Abstract
Antibiotic resistance development has been linked to the bacterial SOS stress response. In Escherichia coli, fluoroquinolones are known to induce SOS, whereas other antibiotics, such as aminoglycosides, tetracycline, and chloramphenicol, do not. Here we address whether various antibiotics induce SOS in Vibrio cholerae. Reporter green fluorescent protein (GFP) fusions were used to measure the response of SOS-regulated promoters to subinhibitory concentrations of antibiotics. We show that unlike the situation with E. coli, all these antibiotics induce SOS in V. cholerae.
INTRODUCTION
The emergence of multiply resistant bacteria has been correlated with widespread antibiotic usage. It has been noted that sub-MICs of antibiotics induce several changes in gene expression (8). Fluoroquinolones (FQs), beta-lactams, and trimethoprim (TMP) induce the SOS stress response in Escherichia coli (19, 23, 25), resulting in increased mutation frequency (17, 25), whereas other antibiotics, such as aminoglycosides (AGs), chloramphenicol (CAM), rifampin (RIF), or tetracycline (TCN), do not (23). However, TCN induces mutagenesis requiring SOS-regulated DNA polymerases (7), suggesting a link between tetracycline and SOS. Interestingly, AGs, as well as FQs and mitomycin C (MMC), induce the competence regulon (com) in Streptococcus (21). Streptococcus does not have any homologue for the SOS repressor LexA; however, most of the DNA repair genes, including recA, belong to the com regulon, considered a parallel of SOS in Streptococcus.
A recent study demonstrated how beta-lactams, FQs, and AGs stimulate production of reactive oxygen species (ROS) in bacteria (12). ROS can damage DNA and induce mutagenesis, leading to multiple resistances. Damaged DNA is a potent SOS inducer, suggesting that all antibiotics have the potential to induce the bacterial stress response, which is a fundamental mechanism of adaptation and resistance development.
Vibrio cholerae is a Gram-negative human pathogen which can be deadly if not treated appropriately with antibiotics (e.g., TCN, TMP, FQs, and sometimes CAM, according to WHO recommendations). AGs are also generally used against Gram-negative bacteria, although not specifically against cholera infections. All strains of V. cholerae carry a chromosomal superintegron (SI), composed of an array of promoterless adaptive gene cassettes, that can be recombined and summoned when necessary through the action of the integrase IntIA (3), which is regulated by SOS (5, 11).
The V. cholerae response to sub-MICs of antibiotics has not been thoroughly studied. In this study we addressed whether antibiotics that do not induce SOS in E. coli might act differently in V. cholerae.
Results and discussion.
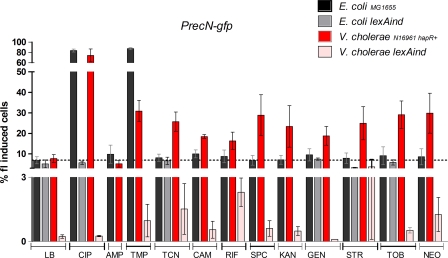
We constructed V. cholerae and E. coli reporter strains carrying the green fluorescent protein gene (gfp) fused to the recN promoter. RecN expression is upregulated during SOS induction in both bacteria (1). Figure 1 shows the percentage of GFP-induced cells after treatment with sub-MICs of specified antibiotics. Sub-MICs were determined as concentrations 100-fold lower than the MIC for each bacterium (Fig. 1). Bacteria were cultured overnight with specified antibiotics on Mueller-Hinton (MH) medium, and the percentage of fluorescence-induced cells was determined by flow cytometry as described previously (1).
Fig. 1.
SOS induction by subinhibitory concentrations of antibiotics in E. coli and V. cholerae. Bacteria were grown overnight in MH supplemented with sub-MICs of specified antibiotics as follows (final concentrations [μg ml−1] of drug for organism [E. coli, V. cholerae]): ciprofloxacin (CIP), 0.05, 0.005; ampicillin (AMP) and rifampin (RIF), 0.05, 0.01; trimethoprim (TMP), 0.05, 0.005; tetracycline (TCN), 0.15, 0.015; chloramphenicol (CAM), 0.15, 0.005; spectinomycin (SPC), kanamycin (KAN), and streptomycin (STR), 0.2, 0.05; gentamicin (GEN), tobramycin (TOB), and neomycin (NEO), 0.1, 0.01. The samples were washed in PBS, and the fluorescence was measured using the FACSCalibur device (1). The percentage of fluorescent cells is represented on the y axis. Each measurement was reproduced at least four times. In order to test whether the means of two groups were different, we first tested the equality of their variance with a Fisher test. When the variances of two groups were found to be significantly different, an unequal-variance t test was performed. Otherwise, a simple t test was performed. The chosen significance threshold was 0.05 for all tests.
