Abstract
The geometric mean 50% inhibitory concentration (IC50) for Proveblue, a methylene blue complying with the European Pharmacopoeia, was more active on 23 P. falciparum strains than chloroquine, quinine, mefloquine, monodesethylamodiaquine, and lumefantrine. We did not find significant associations between the Proveblue IC50 and polymorphisms in the pfcrt, pfmdr1, pfmdr2, pfmrp, and pfnhe-1 genes or the copy numbers of the pfmdr1 and pfmdr2 genes, all of which are involved in antimalarial resistance.
INTRODUCTION
In 1891, Guttmann and Ehrlich were the first to report the antimalarial properties of a synthetic thiazine dye, methylene blue (MB), when they described the clinical cure of two patients after oral administration of MB (11). Cardamatis wrote in Progrès Médical that he had found MB to be very effective in the early stages of severe malaria cachexia in cases resistant to quinine (4). MB has shown in vitro activity against Plasmodium falciparum strains (2, 10) and isolates (1) and in vivo activity against P. vinckei and P. yoelii parasites (5).
Currently, there is no available MB across the world that complies with the European Pharmacopoeia. Indeed, up to now, the pharmaceutical use of MB has been stymied by contamination with organic impurities as well as heavy metals of recognized toxicity. Provence Technologies and its subsidiary, Provepharm, have conducted 4 years of research that resulted in the first European Pharmacopoeia-grade MB, Proveblue, obtained using a new innovative synthetic pathway with a heavy-metal-free process involving pharmaceutical-grade reagents (patent application no. FR06/06330 [July 2006, France], which has been extended to the international PCT reference PCT/FR/2007/001193). The sum of metals is <20 ppm, the quantity of azure B is <2% (the most important impurity in MB), and the quantity of other impurities is <0.5%. An analysis performed on commercial MB by an independent laboratory showed that officinal MB contained a quantity of cadmium higher than the level authorized by the European Pharmacopoeia. The industrial MB contained 94.45% of MB, while the European Pharmacopoeia requires a greater than 95% quantity of MB. Moreover, it contained 5.55% of azure B impurities, while the required quantity is below 5%. Those results showed that the currently marketed officinal and industrial MB do not comply with European Pharmacopoeia standards.
The aim of the present work was to assess the following: (i) the in vitro activity of Proveblue in comparison to that of commercial MB, to ensure that the previously described antimalarial activity was due to MB and not to contaminants; (ii) the in vitro activity of Proveblue in comparison to those of standard antimalarial drugs such as chloroquine (CQ), quinine (QN), monodesethylamodiaquine (MDAQ) (the active metabolite of amodiaquine), mefloquine (MQ), lumefantrine (LMF), and dihydroartemisinin (DHA) (the active metabolite of artemisinin derivatives); (iii) the in vitro cross-resistance between Proveblue and standard antimalarial drugs; and (iv) the potential association of in vitro Proveblue responses with genetic polymorphisms in genes that are known or supposed to be associated with reduced quinoline susceptibility, such as the P. falciparum chloroquine resistance transporter gene (pfcrt), the P. falciparum multidrug resistance-associated protein gene (pfmrp), the P. falciparum multidrug resistance protein 1 gene (pfmdr1), the P. falciparum Na+/H+ exchanger gene (pfnhe1), and the P. falciparum multidrug resistance protein 2 gene (pfmdr2) (3, 13, 14, 16).
Materials and methods.
A total of 23 preidentified parasite strains from a wide panel of countries (Brazil, Cambodia, Cameroon, Comoros, Djibouti, the Gambia, French Guyana, Honduras, Indochina, Niger, Republic of Congo, Senegal, Sierra Leone, Sudan, Thailand, and Uganda) (15, 21) were maintained in culture as previously described (27) and verified using PCR genotyping of polymorphic genetic markers, including msp1, msp2, and microsatellite loci (6, 12). Each strain was tested for antimalarial activity in 6 to 21 experiments.
Proveblue was obtained from Provepharm SAS (Marseille, France). The two standard MB varieties were purchased from Cooper (France), one of “officinal” quality from a purified source (MBO) and the other of “industrial” quality (MBI).
The three methylene blue dyes were assessed in blind tests. The in vitro isotopic microtest used was previously described (21).
The methods for single nucleotide polymorphism (SNP) identification of pfcrt, pfmdr1, pfmdr2, and pfmrp (27), for the identification of pfnhe-1 microsatellite profiles (9, 16), and for the estimation of the copy numbers of pfmdr1 and pfmdr2 (27) were previously described.
