Abstract
Background
Most medullary thyroid carcinomas (MTC) recur or progress despite optimal surgical resection. Current targeted-therapies show promise but lack durable efficacy and tolerability. The purpose of this study was to build upon previous in vitro work and evaluate Withaferin A (WA), a novel RET inhibitor, in a metastatic murine model of MTC.
Methods
5 million DRO-81-1 human MTC-cells injected in the left posterior neck of Nu/Nu mice uniformly generated metastases to the liver, spleen, and/or lungs. Treatment with WA (8mg/kg/day i.p.×21 days) was started for tumors >100 mm3. Endpoints were survival, tumor>1500 mm3, decreased bodyweight, or body score (all measured thrice weekly).
Results
All controls (saline; n=5) died or deteriorated from metastatic disease by 7 weeks post injection. All treated animals were alive,(WA; n=5), having tumor regression and growth-delay without toxicity or weight-loss at 6 wks post treatment; p<0.01. Tumor cells treated with WA demonstrated inhibition of total and phospho-RET levels by Western-Blot analysis in a dose-dependent manner (almost complete inhibition with 5uM WA treatment) as well as potent inhibition of phospho-ERK and phospho-AKT levels.
Conclusions
Withaferin A is a novel natural-product RET-inhibitor with efficacy in a metastatic murine model of MTC. Further long-term efficacy/toxicity studies are warranted to evaluate this compound for clinical translation.
INTRODUCTION
Medullary thyroid cancers originate from neural crest-derived parafollicular C-cells in the thyroid gland. While they represent a small portion of thyroid malignancies (4–9%) they remain a challenge to treat as more than 50% will recur or progress despite optimal surgical therapy.1,2 Unlike well-differentiated thyroid cancers, these tumors are not sensitive to conventional radioiodine-based therapy and no survival benefit has been shown with radiation therapy. External beam radiation does, however, carry a high incidence of complications including radiation fibrosis and injury to the aerodigestive tract and vascular structures in the neck.3
Once a diagnosis of MTC is confirmed histologically by fine needle aspiration and a metastatic work-up is completed, patients should have primary surgical resection with lymph node evaluation. Total thyroidectomy is the appropriate treatment for the primary tumor, accompanied by a central node neck (levels VI and VII) dissection and possibly additional functional neck dissection (levels II–V) if there is concern for more cervical nodal involvement. The goal of this operation has been to remove all thyroid and nodal tissue of concern. Detection of recurrent disease is often made by the development of a palpable recurrence or by following patients postoperatively with serial serum calcitonin levels.2,4 Once recurrent disease is established, reoperation is the initial treatment of choice with adjuvant therapy trials reserved for patients who are not operative candidates.
Standard chemotherapy regimens with agents effective in other solid tumors such as doxorubicin, dacarbazine, irinotecan or capecitabine have limited efficacy in MTC. Response rates are often temporary and occur in less than 10–20% of patients without long-term benefit. Additionally, these drugs carry moderate systemic toxicities.5 Chemotherapy failure in some circumstances has been ascribed in part to the overexpression by MTC of the multidrug resistance 1 (MDR1) gene, encoding a transmembrane glycoprotein (P-gp) that antagonizes intracellular accumulation of cytotoxic agents.6–7
While 20–25% of MTC’s are due to hereditary germline mutations in RET such as in multiple endocrine neoplasia type 2 (MEN 2A or MEN 2B) or familial medullary thyroid carcinoma (FMTC), a majority (75–80%) are due to sporadic mutations in this proto-oncogene. The RET (rearranged during transfection) protooncogene is located in the pericentromeric region of chromosome 10q11.2 and encodes a receptor tyrosine kinase. It is expressed mostly in neuroendocrine cells of the thyroid and adrenal gland, neural parasympathetic and sympathetic ganglion cells, and cells of the urogenital tract and testis germ cells.8
Gain of function mutations in RET have been demonstrated to lead to MTC tumor development. Activation of key regulatory pathways responsible for C-cell proliferation and differentiation such as the Ras/ERK and p38 mitogen-activated protein kinase pathways and the phosphatidylinositol 3-kinase(PI3K)/Akt pathway occurs mainly through Tyr1062.6,9–10 Because RET is a growth factor receptor with limited expression, and there are both germline and somatic mutations, it has been an attractive candidate for targeted therapy. There has been significant experimental evidence showing that RET inhibition leads to growth inhibition and apoptosis in MTC cells.10 It is through a better understanding of the various RET mutations that newer targeted therapies have been developed to selectively inhibit RET activation and phosphorylation of the kinase domain. Several targeted kinase inhibitors are currently being evaluated in clinical phase I, II and III trials.11–13 There are a number of RET kinase inhibitors which share the property of binding to the RET ATP-binding pocket; these include vandetanib, sorafenib, sunitinib, imatinib, pazopanib, axitinib, motesanib, gefitinib and XL 184.14–15 Many of these small molecules are not only RET kinase inhibitors but multikinase inhibitors effective on VEGF-R1, VEGF-R2 and VEGF-R3, PDGF-R, and EGF-R, with varying affinities, and thus often affecting multiple signaling pathways.
