Abstract
Stem cells have the potential for self-renewal and differentiation. First stem cell cultures were derived 30 years ago from early developing mouse embryos. These are pluripotent embryonic stem (ES) cells. Efforts towards ES cell derivation have been attempted in other mammalian and non-mammalian species. Work with stem cell culture in fish started 20 years ago. Laboratory fish species, in particular zebrafish and medaka, have been the focus of research towards stem cell cultures. Medaka is the second organism that generated ES cells and the first that gave rise to a spermatogonial stem cell line capable of test-tube sperm production. Most recently, the first haploid stem cells capable of producing whole animals have also been generated from medaka. ES-like cells have been reported also in zebrafish and several marine species. Attempts for germline transmission of ES cell cultures and gene targeting have been reported in zebrafish. Recent years have witnessed the progress in markers and procedures for ES cell characterization. These include the identification of fish homologs/paralogs of mammalian pluripotency genes and parameters for optimal chimera formation. In addition, fish germ cell cultures and transplantation have attracted considerable interest for germline transmission and surrogate production. Haploid ES cell nuclear transfer has proven in medaka the feasibility of semi-cloning as a novel assisted reproductive technology. In this special issue on “Fish Stem Cells and Nuclear Transfer”, we will focus our review on medaka to illustrate the current status and perspective of fish stem cells in research and application. We will also mention semi-cloning as a new development to conventional nuclear transfer.
Keywords: chimera, gene targeting, germ cell, medaka, stem cell, pluripotency, semi-cloning
INTRODUCTION
A stem cell can divide to produce many daughter cells that are identical to each other and to their mother stem cell. This is called self-renewal. A stem cell is also capable of producing specialized cells. This is the developmental potential. The ability of self-renewal and differentiation are the defining properties making stem cells to differ from other cells in the body. Since the establishment of first embryonic stem (ES) cells in mice, stem cells have been the focus of active research because of their enormous potential for basic research, medicine and animal biotechnology.
Work with fish stem cell culture has experienced 20 years. Several reviews have provided the status of fish stem cells 1-3. In this paper, we want to provide a timely update of fish stem cell cultures. After a brief overview on the history of stem cell research, we will focus on the basic knowledge and recent advances of fish stem cell cultures and biotechnology, in particular on haploid ES cell derivation, germ cell culture and transplantation, gene targeting and semi-cloning.
ES cells and ES cell technology
The success in ES cell culture benefited from the experience working with germ cell tumors or teratocarcinomas 4. These tumors consist of undifferentiated cells and differentiated cells of the three germ layers. In culture, these tumors develop into embryonal carcinoma (EC) cells 5. In 1981, two labs independently demonstrated that the inner cell mass (ICM) of mouse blastocyst embryos can - without a tumor formation step - give rise to EK cells or embryo-derived stem cells, the first pluripotent ES cells 6; 7. When injected into recipient mouse blastocysts, these embryo-derived cells gave rise to all tissue types including the germline, contributing to chimeric animals in vivo 8. They could also be induced to differentiate in vitro in the presence of retinoic acid, form embryoid bodies (EB), and shared immunological markers with the ICM of the blastocyst.
Pluripotent mouse ES cells were used to develop the ES cell technology. Homologous recombination (HR) between an endogenous chromosomal copy and a modified version introduced in the form of a HR vector allows for gene targeting (GT), the precise gene replacement at the specific site in the ES cell genome 9. These genetically modified ES cells were introduced back into recipient mouse embryos at the blastocyst stage, generating chimeric embryos. In the chimeras, the ES cells participated in normal embryogenesis and contributed to many organ systems including the germline, leading to the production of ES cell-derived whole animals. Intercrossing between germline chimeras can produce mice heterozygous and homozygous for the desired mutation. Such homozygous mutants are the so-called knockout mice 10. To date, GT is a routine in mouse developmental genetics. In this regard, ES cells are superior to EC cells in the efficiency of germline chimera formation.
The establishment and potential of ES cells and ES cell technology attracted considerable efforts towards the generation of ES cells in many other animals. Ultimately, human ES cells came into being in 1998 11. Like the mouse counterparts, human ES cells have a normal karyotype, express high telomerase activity and cell surface markers characteristic of pluripotent stem cells. Importantly, these human ES cells maintain the developmental potential to form various derivatives of all three embryonic germ layers. The pluripotency makes human ES cells a versatile resource for producing many different cell types in large quantity for the analysis of human development in vitro and more importantly, for cell therapy in regenerative medicine.
FISH ES CELL CULTURE
Mouse ES cells originate from the ICM cells, which tend to differentiate spontaneously. Spontaneous differentiation represents a major challenge for ES cell derivation. Inhibition of spontaneous differentiation in mice is achieved by using a layer of so-called feeder cells 6 or conditioned medium 7. Since the advent of first mouse ES cells, attempts have been made towards ES cell derivation in many different mammalian species. These early attempts were only partially successful in short-term ES cell culture or unsuccessful, raising a question as to whether the ability to achieve ES cells was limited to mouse or whether the culture conditions applied for mouse ES cells were not appropriate to inhibit spontaneous differentiation in other mammalian species.
Work towards fish ES cells dates 20 years ago, by adopting the feeder layer technique in zebrafish 12 and medaka 13. Independently, we developed a feeder-free culture condition for fish ES cell derivation from medaka, leading to the establishment of three ES cell lines MES1 to MES3 in 1996 14. Since mouse is one of the most advanced vertebrates, and medaka is among the most primitive vertebrates, the success in medaka ES cells provided direct evidence for the general possibility to derive ES cells in diverse vertebrate species. Indeed, two years later, human ES cells were obtained from the ICM of the blastocyst of an early-staged embryo 15.