An assessment of the cross-resistance of standard antimalarial drugs with Proveblue was estimated by Pearson's coefficient of correlation (r) and the coefficient of determination (r2). The Kruskal-Wallis test or the Mann-Whitney U test was used, when appropriate, to test for associations between the 50% inhibitory concentration (IC50) and mutations.
Results and discussion.
Twenty-three P. falciparum strains were tested for their in vitro susceptibilities to Proveblue, MBI, MBO, CQ, QN, MQ, MDAQ, LMF, and DHA. The geometric mean IC50 was 3.62 nM (95% confidence interval [CI]= 2.82 to 4.66) for Proveblue, 3.87 nM (CI = 3.08 to 4.87) for MBI, and 3.97 nM (CI = 2.83 to 5.57) for MBO. The same antimalarial activities were observed with the three qualities of MB. Proveblue was significantly more active than the officinal MB (P = 0.0020). Proveblue demonstrated a high antimalarial potency. The mean IC50 for each strain ranged from 0.6 nM to 9.4 nM. These data are consistent with previous findings for MB with organic impurities as well as inorganic impurities (heavy metals), with mean IC50s ranging from 3 to 11 nM on P. falciparum strains (2, 10, 26) and of about 10 nM on Nigerian isolates (1). We proved that the antimalarial activity is not linked to the metal impurities but to the phenothiazine.
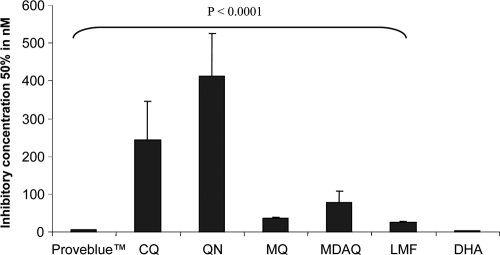
Moreover, this activity appeared to be 20 to 50 times higher than those of standard antimalarial drugs such as CQ, QN, MDAQ, MQ, and LMF (P < 0.0001) (Fig. 1). Proveblue is highly effective on P. falciparum strains with reduced susceptibilities to CQ, QN, MDAQ, or MQ, but it is significantly less active than DHA (P = 0.0004; geometric mean IC50 = 1.77 nM; CI = 1.39 to 2.26).
Fig. 1.
In vitro activity of Proveblue (geometric mean) against 23 P. falciparum strains in comparison to chloroquine (CQ), quinine (QN), monodesethylamodiaquine (MDAQ), mefloquine (MQ), lumefantrine (LMF), and dihydroartemisinin (DHA). Bars represent the 95% confidence interval.
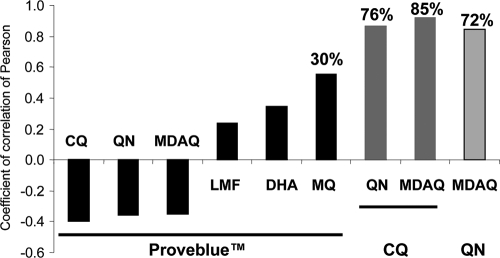
Encouragingly, no significant correlations were found between the Proveblue responses and CQ, QN, MDAQ, LMF, or DHA (Fig. 2), suggesting that no cross-resistance exists between Proveblue and the standard antimalarial drugs. This absence of cross-resistance suggests that Proveblue and CQ, QN, MDAQ, LMF, and DHA have different modes of action or that different mechanisms of resistance are involved. A significant positive correlation was shown between responses to Proveblue and MQ (r = 0.5519; P = 0.0063). Thirty percent of the variation in the response to Proveblue can be explained by variations in the responses to MQ.
Fig. 2.
In vitro Pearson's coefficients of correlation between the Proveblue responses (IC50) and those to chloroquine (CQ), quinine (QN), monodesethylamodiaquine (MDAQ), mefloquine (MQ), lumefantrine (LMF), and dihydroartemisinin (DHA), between the CQ responses and those to MDAQ and QN and between the QN and MDAQ responses. Coefficients of determination are indicated in boldface.