Two of the more successful agents thusfar in clinical studies are vandetanib and XL-184. Vandetanib is an oral, small-molecule tyrosine kinase inhibitor (ZD6474) which inhibits RET, VEGF-R2, VEGF-R3 and, at higher concentrations, EGF-R.16 Based on site investigator assessments, 20% of patients experienced a partial response, and another 60% experienced stable disease for a disease control rate of 80%.17 XL-184 is currently the only agent in phase III analysis in this disease and is being studied through an international patient cohort in order to obtain necessary power for the study. This multikinase inhibitor selectively inhibits and targets RET, MET and VEGFR2 kinases. Targeted kinase inhibitors, however, are not without toxicities which may vary significantly from drug to drug but typically will include gastrointestinal symptoms such as nausea or diarrhea, alopecia, hypertension, hand-foot syndrome or other skin toxicity, and infections. A majority of patients on these drugs will experience at least some form of toxicity while on study and in some cases this can be dose-limiting.
While several of these targeted inhibitors are moving forward in clinical trial applications, some therapies are now being evaluated as part of multi-drug regimens. In terms of drug development and discovery strategies, the field remains open for a novel agent that has improved efficacy in this population with sustained responses and lower toxicity profiles.
Our group recently identified a novel natural product derivative that has potent selective anticancer activity against thyroid cancers in vitro. This drug compound, Withaferin A, has been shown to inhibit human MTC cell proliferation (TT cells and DRO81-1) in vitro by MTS assay and confirmed by trypan-blue exclusion assay.18 This inhibition occurred at a higher potency than drugs like 17-AAG, a popular heat shock protein 90 inhibitor that is currently in phase 1/2 clinical trials in patients including MTC patients. The purpose of this study is to evaluate the efficacy and toxicity of WA in an in vivo murine xenograft model of MTC using human medullary cancer DRO-81-1 cells that spontaneously metastasize to the lung, liver and spleen. This is a true medullary cancer cell line and has not been shown to exhibit potential genetic cross-contamination with melanoma or colon cancer cells as many well-used thyroid cell lines were recently demonstrated to contain.19–20 This cell line also produces the hormone calcitonin allowing for biochemically quantitative assessment of in vivo disease to supplement visible tumor volume measurements and in culture will grow much more rapidly than standard TT cells (observational data not shown). In addition to generating efficacy and survival data, we also plan to evaluate the clinical toxicity of this compound as defined by changes in body weight, body score and tumor volume. Western-Blot protein analysis of tumor tissues will be performed to evaluate RET and AkT phosphorylation and determine if there is dose-related inhibition of key regulatory pathways in MTC cells by WA.
MATERIALS AND METHODS
Cell Culture
Human MTC DRO81-1 cells were obtained through a generous donation from Dr. Jillard (University of California – Los Angeles, CA). TT cells were purchased from American Type Culture Collection (ATCC). TT cells were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO.), 100 IU/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich, St Louis, MO). DRO 81-1 cells were maintained on DMEM media (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat inactivated FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2 in air at 37 °C. Cells were treated at exponential growth phase (50–70% confluence).