Our data confirm that FQs and TMP are strong SOS inducers in E. coli (Fig. 1, black bars). The isogenic lexAind strain, which is not inducible for SOS (due to an uncleavable LexA repressor) corroborated that the increase in GFP expression was indeed due to SOS induction (Fig. 1, gray bars). Conversely, AGs, TCN, and CAM do not induce SOS in E. coli. Supporting this fact, abrogation of SOS (lexAind strain) does not impact fluorescence values obtained with TCN and AGs. In order to examine how these antibiotics act on V. cholerae SOS, we used the quorum-sensing-proficient N16961 hapR+ strain (4, 16), because the overall SOS level (even without antibiotics) was three to five times higher (and thus easier to detect) in the hapR+ context than in the hapR-deficient mutant (Fig. 1, red versus pink bars for MH medium; see also Fig. S1 in the supplemental material). Besides, many other natural strains of V. cholerae, such as the classical O1 strain responsible for the 6th pandemic (O395), the O1 strain El Tor MJ1236, and strains A1552, O37, HK1, CA401, and SG21, are hapR+. As expected, ciprofloxacin (CIP) (an FQ) and TMP strongly induced SOS (Fig. 1, red bars). Strikingly, AGs, CAM, RIF, and TCN also induced SOS in V. cholerae, although to a lower degree than FQs and TMP. GFP expression was dramatically reduced in the lexAind strain regardless of the antibiotic, confirming that sub-MIC antibiotics induce SOS in V. cholerae. SOS induction by beta-lactams is variable, depending on experimental conditions (15, 18), and ampicillin did not induce SOS in our PrecN-gfp fusion strains.
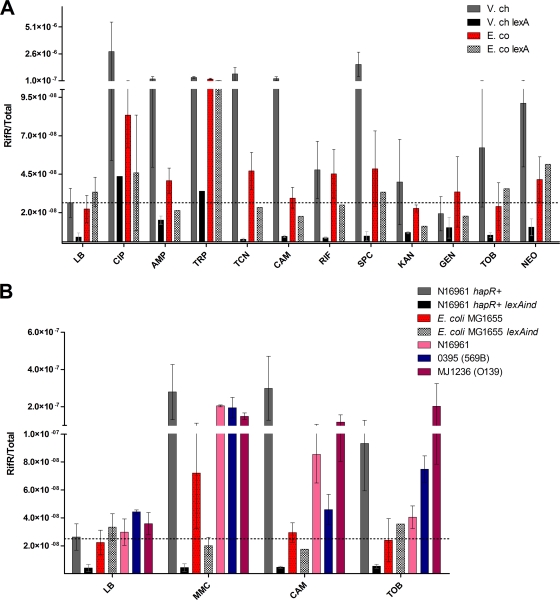
SOS is also known to induce mutagenesis. A major pathway is through the activation of the translesion synthesis polymerases (such as DinB/VC2287) that lack proofreading activity and that belong to the SOS regulon in both bacteria. We determined the effect of incubation with sub-MIC antibiotics on mutation frequency, using RIF resistance appearance as a reporter. Indeed, spontaneous point mutations in the gene encoding the β-subunit of RNA polymerase (rpoB) are known to give rise to this phenotype and are commonly used for such testing (24).
As expected, mutagenesis was strongly induced by CIP (FQ) and TMP in the E. coli strain MG1655 (Fig. 2A). TCN, CAM, RIF, and spectinomycin (SPC) also slightly induced mutation frequency, suggesting a low effect of these antibiotics on SOS, which was undetectable by flow cytometry. It is important to note here that mutagenesis may not exclusively be due to SOS, as other pathways exist (2, 20).
Fig. 2.
Spontaneous mutation frequency after treatment with subinhibitory concentrations of antibiotics. Bacteria were grown as described in Fig. 1. Appropriate dilutions were plated on Luria broth, and 1 ml of culture was centrifuged and plated on 100-μg/ml rifampin plates. The mutation frequency, corresponding to the rifampin-resistant CFU count over the total number of CFU, was measured and represented on the y axis for each antibiotic. (A) E. coli MG1655 and V. cholerae N16961 hapR+. (B) Comparison of various V. cholerae strains.
Notably, in addition to the strong SOS inducers (CIP and TMP), ampicillin (AMP), TCN, and CAM strongly increased the mutation frequency in V. cholerae N16961 hapR+. Some AGs (SPC, tobramycin [TOB], and neomycin) also significantly induced mutagenesis, whereas gentamicin and kanamycin did not consistently affect mutation frequency. No increase was observed in the isogenic lexAind strain, showing that increased frequencies in wild-type V. cholerae were indeed due to the induction of SOS (Fig. 2A). To ascertain that the effect of sub-MICs of antibiotics is a general property of V. cholerae, we also tested the mutagenic effect of selected antibiotics on O1 classical (0395/569B) and O139 (MJ1236) strains of V. cholerae, which differ from N16961 according to whole genome sequencing data comparison. MMC was used as a control SOS inducer. All V. cholerae strains show increased mutation frequency after CAM and TOB treatment, whereas no effect is observed in E. coli (Fig. 2B). Unlike E. coli, V. cholerae is thus prone to SOS response activation by a wide set of antibiotics at sub-MICs. We conclude that E. coli should not be taken as a paradigm regarding the effects of antibiotics on bacteria.