The differences in the drug uptakes and/or modes of action of Proveblue and the other commonly used antimalarial drugs are reinforced by the lack of a significant association between the Proveblue IC50 (0.0556 < P < 0.8248) and the polymorphisms or copy numbers of genes involved in quinoline resistance, such as pfcrt for CQ and MDAQ (24), pfmdr1 for MQ (23), and LMF (8), pfnhe-1 for QN (16, 22), and pfmrp for CQ and QN (25) (Table 1), while significant associations were shown between the CQ, MDAQ, QN, and MQ responses and polymorphisms in the pfcrt gene and between the MDAQ and QN responses and polymorphisms in the pfmrp gene (not shown).
Table 1.
Associations of the in vitro responses (IC50) of Proveblue and the polymorphisms in the pfcrt, pfmdr1, pfmrp, pfnhe1, and pfmrp2 genes and copy numbers of the pfmdr1 and pfmdr2 genes of 23 strains of Plasmodium falciparuma
| Genotype | P value | Significance |
|---|---|---|
| pfnhe-1 ms4760 profiles | 0.2975 | NS |
| pfnhe-1, no. of DNNND repeats | 0.1768 | NS |
| pfnhe-1, no. of NHNDNHNNDDD repeats | 0.1026 | NS |
| Mutation in codon 72 of the pfcrt gene | 0.7628 | NS |
| Mutation in codon 74 of the pfcrt gene | 0.1011 | NS |
| Mutation in codon 75 of the pfcrt gene | 0.1011 | NS |
| Mutation in codon 76 of the pfcrt gene | 0.1654 | NS |
| Mutation in codon 220 of the pfcrt gene | 0.1210 | NS |
| Mutation in codon 271 of the pfcrt gene | 0.2370 | NS |
| Mutation in codon 326 of the pfcrt gene | 0.2911 | NS |
| Mutation in codon 356 of the pfcrt gene | 0.9394 | NS |
| Mutation in codon 371 of the pfcrt gene | 0.1011 | NS |
| Mutation in codon 86 of the pfmdr1 gene | 0.5347 | NS |
| Mutation in codon 184 of the pfmdr1 gene | 0.0926 | NS |
| Mutation in codon 1034 of the pfmdr1 gene | 0.1939 | NS |
| Mutation in codon 1042 of the pfmdr1 gene | 0.2962 | NS |
| Mutation in codon 1246 of the pfmdr1 gene | 0.1554 | NS |
| Copy no. of the pfmdr1 gene (1 and >1) | 0.5977 | NS |
| Mutation in codon 208 of the pfmdr2 gene | 0.0676 | NS |
| Mutation in codon 423 of the pfmdr2 gene | 0.4940 | NS |
| Copy no. of the pfmdr2 gene (only 1) | ND | |
| Mutation in codon 191 of the pfmrp gene | 0.5793 | NS |
| Mutation in codon 437 of the pfmrp gene | 0.5793 | NS |
Values are based on the Mann-Whitney U test or the Kruskal-Wallis test. NS, not significant; ND, not determined.
Twenty-three strains may not be a sufficient number on which to base definitive conclusions. The validity of the conclusions should be further investigated by analyzing more strains or isolates. Nevertheless, the high level of activity of Proveblue against all of the P. falciparum strains with reduced susceptibility to CQ, QN, MDAQ, or MQ, the absence of cross-resistance, and the lack of association with the proteins involved in quinoline resistance suggest that Proveblue could be a good partner in combination with current antimalarial drugs. Recent trials with different partner drugs, such as amodiaquine and artesunate (7, 28), conducted with children and adults in Burkina Faso provided evidence that MB (despite not complying with the European Pharmacopoeia) is safe and relatively effective in uncomplicated falciparum malaria. Nevertheless, the combination of MB and CQ is safe but not effective in malaria (17–20). In addition, MB showed a gametocytocidal effect that appeared to act on both existing and developing P. falciparum gametocytes (7).
Acknowledgments
The conclusions of this article were not financially influenced.
Footnotes
Published ahead of print on 22 February 2011.