Western Blot Analysis
MTC cells, after treatment, were washed using cold 1X phosphate-buffered saline (PBS) lysed using RIPA lysis buffer (0.5% Nonidet P-40, 100 mM, 10 mM Tris (pH 7.5), 1 mM NaF, 1mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM sodium orthovanadate) supplemented with 1:100 dilution of each of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO). Cell lysate was incubated on ice for 30 min, and then centrifuged (14,000×g for 20 min) to obtain clear cell lysate. Mouse tumor tissue was collected from control (DMSO treated) and withaferin A treated animals 21 days after start of drug treatment. Tumor tissues were stored at −80°C if they are not used immediately. Tumor tissues were weighted and diced into small pieces. Tumor tissue was homogenized using dounce homogenizer and RIPA buffer (3.0 mL/gr of tumor tissue) containing 1:100 dilution of each of protease inhibitor and phosphatase inhibitor. Tissue homogenate was incubated on ice for 30 min and then centrifuged (14,000×g, for 20 min). Cell lysate as well as tissue homogenate protein concentrations were determined using a bicinchoninic assay kit (Pierce, Rockford, IL).
Equal quantities of proteins (30–60 μg) were mixed with sample buffer and were denatured by boiling for 5 minutes and separated by 8–12% SDS-PAGE, transferred onto nitrocellulose membranes, then blocked and exposed to primary and secondary antibodies as described. The following primary antibody dilutions were used: phospho-RET (1:500), phospho-AKT(1:1000), phospho-ERK (1:1000), Total Akt (1:1000), uncleaved caspase 3 (1:1000); total RET (1:500), total ERK2 (1:5,000), beta-actin (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibody incubations were kept overnight at 4°C and membranes were washed and incubated with a 1:15,000 dilution of horseradish peroxidase-conjugated anti-mouse secondary antibody anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were developed by Pico West chemiluminescence substrate (Pierce) according to the manufacturers’ directions.
Xenograft Tumor Model and Withaferin A Administration
Animal care and treatment were performed based our animal experimental protocol (2008.1727) approved by the University of Kansas Medical Center Animal Care and Use Committee. Sixteen female nude athymic, nu/nu mice (Charles Rivers, Wilmington, Maryland, USA) received a subcutaneous (s.c.) injection of (5×106 cells) DRO 81-1 cells into the left posterior triangle of the neck. Mice with tumor size ≥5mm in diameter (approximately 100mm3 volume) were randomly divided into two groups treated either with DMSO or withaferin A (Chromadex, Irvine, CA) for the study. Withaferin A was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) at a stock concentration of 20mM and stored at −80°C. DMSO was used as control at the same level (v/v) as was used in the WA treated animals. Fresh WA solution was made daily for each injection. The treatment group of mice received intraperitoneal injection of 8 mg/kg/day WA for 21 consecutive days. The control group was injected with vehicle (DMSO) diluted in PBS. The mice were weighed three times weekly to assess toxicity of the treatments including bodyscore measurement, and the tumors were measured at that time using digital calipers, with measurements performed by the same 2 independent laboratory personnel on each day. MTC tumor volume was calculated from the two-dimensional caliper measurements using the following formula: tumor volume = length × (width)2 × π/6. Survival of animals in both groups was followed prospectively for up to 12 weeks after commencement of injections. If the tumor volume exceeded 1500 mm3 animals were sacrificed. From each group 3 animals were randomly euthanized on the last day of drug treatment to evaluate tumor burden (primary and metastatic) as well as evaluate tumor tissue for inhibition of RET protein expression and phosphorylation, AkT phosphorylation, and ERK phosphorylation. On the final day of the study, all mice lasting the full duration of the study or exhibiting either weight or body score deterioration or tumor volume greater than the maximum allowed were euthanized by carbon dioxide inhalation. The s.c. tumor was removed and snap frozen in liquid nitrogen for future protein analysis.