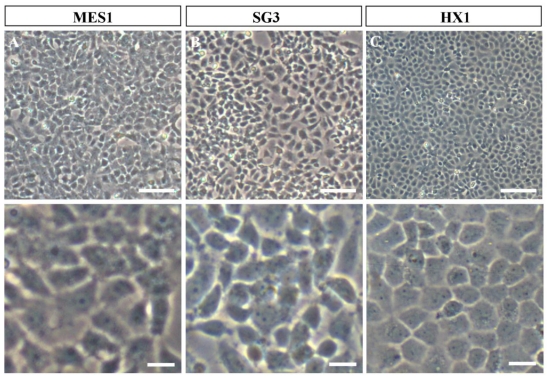
In our feeder-free culture system, an essential component of ES cell-conducive medium is the fish embryo extract from medaka, which together with basic fibroblast growth factor and fish serum is able to support self-renewal of disassociated midblastula embryo (MBE) cells on a gelatin-coated culture dish 14. MES1 was chosen for detailed characterization over 1 year of culture with more than 100 passages and found to display all features characteristic to mouse ES cells. These include stable growth, a typical ES cell phenotype (a round/polygonal shape, a small size, large nuclei and prominent nucleoli) (as illustrated in Fig. 1A), high alkaline phosphatase activity (a general marker of mouse ES cells), a normal karyotype and the ability for spontaneous differentiation into various cell types including pigment cells, muscle cells, nerve cells and fibroblasts 14. Importantly, MES1 can undergo clonal growth, forming compacted cell colonies of undifferentiated ES cells capable of expansion into ES cell clones.
Fig 1.
Phenotypes of growing medaka stem cell lines in culture. All cells were grown on gelatin-coated substrata in the absence of feeder layer cells. Similar phenotypes are seen for all the three cell lines. (A) MES1, a diploid ES cell line from fertilization blastulae. (B) SG3, a male germ stem cell line from the adult testis. (C) HX1, haploid ES cell line from gynogenetic blastulae. Scale bars, 50 μm (top) and 10 μm (bottom).
Derivation and initial characterization by using above criteria represent a first step in the derivation of new ES cells. Several independent experiments are required to investigate the pluripotency in vitro and in vivo, which in mouse ES cell culture is usually manifested by differentiation, in particular induced differentiation via EB formation. ES cells in a monolayer are dissociated into single cells and small clusters, seeded in bacteriological dishes for suspension culture to allow them to form cell aggregates that in turn develop into three-dimensional spheres called EB. EB may display similar structures to early developing embryos consisting of three germ layers. This way, ES cells within the EB are induced to undergo differentiation into various cell types. MES1 can form EB and shows apparent differentiation as evidenced by enhanced melanocyte differentiation 14 and expression of differentiation genes (see below). MES1 is easy for directed differentiation. When transfected with a vector expressing the microphthalmia transcription factor (mitf), MES1 specifically develops into melanocytes by morphology, melanosome motility and gene expression patterns of completely differentiated pigment cells 16. Therefore, medaka ES cells represent an excellent model for directed differentiation.
Undifferentiated mouse ES cells specifically express a set of the so-called pluripotency genes, such as oct4, nanog, klf4, sox2, myc, ronin, sall4, tcf3 (tcf7l1) 17. In mammals, the expression of these pluripotency genes has routinely been used to characterize stem cell cultures at the molecular level. Their homologs/paralogs are present also in fish. For example, medaka homologs/paralogs of the nanog 18, oct4 19 and other genes have been identified 20. Evidence for these genes as pluripotency markers in vitro came from the observation in medaka haploid ES cells that the expression of nanog and oct4 is strong in undifferentiated culture but dramatically down-regulated and even lost upon induced differentiation 20. In this issue, Wang et al. report that a set of seven medaka pluripotency genes (e.g. nanog and oct4), which all exhibit strong expression in medaka diploid ES cells 21. Rao et al. report that the lack of expression of tert splicing variants is also a pluripotency indicator of undifferentiated ES cells, although telomerase activity and expression of tert RNA (encoding the telomerase reverse transcriptase, namely the catalytic subunit of telomerase) is versatile in vivo and present in all the seven stable medaka cell lines 22. These data suggest that the pluripotency genes and telomerase activity are general markers for ES cell cultures from fish to mammals.
Mouse ES cells upon differentiation express molecular markers of various lineages and cell types. Expression of these so-called differentiation genes is often used to detect ES cell differentiation by RT-PCR analysis. These include ectodermal markers nf200, mesodermal markers ntl and atn2, endodermal markers sox17 and hnf3b, and neural crest markers sox10 and mitf. Interestingly, the expression of these genes becomes activated or dramatically up-regulated in medaka diploid and haploid ES cells following induced differentiation 20.
To detect differentiation at the single cell level, immunostaining is a routine in mammalian ES cell culture. Here, ES cells are subject to suspension culture for EB formation to induce differentiation. After several days of suspension culture, EBs are replated to adherent culture, and single cells become clearly visible. After fixation, these cells are detected in situ by using antibodies against markers specific to particular types of cells. Many antibodies against mammalian molecules are commercially available. For use in fish, these antibodies must be tested for cross-activity and staining specificity. Recently, we have demonstrated the suitability of this immunochemistry approach in medaka ES cells 20. Specifically, five commercial antibodies delivered clear staining. They are against NF200 and Map2, glial fibrillary acidic protein (GFAP), panCK and Actinin2, which detect neurons, astrocytes, squamous epithelia and striated mature muscles, respectively.
