being more significant in the absence of albumin. These differences between the in vitro and in vivo activity effects of serum on echinocandin drugs need to be studied further.
RPMI 1640 with 50 mg/ml of BSA is better than RPMI 1640 alone at revealing Candida sp. FKS mutants.
Multiple reports have demonstrated the excellent in vitro activity of echinocandin drugs against clinical Candida sp. isolates (25, 26). However, these studies also demonstrated a bimodal echinocandin MIC distribution, with strains from highly susceptible species exhibiting elevated MIC values. Furthermore, they highlighted the presence of Candida spp. with RES phenotypes (8, 11, 21, 29, 32, 34). However, there are no convincing data linking high echinocandin MIC values and clinical failure, leading to skepticism regarding the value of routine echinocandin MIC testing for resistance (3, 14, 18, 22, 31). Moreover, it has been suggested that there is a better correlation between the presence of certain FKS hot spot mutations and echinocandin clinical failure than between high MIC values alone and echinocandin clinical failure (27). The CLSI published the echinocandin susceptibility breakpoint (≤2 μg/ml) (7), but multiple reports have shown that this breakpoint may misclassify as echinocandin susceptible many of the Candida sp. fks hot spot mutants isolated during or after echinocandin therapy (2, 14–16, 35, 40). The CLSI committee is revisiting the issue of breakpoints for echinocandins to reflect these concerns in the literature (30).
Lately, a variety of suggestions have been made to improve the ability of in vitro susceptibility testing to detect FKS mutants (2, 9, 15, 16), including the use of RPMI 1640 with 50% human serum to distinguish C. albicans and C. glabrata fks mutants from WT strains and to normalize the behavior of all three echinocandin drugs (15, 16, 24). Yet the standardization of human serum is too difficult to allow its incorporation into a standard assay. To overcome this issue, we have used highly pure fatty-acid-free BSA as a surrogate for human serum albumin. It was evident that the use of RPMI-BSA differentially increased echinocandin MIC values, clearly separating the WT and ER populations (Fig. 3). Also, the addition of 50 mg/ml of BSA to RPMI 1640 showed no MIC range overlaps and the lowest number of VME. Moreover, this work reinforces our group's previous suggestion that CSF should be used as a marker of the RES phenotype (2, 15, 16) and that the original CLSI breakpoint for echinocandins requires revision in order to identify all or most of the fks hot spot mutants. On the other hand, the ANF and MCF WT-UL values (0.12 μg/ml) were similar to the MIC and IC50 breakpoints (0.25 and 0.5 μg/ml) proposed previously by our group for C. albicans and C. glabrata (15, 16).
C. parapsilosis, C. orthopsilosis, and C. metapsilosis strains showed MIC values similar to those for the ER population.
C. parapsilosis sensu lato species showed a MIC90 value more than 16-fold higher than the MIC90s of other Candida spp. (11, 28). Moreover, none of the echinocandin drugs showed fungicidal activity against C. parapsilosis (4). This lower activity of echinocandin drugs against these species has been linked to a naturally occurring amino acid substitution in hot spot 1 of FKS1 (13, 27). Despite all these in vitro and genetic data, most infections caused by C. parapsilosis sensu lato species respond to echinocandin therapy (20, 25, 36). The data presented in this work demonstrated that C. parapsilosis sensu lato species showed echinocandin MIC values similar to those for other Candida sp. fks mutants regardless of the medium used. However, 50 mg/ml of BSA or 50% human serum made MCF and ANF behave differently from CSF with these species. BSA reduced the ANF and MCF MIC range for the IRES population and displaced it to higher MIC values (≥8 μg/ml). On the other hand, CSF MIC values were not reduced, and the MIC50 shift was lower for this drug. These results suggest that CSF is more active than ANF and MCF against C. parapsilosis sensu lato species in vitro. Our group has shown that the glucan synthase complexes of these species are 20-fold more sensitive to CSF than to the other two echinocandin drugs (13). Moreover, a recently published paper showed that 83% of the C. parapsilosis strains not responding to MCF therapy had MCF MICs of ≥4 μg/ml and that those isolates had CSF MICs ranging from 0.5 to 1 μg/ml (35). The results of that work reinforce the notion that C. parapsilosis, C. metapsilosis, and C. orthopsilosis behave as weakly echinocandin resistant organisms in vitro but that other factors influence their behavior as weak pathogens. One possibility is a potential fitness cost to the Candida sp. cells harboring FKS hot spot mutations (or polymorphisms) due to a decreased Vmax of the GS complex (13, 15, 16).