Calcitonin ELISA assay for serum evaluation
Mouse serum calcitonin levels were measured using The Calcitonin Immunoassay from ALPCO Diagnostics (Salem, NH). The calcitonin assay is a two-site ELISA [Enzyme-Linked ImmunoSorbent Assay] for the measurement of the biologically intact 32 amino acid chain of Calcitonin. It utilizes two different mouse monoclonal antibodies to calcitonin specific for well-defined regions on the calcitonin molecule. The serum samples were tested along with standard concentrations of calcitonin and positive and negative control in duplicates according to the manufacturer’s instruction.
Statistical Analysis
Evaluation of multiple variables was performed using analysis of variance (ANOVA) with Bonferroni post hoc testing via a standard statistical analysis software package (SPSS version 17.0, SPSS, Chicago, IL). A p-value of < 0.05 was considered significant.
RESULTS
Efficacy of Withaferin A in a Murine Xenograft MTC model
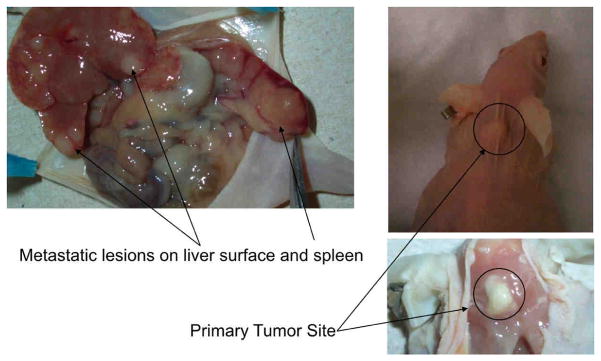
Nude mice (n=16) were injected with 5 million DRO 81-1 cells in the posterior triangle of the left neck with 100% of animals developing primary tumors. Animals were split evenly into a control group (n=8; injected with saline) or a WA treatment group (n=8). All control animals in this experiment developed abdominal metastatic disease to the liver and spleen with three animals also demonstrating lung metastases. Tumors in all the mice reached over 100 mm3 within 2-weeks following cell injections and drug treatment was started on all animals at this 2-week post-injection timepoint. From each group, 3 animals were randomly euthanized on the last day of drug treatment to evaluate tumor burden (primary and metastatic) as well as tumor tissue protein levels described in detail below. All of the remaining control animals (n=5) developed progressively enlarging tumor masses with increased disease burden. This tumor burden resulted in deterioration of bodyweight (>10% loss from baseline) as well as a decline in body score within 6–7 weeks post-injection leading to natural death or euthanasia as per standard animal protocol monitoring. A representative control animal at autopsy is demonstrated in Figure 1A with a view of both the primary tumor implant as well as intra-abdominal metastases. In contrast to the control group, all of the treated animals (n=5) were alive at 6 weeks-post induction of therapy, without evidence of weight loss or body-score deterioration. The remaining three treated animals that were euthanized on the last day of treatment demonstrated no evidence of intra-abdominal metastases grossly or histologically. A gross comparison of three of these 3-week post-treatment (5-weeks post-injection of tumor cells) animals is shown in Figure 1B. The control animal on the left has a large primary tumor with evidence of weight-loss (spine more prominent) while the treated animals (2 on the right) have almost completely regressed tumors with no clinical toxicity as evidenced by normal weight, body score and activity levels.
Figure 1. Neck Xenografts of DRO81-1 MTC cells in Nu/Nu mice develop metastatic disease similar to human MTCs.