Pluripotency in vivo is evaluated by ES cell transplantation into early developing embryos for chimera formation, a broadly used method to test the developmental pluripotency of stem cells in vivo. Wakamatsu and colleagues initially established the conditions for chimera formation on the basis of the procedure developed for transplanting non-cultivated medaka blastomeres obtained from a deep layer 23. The resulting chimeras produced by transplanting non-cultivated medaka blastomeres from whole blastulae displayed donor-derived wild-type melanocytes 24. Interestingly, we have recently found that the host accessibility to ES cell contribution displays dramatic strain differences 25. For example, MES1 produces melanocytes in i1 but not i3 albino strain. More intriguingly, upon γ-irradiation, i1 becomes inaccessible and i3 becomes accessible for MES1 contribution into melanocytes. Hence, for chimera formation by using pigmentation as a marker of ES cell differentiation, different host strains should be tested. Similar studies have also been carried out in the mouse. Subsets of the 129, PO, and CBA/Ca ES lines, as well as the single C57BL/6 line, were assayed for chimera formation and germline transmission by blastocyst injection. Satisfactory rates of chimerism and germ-line transmission were obtained with all three genotypes of ES cells, with the profound effect that the choice of the genotype of the host blastocyst is evident for the efficiency of chimerism and germline transmission 26.
Besides a suitable culture condition conducive to ES cell self-renewal rather than differentiation, there are strain differences for ES cell derivation. A survey in medaka demonstrated that only 5 out of 11 strains and species are permissive for ES cell culture, one of them is HB32C from which MES1 originated 24. Therefore, the initial success in the first medaka ES cells was a lucky event in terms of having chosen a permissive strain. Our recent work has added i1 to the list of permissive strains (see below). This phenomenon mimics the situation in the mouse. Mouse ES cells were derived most frequently from the permissive strain 129, although ES cells were successfully isolated also from non-permissive strains such as CBA/Ca 26; 27.
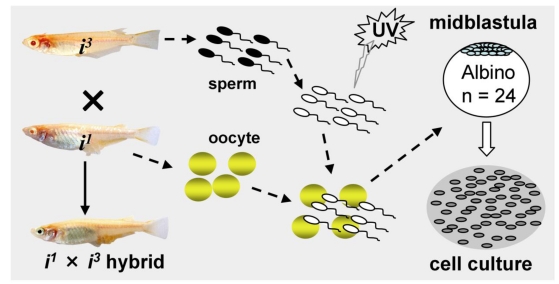
Most recently, we have obtained first ever haploid ES cell lines HX1 to HX3 from medaka 20. The condition for medaka haploid ES cell derivation is similar to that for diploid MES1, but by using haploid blastula embryos for cell initiation instead of fertilization diploid blastulae (Fig. 2). Sperm of i3 strain are treated with an elaborated dose of ultra-violet light irradiation to such an extent to destroy their nuclei but retain their ability to trigger egg activation. These genetically inactivated sperm are mixed with mature oocytes of i1 strain for artificial insemination, resulting in eggs only with a haploid female nucleus. Such embryos undergo all-female embryogenesis called gynogenesis. Until the midblastula stage, the gynogenetic haploid embryos were dissociated into single cells and seeded for feeder-free culture on gelatin-coated substrata. Details for induced haploid gynogenesis and cell derivation have been described 28. Notably, haploid ES cell derivation is more demanding and additionally requires media conditioned by MES1 and MO1, a medaka ovary-derived cell line. However, once established, haploid ES cells are not different from diploid ES cells in conditions for maintenance. Haploid ES cells (Fig. 1C) show all characteristics of MES1, including stable and competitive growth, genetic stability and pluripotency in vitro and in vivo 20.
Fig 2.
Procedures for medaka haploid ES cell derivation. UV-irradiation destroys the genetic material of sperm without compromising their ability to trigger egg activation for haploid gynogenesis. Haploid embryos are dissociated at the midblastula stage into single cells for cell culture initiation.
Mammalian ES cells are usually derived from the ICM or epiblast of blastocyst-stage embryos. The two extraembryonic lineages at this stage can also give rise to stem cell cultures, namely trophoblast stem cells from the trophectoderm 29 and primitive endoderm stem cells 30. None of these stem cells is totipotent in gene expression profile and lineage contribution in chimeric embryos. It is unknown whether embryos prior to the blastocyst stage can be cultivated towards totipotent stem cell cultures. In this issue, Li et al. report that even 32-cell cleavage embryos of medaka are capable of generating ES-like cell cultures 31, pointing to the possibility to derive stem cell culture from fish embryos at earlier stages.
ES cell cultures in Zebrafish and other fish species
Collodi's lab majors in zebrafish ES cell culture and has obtained cell cultures from blastula-stage embryos by adopting the mouse feeder layer technique. The feeder cells they have used include zebrafish embryonic fibroblasts 12, buffalo rat liver cells 32 and the rainbow trout spleen cell line RTS34st 33; 34. They also demonstrated that short-term zebrafish embryo cell cultures were able to produce germline chimeras 34. The germline competence was further extended to several weeks and multiple passages in culture 35. By using the feeder-free culture condition, we have also obtained a zebrafish cell line from the blastula-stage embryos, which is being investigated for pluripotency (unpublished data). Therefore, both feeder layer and feeder-free conditions seem to work in zebrafish ES cell culture.