SOS induction by antibiotics has to be taken seriously, as it can result in undesired changes in the behavior of bacteria and their adaptation to hostile environments. Examples are numerous: SOS can induce the expression of the cholera toxin (22), can trigger SI cassette rearrangements conferring resistance to an originally sensitive V. cholerae (1), and may lead to persistence and eventually the development of multidrug resistance (9, 10, 13).
In conclusion, activation of SOS appears to be a major threat to the efficiency of antibiotic treatments. An engineered bacteriophage that suppresses the SOS network has been reported to enhance killing by antibiotics in E. coli and to increase survival of infected mice (14). Another example in treatment failure due to SOS is the resistance to FQs mediated by translesional DNA-polymerase-dependent mutations appearing upon SOS induction (6). Consequently, the inhibition of the SOS response in pathogens by the use of “anti-SOS” components could be promising for prevention of resistance development.
Supplementary Material
Acknowledgments
We thank David Bikard for his help with statistical analysis of the flow cytometry data.
This work was supported by the Institut Pasteur, the Centre National de la Recherche Scientifique (CNRS-URA 2171), the French National Research Agency (ANR-08-MIE-016), the EU (NoE EuroPathoGenomics, LSHB-CT-2005-512061). and the Fondation pour la Recherche Médicale (équipe FRM 2007). Z.B. is supported by a postdoctoral fellowship from the Roux Foundation and a DIM Malinf fellowship (Conseil régional d'Île-de-France).
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 7 February 2011.
REFERENCES
- 1. Baharoglu Z., Bikard D., Mazel D. 2010. Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 6:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balashov S., Humayun M. Z. 2002. Mistranslation induced by streptomycin provokes a RecABC/RuvABC-dependent mutator phenotype in Escherichia coli cells. J. Mol. Biol. 315:513–527 [DOI] [PubMed] [Google Scholar]
- 3. Biskri L., Bouvier M., Guerout A. M., Boisnard S., Mazel D. 2005. Comparative study of class 1 integron and Vibrio cholerae superintegron integrase activities. J. Bacteriol. 187:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blokesch M., Schoolnik G. K. 2008. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J. Bacteriol. 190:7232–7240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cambray G., Guerout A. M., Mazel D. 2010. Integrons. Annu. Rev. Genet. 44:141–166 [DOI] [PubMed] [Google Scholar]
- 6. Cirz R. T., Romesberg F. E. 2006. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 50:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen S. E., Walker G. C. 2010. The transcription elongation factor NusA is required for stress-induced mutagenesis in Escherichia coli. Curr. Biol. 20:80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies J., Spiegelman G. B., Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9:445–453 [DOI] [PubMed] [Google Scholar]
- 9. Dorr T., Lewis K., Vulic M. 2009. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 5:e1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorr T., Vulic M., Lewis K. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 8:e1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerin E., et al. 2009. The SOS response controls integron recombination. Science 324:1034. [DOI] [PubMed] [Google Scholar]
- 12. Kohanski M. A., DePristo M. A., Collins J. J. 2010. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 37:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372 [DOI] [PubMed] [Google Scholar]
- 14. Lu T. K., Collins J. J. 2009. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. U. S. A. 106:4629–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maiques E., et al. 2006. β-Lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J. Bacteriol. 188:2726–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meibom K. L., Blokesch M., Dolganov N. A., Wu C. Y., Schoolnik G. K. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827 [DOI] [PubMed] [Google Scholar]
- 17. Mesak L. R., Davies J. 2009. Phenotypic changes in ciprofloxacin-resistant Staphylococcus aureus. Res. Microbiol. 160:785–791 [DOI] [PubMed] [Google Scholar]
- 18. Mesak L. R., Miao V., Davies J. 2008. Effects of subinhibitory concentrations of antibiotics on SOS and DNA repair gene expression in Staphylococcus aureus. Antimicrob. Agents Chemother. 52:3394–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller C., et al. 2004. SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science 305:1629–1631 [DOI] [PubMed] [Google Scholar]
- 20. Perez-Capilla T., et al. 2005. SOS-independent induction of dinB transcription by beta-lactam-mediated inhibition of cell wall synthesis in Escherichia coli. J. Bacteriol. 187:1515–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prudhomme M., Attaiech L., Sanchez G., Martin B., Claverys J. P. 2006. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313:89–92 [DOI] [PubMed] [Google Scholar]
- 22. Quinones M., Kimsey H. H., Waldor M. K. 2005. LexA cleavage is required for CTX prophage induction. Mol. Cell 17:291–300 [DOI] [PubMed] [Google Scholar]
- 23. Shaw K. J., et al. 2003. Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents. J. Mol. Microbiol. Biotechnol. 5:105–122 [DOI] [PubMed] [Google Scholar]
- 24. Taddei F., Matic I., Radman M. 1995. cAMP-dependent SOS induction and mutagenesis in resting bacterial populations. Proc. Natl. Acad. Sci. U. S. A. 92:11736–11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ysern P., et al. 1990. Induction of SOS genes in Escherichia coli and mutagenesis in Salmonella typhimurium by fluoroquinolones. Mutagenesis 5:63–66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.