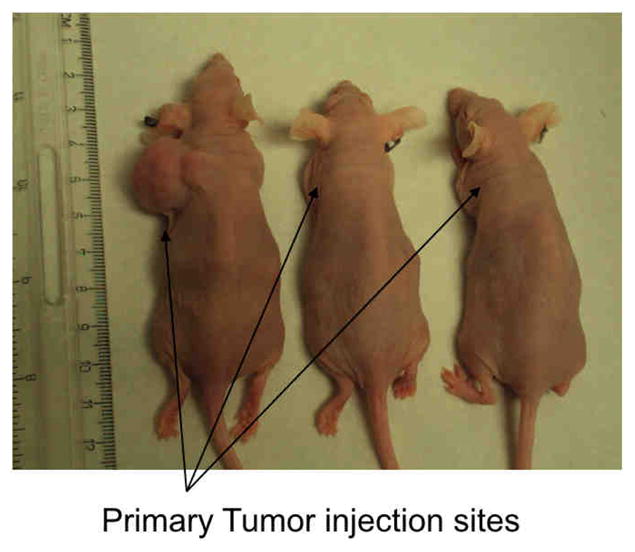
MTC xenografts in NU/NU mice were created by injection in the left posterior neck with 5 million DRO81-1 cells. All of the control animals (n=5) developed progressively enlarging tumor masses with increased disease burden resulting in deterioration of bodyweight (>10% loss from baseline) as well as decline in body score within 6–7 weeks post-injection leading to natural death or euthanasia as per standard animal protocol monitoring. A representative control animal at autopsy is demonstrated in Figure 1A with a view of both the primary tumor implant as well as intra-abdominal metastases. This xenograft model reproducibly metastasizes to the liver, lungs and spleen in a similar pattern to human MTC. A gross comparison of three of these 3-week post-treatment (5-weeks post-injection of tumor cells) animals is shown in Figure 1B. The control animal on the left has a large primary tumor with evidence of weight-loss (spine more prominent) while the treated animals (2 on the right) have almost completely regressed tumors with no clinical toxicity as evidenced by normal weight, body score and activity levels.
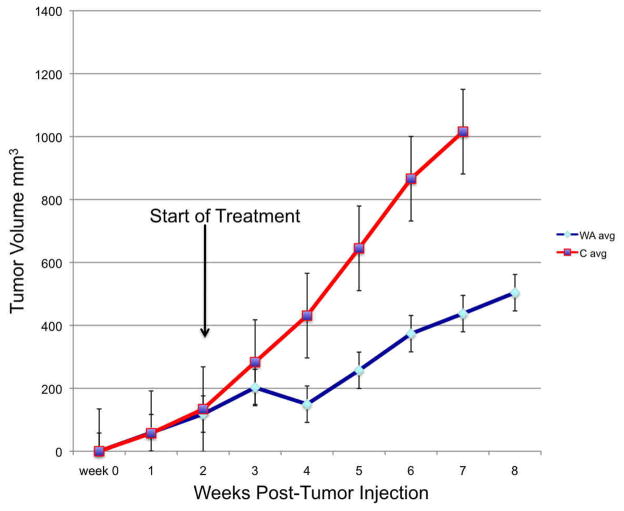
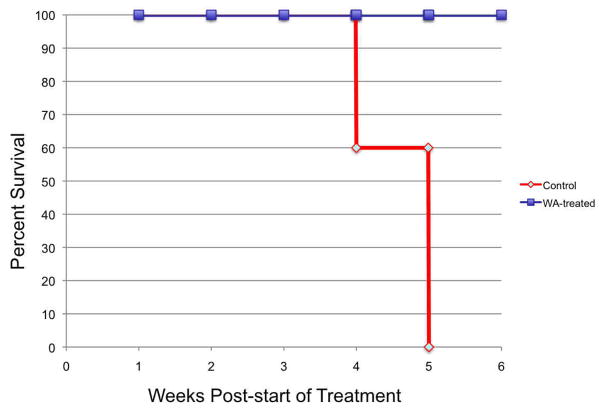
Drug efficacy analysis is plotted out for 8 weeks post-initial tumor injection (since all of the control animals (n=5) have died by 7 weeks post-injection and all of the treated animals (n=5) are alive with tumor). Comparatively even in this small group, treated animals demonstrated a significant survival advantage at this time-point over controls (p<0.01). Tumor growth progression is demonstrated in Figure 2A depicted as an average tumor volume for each group by week (average of all 5 animals per group) with standard error bars. Survival times are depicted in Figure 2B using a Kaplan-Meier format. As a group the treated animals demonstrated a uniform delay-in growth of tumors with 80% of animals demonstrating an initial significant regression in tumor volume at 2 weeks into therapy (week 4 post-tumor implantation) which was followed by eventual disease progression, albeit at a 3 to 4-week growth-delay compared to controls and at a lower growth rate trajectory.