The application of feeder-free culture system has led to the establishment of ES-like cells in several marine fish species, including the gilthead sea bream (Sparus aurata) 36, red sea bream (Pagrus major) 37, sea perch (Lateolabrax japonicus) 38, Asian sea bass (Lates calcarifer) 39, and Atlantic cod (Gadus morhua) 40. Long-term culturing of turbot (Scophtalmus maximus) ES-like cells on gelatin-coated substrata was also demonstrated 41.
FISH GERM CELL CULTURE
Germ cells including primordial germ cells (PGCs) and gonadal germ stem cells - spermatogonia in the testis and oogonia in the ovary - have attracted considerable interest in the development of germline-competent stem cell cultures. A first success in fish germ stem cell culture is the establishment of SG3, a cell line of male germ stem cells spermatogonia we have generated from a mature medaka testis 42. SG3 originates from normal spermatogonia without immortalization, which has been reported as a prerequisite for mouse spermatogonial culture 43. SG3 (Fig. 1B) retains stable proliferation, diploid karyotype, and phenotype and gene expression pattern characteristic of spermatogonial stem cells. Under appropriate culture conditions, SG3 undergoes meiosis and spermiogenesis to generate motile sperm in the test tube 42. Therefore, the ability of continuous proliferation and sperm production in culture is an intrinsic property of medaka spermatogonial stem cells and that immortalization apparently is not required for deriving male germ cell cultures. The culture condition used for SG3 derivation is similar to that for medaka ES cell derivation, strongly suggesting the suitability of the feeder-free culture conditions for cultivating fish stem cells of different sources.
The success in the medaka spermatogonial stem cell culture has provoked an increasing interest in other species. For example, two years after establishment of SG3, pluripotent spermatogonial stem cells were derived from adult mouse testis without immortalization 44. In fish, transplantable type-A spermatogonia isolated from a trout transgenic line have been found to maintain the survival and mitotic activity in culture 45. Most recently, medaka female germ stem cells have been identified in the ovary 46, which raises the possibility to develop female germ stem cell cultures in this organism. In addition, interest is increasing in the derivation of gonadal somatic cell cultures towards the analysis of soma-germ cell interactions in vitro. For instance, a somatic cell line has been established from a juvenile testis of the half-smooth tongue sole, Cynoglossus semilaevis 47.
Primordial germ cells
The first stem cells of the germline are PGCs, which are set aside early in development 48. They solely colonize the gonad for egg or sperm production. PGCs hold promise to develop into pluripotent stem cell cultures. Since the identification of zebrafish vasa as a molecular marker of fish germ cells 49, interest and progress in fish germ cell biology have been steadily increasing. Homologs of vasa as well as boule, dazl, dead end and nanos have been identified in several fish species 50. More importantly, the vasa and/or nanos promoter have been successfully used for the generation of transgenic lines in several fish species including medaka 51, zebrafish 52 and trout 53. These transgenic fishes express green (GFP) or red fluorescent protein (RFP) specifically in germ cells including PGCs, allowing for visualization of PGCs in developing embryos, and more importantly, easy isolation for culture. We have shown that medaka PGCs from early gastrula embryos can survive, divide and move around 51. PGCs from a zebrafish vasa::RFP transgenic line can be cultured for up to 4 months 52. In medaka, dissociated cells from early gastrulae show PGC survival, proliferation and motility 51. Future work is needed to determine whether fish PGCs can give rise to stable cell cultures and retain the ability for germline transmission.
STEM CELL TECHNOLOGIES
Fish stem cells have the potential for use in various biotechnologies. Among them, gene targeting, germ cell transplantation and semi-cloning by nuclear transfer have attracted considerable interest and progress.
Gene targeting
GT combined with the mouse ES cells serve as the basis of the knockout technology. Though GT events are highly desirable, they are rare compared to random integration. A procedure called positive-negative selection (PNS) on the basis of drug selection can greatly facilitate the elimination of random events, leading to enrichment for homologous recombinants in mouse ES cells 54. Conditions for gene transfer and drug selection in MES1 have been optimized as a step towards gene targeting in fish. It was found that expression of selectable genes encoding resistance for neomycin (neo), hygromycin (hyg) or puromycin (pac) conferred indeed resistance to G418, hygromycin or puromycin for positive selection, while the herpes simplex virus thymidine kinase (tk) expression provided sensitivity to gancyclovir for negative selection. Therefore, PNS works also in MES1 55. More importantly, MES1 cells after gene transfer and long-term drug selection retain the developmental pluripotency 56. MES1 cells seem to possess adequate homologous recombination (HR) activity as evidenced by the appearance of sister-chromatid exchanges following sister-chromatid differential staining. The presence of cellular HR activity is a prerequisite to GT. Recently, we have shown that baculovirus transduction leads to a high efficiency of gene delivery into medaka ES cells without compromising pluripotency 57. By using a GT vector containing medaka p53 gene as a model, HR cell clones have reproducibly been obtained (data unpublished), demonstrating the possibility of ES cell technology in medaka. Collodi and his colleagues reported successful GT by HR in zebrafish ES cells 58.