Overall, it is apparent that 50 mg/ml of BSA mimicked the effect of human serum, altering the antifungal properties of the echinocandin drugs. RPMI-BSA is a viable alternative to human serum for in vitro susceptibility testing standardization. Most importantly, RPMI-BSA is able to distinguish between WT and ER populations when one is using the existing susceptibility breakpoint of <2 μg/ml (or a lower susceptibility breakpoint, once it is redefined by the CLSI). In conclusion, the addition of BSA to RPMI 1640 could be considered a modification of CLSI echinocandin susceptibility testing. However, the optimum testing conditions have to be established, and multilaboratory reproducibility studies are essential for proposing such a modification. Collaborative studies will be arranged in the near future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maiken Cavling Arendrup for critical reading of the manuscript and helpful suggestions. We are grateful to Astellas, Merck, and Pfizer for providing micafungin, caspofungin, and anidulafungin as pure substances.
This work was supported by a grant to D.S.P. from NIH (AI069397).
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 7 March 2011.
REFERENCES
- 1. Abe F., et al. 2008. Role of plasma proteins in pharmacokinetics of micafungin, an antifungal antibiotic, in analbuminemic rats. Antimicrob. Agents Chemother. 52:3454–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arendrup M. C., et al. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27–A3, Etest, disk diffusion, and agar dilution methods with RPMI and Iso-Sensitest media. Antimicrob. Agents Chemother. 54:426–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett J. E. 2006. Echinocandins for candidemia in adults without neutropenia. N. Engl. J. Med. 355:1154–1159 [DOI] [PubMed] [Google Scholar]
- 4. Cantón E., Espinel-Ingroff A., Peman J., del Castillo L. 2010. In vitro fungicidal activities of echinocandins against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis evaluated by time-kill studies. Antimicrob. Agents Chemother. 54:2194–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castanheira M., et al. 2010. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob. Agents Chemother. 54:2655–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleary J. D., Garcia-Effron G., Chapman S. W., Perlin D. S. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27–A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Cuenca-Estrella M., et al. 2009. Activity profile in vitro of micafungin against Spanish clinical isolates of common and emerging species of yeasts and molds. Antimicrob. Agents Chemother. 53:2192–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desnos-Ollivier M., et al. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodgson A. R., Pujol C., Denning D. W., Soll D. R., Fox A. J. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J. Clin. Microbiol. 41:5709–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espinel-Ingroff A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents, and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121–136 [PubMed] [Google Scholar]
- 12. Gamarra S., et al. 2010. Mechanism of the synergistic effect of amiodarone and fluconazole in Candida albicans. Antimicrob. Agents Chemother. 54:1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Effron G., Katiyar S. K., Park S., Edlind T. D., Perlin D. S. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Effron G., Kontoyiannis D. P., Lewis R. E., Perlin D. S. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Effron G., Lee S., Park S., Cleary J. D., Perlin D. S. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Effron G., Park S., Perlin D. S. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa J., et al. 2009. Antifungal activity of micafungin in serum. Antimicrob. Agents Chemother. 53:4559–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kartsonis N., et al. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katiyar S., Pfaller M., Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuse E. R., et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 21. Messer S. A., et al. 2006. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J. Clin. Microbiol. 44:324–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mora-Duarte J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 23. Odabasi Z., Paetznick V., Rex J. H., Ostrosky-Zeichner L. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paderu P., et al. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 26. Park S., et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perlin D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaller M. A., et al. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaller M. A., et al. 2006. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 44:3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfaller M. A., et al. 2010. Clinical breakpoints for the echinocandins and Candida, revisited, abstr.M-369. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 31. Pfaller M. A., et al. 2005. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob. Agents Chemother. 49:4795–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfaller M. A., Diekema D. J., Messer S. A., Hollis R. J., Jones R. N. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfaller M. A., et al. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfaller M. A., et al. 2003. Caspofungin activity against clinical isolates of fluconazole-resistant Candida. J. Clin. Microbiol. 41:5729–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfeiffer C. D., et al. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reboli A. C., et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472–2482 [DOI] [PubMed] [Google Scholar]
- 37. Robles J. C., Koreen L., Park S., Perlin D. S. 2004. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discriminating among strains of Candida albicans. J. Clin. Microbiol. 42:2480–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stone J. A., et al. 2004. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob. Agents Chemother. 48:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tavanti A., Gow N. A., Senesi S., Maiden M. C., Odds F. C. 2003. Optimization and validation of multilocus sequence typing for Candida albicans. J. Clin. Microbiol. 41:3765–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson G. R., et al. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiederhold N. P., Grabinski J. L., Garcia-Effron G., Perlin D. S., Lee S. A. 2008. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob. Agents Chemother. 52:4145–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiederhold N. P., Lewis R. E. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313–1333 [DOI] [PubMed] [Google Scholar]
- 43. Wiederhold N. P., et al. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.