Figure 2. Withaferin A has in vivo efficacy against DRO 81-1 xenografts without toxicity.
Drug efficacy analysis is plotted out for 8 weeks post-initial tumor injection (since all of the control animals (n=5) have died by 7 weeks post-injection and all of the treated animals (n=5) are alive with tumor). Comparatively even in this small group, treated animals demonstrated a significant survival advantage at this time-point over controls (p<0.01). Tumor growth progression is demonstrated in Figure 2A depicted as an average tumor volume for each group by week (average of all 5 animals per group) with standard error bars. Survival times are depicted in Figure 2B using a Kaplan-Meier format. As a group the treated animals demonstrated a uniform delay-in growth of tumors with 80% of animals demonstrating an initial significant regression in tumor volume at 2 weeks into therapy (week 4 post-tumor implantation) which was followed by eventual disease progression, albeit at a 3 to 4-week growth-delay compared to controls and at a lower growth rate trajectory.
Analysis of In vivo and In Vitro Regulatory Proteins
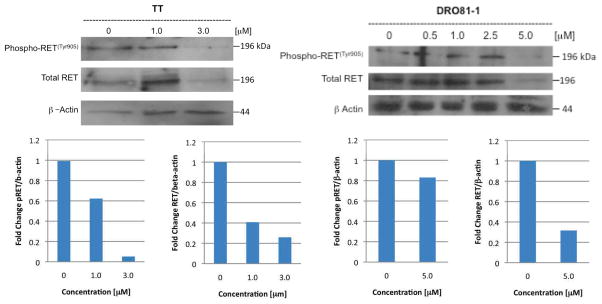
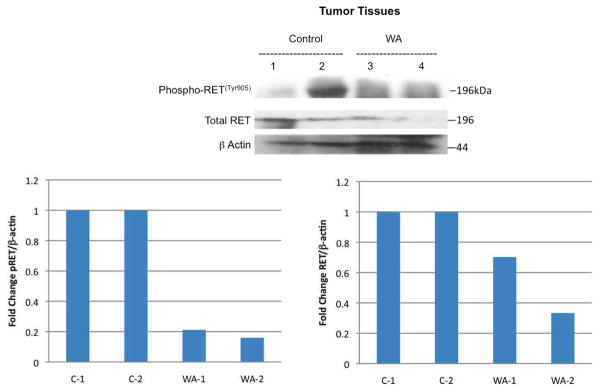
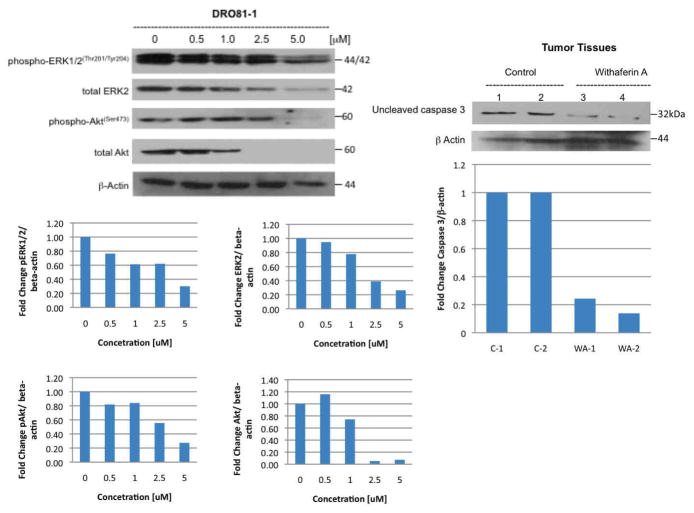
Ex-vivo tumor specimens confirmed histologically were evaluated for RET, phospho-RET and uncleaved caspase 3 protein expression during treatment compared to controls and to DRO 81-1 in vitro data with WA at similar concentrations. Both DRO 81-1 and TT cells in vitro demonstrated a dose-dependent inhibition of both phospho-RET and total RET levels (normalized by densiometry to beta-actin levels; Figure 3) with WA treatment, exhibiting an almost complete inhibition at 3 μM WA concentrations (p<0.02). Ex vivo tumors also demonstrated inhibition of both total-RET and phospho-RET expression levels in the treated group (equivalent to 3 μM in vitro levels) compared to controls (Figure 4) as well as reduction of uncleaved caspase 3 (Figure 3 right) levels suggesting cleavage of caspase 3 and induction of apoptosis. This is consistent with previous in vitro work demonstrating induction of apoptosis by WA in thyroid cancer cells including DRO 81-1 cells.18
Figure 3. Withaferin A inhibits RET protooncogene in human medullary thyroid cancer cells in vitro.