Germ cell transplantation
Yoshizaki's laboratory majors in germ cell transplantation for surrogate production in salmonids. For this, they cloned the trout vasa, and used the vasa promoter to drive GFP expression exclusively in PGCs and gonadal germ cells to generate a transgenic line. They established procedures for isolating PGCs from transgenic larvae and adult germ cells from the testis and ovary for transplantation into trout and salmon. They transplanted these germ cells into the PGC migratory route of developing trout and salmon embryos, thereby obtained germline chimeras that produced offspring from transplanted germ cells in a homologous and heterologous host. Interestingly, transplanted germ cells produce sperm or eggs according to the host sex but independently of the sex of their origin. These pioneer experiments demonstrate the possibility of surrogate production of aquaculture broodstock by germ cell transplantation. Future work will further underscore the importance of this approach by establishing the ability to cultivate and cryopreserve germ cells. For example, germ cells could be transplanted between two different salmonid species, resulting in production of donor-derived offspring 59. Testicular germ cells containing spermatogonial stem cells isolated from adult male rainbow trout (Oncorhynchus mykiss) were transplanted into the peritoneal cavity of newly hatched fry of both sexes. By differentiating into spermatozoa in male recipients and fully functional eggs in female recipients, they were able to produce normal offspring 60. Further work involved transplantation of spermatogonia of rainbow trout into induced triploid fry of masu salmon. These transplanted spermatogonia underwent spermatogenesis in male but oogenesis in female recipients. The triploid salmon recipients are sterile on themselves but produced only donor-derived sperm or eggs, thus trout offspring 61. This approach might be extended to propagate/restore a population of endangered species in conservation biology.
Semi-cloning
Although short-term zebrafish ES cell culture have been used for germline chimera production 34, germline transmission of long-term ES cell culture has not yet been achieved in fish. Zebrafish PGCs are specified cell-autonomously at as early as the 4-cell stage 49. Medaka appears to specify its PGCs also cell-autonomously 62. These raise a question whether fish ES cells lose their germline competence or whether the fish germline is inaccessible to ES cell contribution. As alternates to germline chimera production, nuclear transplantation has been developed in zebrafish 63; 64 and medaka 65. Transplantation of epithelial nuclei from feeding tadpoles into enucleated egg of the Xenopus led to fertile frogs 66; 67. In fish, nuclear transfer by using cultured cells succeeded in the carp as early as in 1986 (republished 68), exactly 10 years prior to the creation of Dolly - the first cloned sheep 69. In medaka, test-tube sperm production from long-term cultured spermatogonia is also possible 42, pointing to the possibility for artificial insemination in the future. The recent establishment of medaka haploid ES cells provides an excellent opportunity for direct nuclear transfer into normal eggs without the need for enucleation. This is semi-cloning (SC). SC was originally proposed as a novel artificial reproductive technology to combat human infertility, and has remained as a scientific fiction 70. In this approach, a mitotic haploid nucleus is transferred to a mature egg without removal of its nucleus, leading to the combination of a haploid somatic nucleus from one parent and a haploid gamete nucleus from the other parent. This is a mosaic egg. By transplanting haploid ES cell nuclei into non-enucleated mature oocytes, we generated Holly, the first semi-cloned fish in the world. Holly shows normal fertility and germline transmission over three generations, providing evidence that mosaic oocytes can generate viable and fertile offspring. Importantly, stable gene transfer does not compromise the ability of haploid ES cells for Holly production 20. In fish, SC represents a first approach for germline transmission of cultured cells even after genetic modifications. In this regard, it deserves to note that the efficiency of SC by haploid ES cell nuclear transfer is comparable to that of sperm nuclear transfer 71.
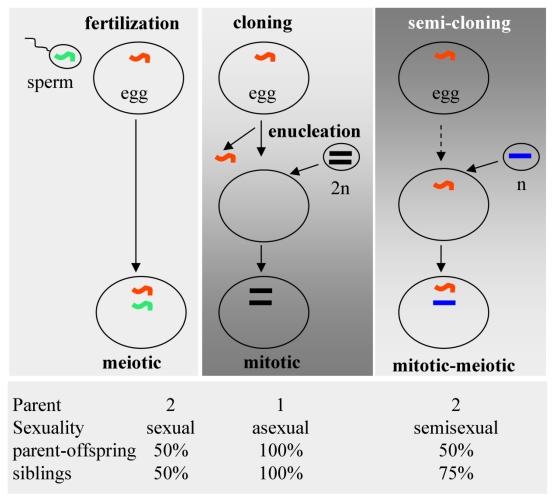
Compared to conventional cloning via somatic cell nuclear transfer (SCNT), SC features a high efficiency and biparental contribution (Fig. 3). Fertilization involves the fusion of a meiotic sperm nucleus with a meiotic egg nucleus. In SCNT, a somatic nucleus is transferred into an enucleated egg. Dolly was created this way. In the SC procedure, a haploid mitotic nucleus is transferred into a non-enucleated egg, producing a mitotic-meiotic mosaic egg (Fig. 3, top), as has been shown by the creation of Holly. Fertilization involves biparental contribution, with offspring being 50% genetically identical to either of parents and to each other. SCNT involves asexual reproduction, producing offspring 100% identical to the donor and to each other. SC combines a nucleus from one parent and a nucleus from the other, generating offspring 50% identical to both parents each but 75% identical to each other (Fig. 3, bottom). This is semisexual reproduction, which is more mimicking the natural fertilization process. Importantly, SC establishes a clear parent-progeny relationship, thus appears to be of less ethical concern for assisted reproduction to treat human infertility.
Fig 3.
Reproductive technologies. (Top) Schematic illustration of fertilization, classical cloning and semi-cloning. (Bottom) Parent-progeny relationships.