REFERENCES
- 1. Ademowo O. G., Nneji C. M., Adedapo A. D. A. 2007. In vitro antimalarial activity of methylene blue against field isolates of Plasmodium falciparum from children in Southwest Nigeria. Indian J. Med. Res. 126:45–49 [PubMed] [Google Scholar]
- 2. Akoachere M., et al. 2005. In vitro assessment of methylene blue on chloroquine-sensitive and-resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob. Agents Chemother. 49:4592–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alibert-Franco S., Pradines B., Mahamoud A., Davin-Regli A., Pagès J. M. 2009. Efflux mechanism, an attractive target to combat multidrug resistant Plasmodium falciparum and Pseudomonas aeruginosa. Curr. Med. Chem. 16:301–317 [DOI] [PubMed] [Google Scholar]
- 4. Anonymous. 1900. Methylene blue in grave malaria cachexia. JAMA 34:1409. [DOI] [PubMed] [Google Scholar]
- 5. Atamna H., et al. 1996. Mode of antimalarial effect of methylene blue and some of its analogues on Plasmodium falciparum in culture and their inhibition of P. vinckei petteri and P. yoelii nigeriensis in vivo. Biochem. Pharmacol. 51:693–700 [DOI] [PubMed] [Google Scholar]
- 6. Bogreau H., et al. 2006. Genetic diversity and structure of African Plasmodium falciparum populations in urban and rural areas. Am. J. Trop. Med. Hyg. 74:953–959 [PubMed] [Google Scholar]
- 7. Coulibaly B., et al. 2009. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomized controlled trial. PLoS One 4:5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dokomajilar C., Nsobya S. L., Greenhouse B., Rosenthal P. J., Dorsey G. 2006. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob. Agents Chemother. 50:1893–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferdig M. T., et al. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985–997 [DOI] [PubMed] [Google Scholar]
- 10. Garavito G., et al. 2007. Blood schizontocidal activity of methylene blue in combination with antimalarials against Plasmodium falciparum. Parasite 14:135–140 [DOI] [PubMed] [Google Scholar]
- 11. Guttmann P., Ehrlich P. 1891. Ueber die wirkung des methylenblau bei malaria. Berlin Klin. Wochenschr. 28:953–956 [Google Scholar]
- 12. Henry M., et al. 2006. Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am. J. Trop. Med. Hyg. 75:146–151 [PubMed] [Google Scholar]
- 13. Henry M., Alibert S., Rogier C., Barbe J., Pradines B. 2008. Inhibition of efflux of quinolines as new therapeutic strategy in malaria. Curr. Top. Med. Chem. 8:563–568 [DOI] [PubMed] [Google Scholar]
- 14. Henry M., et al. 2006. Chloroquine resistance reversal agents as promising antimalarial drugs. Curr. Drug Targets 7:935–948 [DOI] [PubMed] [Google Scholar]
- 15. Henry M., et al. 2008. In vitro activity of ferroquine is independent of polymorphisms in transport protein genes implicated in quinoline resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 52:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henry M., et al. 2009. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob. Agents Chemother. 53:1926–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandi G., et al. 2005. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop. Med. Int. Health 10:32–38 [DOI] [PubMed] [Google Scholar]
- 18. Meissner P. E., et al. 2008. Marked difference in the prevalence of chloroquine resistance between urban and rural communities in Burkina Faso. Acta Trop. 105:81–86 [DOI] [PubMed] [Google Scholar]
- 19. Meissner P. E., et al. 2006. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar. J. 5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meissner P. E., et al. 2005. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso [ISRCTN27290841]. Malar. J. 4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parquet V., et al. 2009. Atorvastatin is a promising partner for antimalarial drugs in treatment of Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 53:2248–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pradines B., et al. 2010. Quinine-resistant malaria in traveler returning from Senegal, 2007. Emerg. Infect. Dis. 16:546–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price R. N., et al. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ursing J., Kofoed P. E., Rodrigues A., Rombo L., Gil J. P. 2007. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am. J. Trop. Med. Hyg. 76:844–848 [PubMed] [Google Scholar]
- 25. Ursing J., Zakeri S., Gil J. P., Bjorkman A. 2006. Quinoline resistance associated polymorphisms in the pfcrt, pfmdr1 and pfmrp genes of Plasmodium falciparum in Iran. Acta Trop. 97:352–356 [DOI] [PubMed] [Google Scholar]
- 26. Vennerstrom J. L., Mackler M. T., Angerhofer C. K., Williams J. A. 1995. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob. Agents Chemother. 39:2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wurtz N., et al. 2010. Synergy of mefloquine activity with atorvastatin, but not chloroquine and monodesethylamodiaquine, and association with the pfmdr1 gene. J. Antimicrob. Chemother. 65:1387–1394 [DOI] [PubMed] [Google Scholar]
- 28. Zoungrana A., et al. 2008. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS One 3:1630. [DOI] [PMC free article] [PubMed] [Google Scholar]