PERSPECTIVE
Differentiated mouse cells can be reprogrammed into induced pluripotent stem (iPS) cells by forced expression of pluripotency transcription factors 72. Researchers independently succeeded in obtaining iPS from human fibroblast cells 73, whereas iPS again can be directly differentiated, e.g. into hepatic cells using a limited number of cytokines 74. It will be interesting to see whether the use of enforced expression of transcription factors can also produce iPS cells in fish. Fish germ cell biology and biotechnology will continue to progress in the near future towards stable culture capable of genetic alterations, cryostorage and germline transmission. It is anticipated that GT will fully be developed in fish, in particular in medaka by using its haploid ES cells for direct genetic phenotypic analyses of null mutations in vitro and for physiological elucidation of gene function in vivo following animal production by SC. Recent progress in fish stem cell culture and transplantation will provide valuable systems and tools for basic research and applications in sustainable aquaculture.
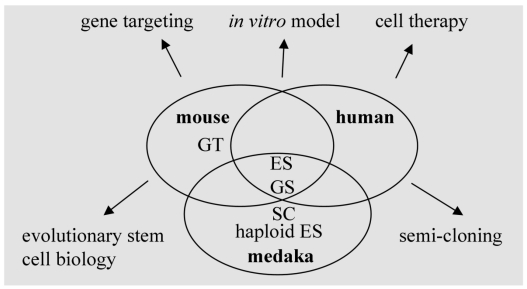
Mouse ES cells show salient differences in growth requirement from their human counterparts. Several organisms with different positions in vertebrate evolution are required to reveal species-specific factors and general mechanisms controlling pluripotency. In this regard, fish models as lower vertebrates represent an important reference to study the conserved mechanisms underlying self-renewal and differentiation (Fig. 4). Future work on the analyses of transcriptomes and proteomes of fish stem cells of various origins will provide valuable information for stem cell research and applications.
Fig 4.
Stem cells and stem cell technology in human and model organisms. ES, embryonic stem cells; GS, germ stem cells; haploid ES, haploid ES cells; GT, gene targeting; SC, semi-cloning.
Acknowledgments
We thank Deng Jiaorong for fish feeding, Drs M. Yi, M. Li and Y. Yan for comments on the manuscript. This work was supported by the Biomedical Research Council of Singapore (R-08-1-21-19-585 and SBIC-SSC-002-2007).
References
- 1.Alvarez MC, Bejar J, Chen S, Hong Y. Fish es cells and applications to biotechnology. Mar Biotechnol (NY) 2007;9:117–127. doi: 10.1007/s10126-006-6034-4. [DOI] [PubMed] [Google Scholar]
- 2.Barnes DW, Parton A, Tomana M, Hwang JH. et al. Stem cells from cartilaginous and bony fish. Methods Cell Biol. 2008;86:343–367. doi: 10.1016/S0091-679X(08)00016-2. [DOI] [PubMed] [Google Scholar]
- 3.Fan L, Collodi P. Zebrafish embryonic stem cells. Methods Enzymol. 2006;418:64–77. doi: 10.1016/S0076-6879(06)18004-0. [DOI] [PubMed] [Google Scholar]
- 4.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: Formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin GR. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209:768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 9.Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 10.Capecchi MR. Generating mice with targeted mutations. Nat Med. 2001;7:1086–1090. doi: 10.1038/nm1001-1086. [DOI] [PubMed] [Google Scholar]
- 11.Prelle K, Zink N, Wolf E. Pluripotent stem cells--model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31:169–186. doi: 10.1046/j.1439-0264.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 12.Collodi P, Kamei Y, Ernst T, Miranda C. et al. Culture of cells from zebrafish (brachydanio rerio) embryo and adult tissues. Cell Biol Toxicol. 1992;8:43–61. doi: 10.1007/BF00119294. [DOI] [PubMed] [Google Scholar]
- 13.Wakamatsu Y, Ozato K, Sasado T. Establishment of a pluripotent cell line derived from a medaka (oryzias latipes) blastula embryo. Mol Mar Biol Biotechnol. 1994;3:185–191. [PubMed] [Google Scholar]
- 14.Hong Y, Winkler C, Schartl M. Pluripotency and differentiation of embryonic stem cell lines from the medakafish (oryzias latipes) Mech Dev. 1996;60:33–44. doi: 10.1016/s0925-4773(96)00596-5. [DOI] [PubMed] [Google Scholar]
- 15.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA. et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 16.Bejar J, Hong Y, Schartl M. Mitf expression is sufficient to direct differentiation of medaka blastula derived stem cells to melanocytes. Development. 2003;130:6545–6553. doi: 10.1242/dev.00872. [DOI] [PubMed] [Google Scholar]
- 17.Do JT, Scholer HR. Regulatory circuits underlying pluripotency and reprogramming. Trends Pharmacol Sci. 2009;30:296–302. doi: 10.1016/j.tips.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Camp E, Sanchez-Sanchez AV, Garcia-Espana A, Desalle R. et al. Nanog regulates proliferation during early fish development. Stem Cells. 2009;27:2081–2091. doi: 10.1002/stem.133. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Sánchez AV, Camp E, Mullor JL. Fishing Pluripotency Mechanisms In Vivo. Int J Biol Sci. 2011;7:410–417. doi: 10.7150/ijbs.7.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi M, Hong N, Hong Y. Generation of medaka fish haploid embryonic stem cells. Science. 2009;326:430–433. doi: 10.1126/science.1175151. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Manali D, Wang T, Bhat N, Hong N, Li Z, Wang L, Yan Y, Liu R, Hong Y. Identification of Pluripotency Genes in the Fish Medaka. Int J Biol Sci. 2011;7:440–451. doi: 10.7150/ijbs.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao F, Wang T, Li M, Li Z, Hong N, Zhao H, Yan Y, Lu W, Yuan Y, Liu L, Guan G, Li C, Hong Y. Medaka tert produces multiple variants with differential expression during differentiation in vitro and in vivo. Int J Biol Sci. 2011;7:426–439. doi: 10.7150/ijbs.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakamatsu Y, Ozato K, Hashimoto H, Kinoshita M. et al. Generation of germ-line chimeras in medaka (Oryzias latipes) Mol Mar Biol Biotechnol. 1993;2:13. [Google Scholar]
- 24.Hong Y, Winkler C, Schartl M. Efficiency of cell culture derivation from blastula embryos and of chimera formation in the medaka (Oryzias latipes) depends on donor genotype and passage number. Dev Genes Evol. 1998;208:595–602. doi: 10.1007/s004270050220. [DOI] [PubMed] [Google Scholar]
- 25.Hong N, Li M, Zeng Z, Yi M. et al. Accessibility of host cell lineages to medaka stem cells depends on genetic background and irradiation of recipient embryos. Cell Mol Life Sci. 2010;67(7):1189–1202. doi: 10.1007/s00018-009-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci U S A. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledermann B, Burki K. Establishment of a germ-line competent c57bl/6 embryonic stem cell line. Exp Cell Res. 1991;197:254–258. doi: 10.1016/0014-4827(91)90430-3. [DOI] [PubMed] [Google Scholar]
- 28.Yi M, Hong N, Hong Y. Derivation and characterization of haploid embryonic stem cell cultures in medaka fish. Nat Protoc. 2010;5:1418–1430. doi: 10.1038/nprot.2010.104. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by fgf4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 30.Debeb BG, Galat V, Epple-Farmer J, Iannaccone S. et al. Isolation of oct4-expressing extraembryonic endoderm precursor cell lines. PLoS One. 2009;4:e7216. doi: 10.1371/journal.pone.0007216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Bhat N, Manali D, Wang D, Hong N, Yi M, Ge R, Hong Y. Medaka Cleavage Embryos Are Capable of Generating ES-Like Cell Cultures. Int J Biol Sci. 2011;7:418–425. doi: 10.7150/ijbs.7.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Bradford CS, Ghosh C, Collodi P, Barnes DW. Es-like cell cultures derived from early zebrafish embryos. Mol Mar Biol Biotechnol. 1995;4:193–199. [PubMed] [Google Scholar]
- 33.Fan L, Crodian J, Collodi P. Culture of embryonic stem cell lines from zebrafish. Methods Cell Biol. 2004;76:151–160. doi: 10.1016/s0091-679x(04)76009-4. [DOI] [PubMed] [Google Scholar]
- 34.Ma C, Fan L, Ganassin R, Bols N, Collodi P. Production of zebrafish germ-line chimeras from embryo cell cultures. Proc Natl Acad Sci U S A. 2001;98:2461–2466. doi: 10.1073/pnas.041449398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan L, Crodian J, Liu X, Alestrom A. et al. Zebrafish embryo cells remain pluripotent and germ-line competent for multiple passages in culture. Zebrafish. 2004;1:21–26. doi: 10.1089/154585404774101644. [DOI] [PubMed] [Google Scholar]
- 36.Bejar J, Hong Y, Alvarez MC. An ES-like cell line from the marine fish sparus aurata: Characterization and chimaera production. Transgenic Res. 2002;11:279–289. doi: 10.1023/a:1015678416921. [DOI] [PubMed] [Google Scholar]
- 37.Chen SL, Ye H, Sha Q, Shi CY. Derivation of a pluripotent embryonic cell line from red sea bream blastulas. J Fish Biol. 2003;63:10. [Google Scholar]
- 38.Chen SL, Sha ZX, Ye HQ, Liu Y. et al. Pluripotency and chimera competence of an embryonic stem cell line from the sea perch (Lateolabrax japonicus) Mar Biotechnol (NY) 2007;9:82–91. doi: 10.1007/s10126-006-6050-1. [DOI] [PubMed] [Google Scholar]
- 39.Parameswaran V, Shukla R, Bhonde R, Hameed AS. Development of a pluripotent ES-like cell line from Asian sea bass (Lates calcarifer)--an oviparous stem cell line mimicking viviparous es cells. Mar Biotechnol (NY) 2007;9:766–775. doi: 10.1007/s10126-007-9028-y. [DOI] [PubMed] [Google Scholar]
- 40.Holen E, Kausland A, Skjaerven K. Embryonic stem cells isolated from atlantic cod (Gadus morhua) and the developmental expression of a stage-specific transcription factor ac-pou2. Fish Physiol Biochem. 2010;36(4):1029–39. doi: 10.1007/s10695-010-9381-z. [DOI] [PubMed] [Google Scholar]
- 41.Holen E, Hamre K. Towards obtaining long term embryonic stem cell like cultures from a marine flatfish, Scophthalmus maximus. Fish Physiol Biochem. 2004;29:245–252. [Google Scholar]
- 42.Hong Y, Liu T, Zhao H, Xu H. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci U S A. 2004;101:8011–8016. doi: 10.1073/pnas.0308668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng LX, Chen Y, Dettin L, Pera RA. et al. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- 44.Guan K, Nayernia K, Maier LS, Wagner S. et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 45.Shikina S, Ihara S, Yoshizaki G. Culture conditions for maintaining the survival and mitotic activity of rainbow trout transplantable type a spermatogonia. Mol Reprod Dev. 2008;75:529–537. doi: 10.1002/mrd.20771. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Wang X, Sha Z, Yang C, Liu S, Wang N, Chen SL. Establishment and Characterization of a Testicular Cell Line from the Half-Smooth Tongue Sole, Cynoglossus semilaevis. Int J Biol Sci. 2011;7:452–459. doi: 10.7150/ijbs.7.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wylie CC. The biology of primordial germ cells. Eur Urol. 1993;23:62–66. doi: 10.1159/000474571. [DOI] [PubMed] [Google Scholar]
- 49.Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue rna is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Li M, Gui J, Hong Y. Fish germ cells. Sci China Life Sci. 2010;53:435–446. doi: 10.1007/s11427-010-0058-8. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Hong N, Xu H, Yi M. et al. Medaka vasa is required for migration but not survival of primordial germ cells. Mech Dev. 2009;126:366–381. doi: 10.1016/j.mod.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Fan L, Moon J, Wong TT, Crodian J, Collodi P. Zebrafish primordial germ cell cultures derived from vasa::Rfp transgenic embryos. Stem Cells Dev. 2008;17:585–597. doi: 10.1089/scd.2007.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizaki G, Takeuchi Y, Sakatani S, Takeuchi T. Germ cell-specific expression of green fluorescent protein in transgenic rainbow trout under control of the rainbow trout vasa-like gene promoter. Int J Dev Biol. 2000;44:323–326. [PubMed] [Google Scholar]
- 54.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: A general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 55.Chen S, Hong Y, Schartl M. Development of a positive-negative selection procedure for gene targeting in fish cells. Aquaculture. 2002;214:67–79. [Google Scholar]
- 56.Hong Y, Chen S, Gui J, Schartl M. Retention of the developmental pluripotency in medaka embryonic stem cells after gene transfer and long-term drug selection for gene targeting in fish. Transgenic Res. 2004;13:41–50. doi: 10.1023/b:trag.0000017172.71391.fa. [DOI] [PubMed] [Google Scholar]
- 57.Yan Y, Du J, Chen T, Yi M. et al. Establishment of medakafish as a model for stem cell-based gene therapy: Efficient gene delivery and potential chromosomal integration by baculoviral vectors. Exp Cell Res. 2009;315:2322–2331. doi: 10.1016/j.yexcr.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Fan L, Moon J, Crodian J, Collodi P. Homologous recombination in zebrafish es cells. Transgenic Res. 2006;15:21–30. doi: 10.1007/s11248-005-3225-0. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi Y, Yoshizaki G, Takeuchi T. Biotechnology: Surrogate broodstock produces salmonids. Nature. 2004;430:629–630. doi: 10.1038/430629a. [DOI] [PubMed] [Google Scholar]
- 60.Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci U S A. 2006;103:2725–2729. doi: 10.1073/pnas.0509218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science. 2007;317:1517. doi: 10.1126/science.1145626. [DOI] [PubMed] [Google Scholar]
- 62.Herpin A, Rohr S, Riedel D, Kluever N. et al. Specification of primordial germ cells in medaka (Oryzias latipes) BMC Dev Biol. 2007;7:3. doi: 10.1186/1471-213X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee KY, Huang H, Ju B, Yang Z, Lin S. Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nat Biotechnol. 2002;20:795–799. doi: 10.1038/nbt721. [DOI] [PubMed] [Google Scholar]
- 64.Siripattarapravat K, Pinmee B, Venta PJ, Chang CC, Cibelli JB. Somatic cell nuclear transfer in zebrafish. Nat Methods. 2009;6:733–735. doi: 10.1038/nmeth.1369. [DOI] [PubMed] [Google Scholar]
- 65.Wakamatsu Y, Niwa K, Kani S, Ozato K. Nuclear transplantation in medaka. Tanpakushitsu Kakusan Koso. 2000;45:2962–2966. [PubMed] [Google Scholar]
- 66.Gurdon JB. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J Embryol Exp Morphol. 1960;8:505–526. [PubMed] [Google Scholar]
- 67.Gurdon JB, Uehlinger V. "Fertile" intestine nuclei. Nature. 1966;210:1240–1241. doi: 10.1038/2101240a0. [DOI] [PubMed] [Google Scholar]
- 68.Chen H, Yi Y, Chen M, Yang X. Studies on the developmental potentiality of cultured cell nuclei of fish. Int J Biol Sci. 2010;6:192–198. doi: 10.7150/ijbs.6.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 70.Tsai MC, Takeuchi T, Bedford JM, Reis MM. et al. Alternative sources of gametes: Reality or science fiction? Hum Reprod. 2000;15:988–998. doi: 10.1093/humrep/15.5.988. [DOI] [PubMed] [Google Scholar]
- 71.Liu T, Liu L, Wei Q, Hong Y. Sperm Nuclear Transfer and Transgenic Production in the Fish Medaka. Int J Biol Sci. 2011;7:469–475. doi: 10.7150/ijbs.7.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 74.Wada N, Wang B, Lin NH, Laslett AL. et al. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J Periodontal Res. 2011 doi: 10.1111/j.1600-0765.2011.01358.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]