Human medullary thyroid cancer TT cells and DRO 81-1 cells were treated with increasing concentration of withaferin A for 24 hr and activity (measured as RET-phosphorylation) and total RET proto-oncogenes levels were analyzed using specific antibodies. Membranes were stripped and reprobed with anti-beta actin antibody to ensure equal protein loading and quantitative densiometry is shown below to compare protein expression as a normalized ratio to beta-actin levels. Withaferin A strongly inhibited Tyr905 phosphorylation of Ret at 3.0 uM concentration in TT cells and it reduced expression of total RET protein expression. In DRO 81-1 cells, both phospho-RET and total RET expression are inhibited at 5μM WA concentrations. Both cell lines demonstrate that Withaferin A is functional RET inhibitor in MTC cells.
Figure 4. Inhibition of RET phosphorylation and total RET protein expression by WA in vivo.
In ex-vivo tumors removed from Nu/Nu mice with DRO81-1 xenograft injections of 5 million cells to the left posterior neck, prosurvival proteins were analyzed by Western Blot analysis and demonstrate in the WA-treated animals both inhibition of phospho-RET and total RET with beta-actin levels demonstrating equal protein loading in lanes. The dose of WA used in the in vivo model is equivalent to 3 μM in vitro dosing. Quantitative densiometry is shown below to compare protein expression as a normalized ratio to beta-actin levels.
Next important kinase-regulated signaling pathways including the RAS/ERK pathway and the IP3K/Akt pathway were evaluated for modulation by WA as these are known to be regulated in part through RET phosphorylation in this cancer cell-type. Figure 5 demonstrates that after 24 hours of WA treatment, DRO 81-1 cells exhibit a dose-dependent inhibition of both phospho-ERK and phospho-Akt as well as both total ERK and total Akt protein levels with significant inhibition of ERK and Akt levels at 2.5 μM WA and almost complete inhibition of Akt levels occurring at 5 μM WA (confirmed quantitatively by denisiometry analysis with ratio to beta-actin levels).
Figure 5. Withaferin A modulates proliferative pathways in medullary thyroid cancer cells in vitro and in vivo.
DRO 81-1 cells were treated in vitro with increasing concentration of withaferin A for 24 hr (left western blot) and levels of phosphorylated ERK1/2, total ERK1/2, phosphorylated Akt and total Akt were examined using specific antibodies. Membranes were stripped and reprobed with anti-beta actin antibody to ensure equal protein loading. Quantitative densiometry is shown below to compare protein expression as a normalized ratio to beta-actin levels. These data demonstrate that withaferin A in a concentration-dependent fashion inhibited ERK1/2 phosphorylation and activity as well as total ERK2 levels. In addition withaferin A inhibited Akt activity and reduced total Akt levels. In ex-vivo tumors removed from Nu/Nu mice with DRO81-1 xenograft injections of 5 million cells to the left posterior neck, prosurvival proteins were analyzed by Western Blot analysis and demonstrate in the WA-treated animals reduction of uncleaved caspase 3 (right blot) indicating its cleavage as a marker of apoptotic cell-death. Mechanistically WA has been shown previously in vitro to induce apoptosis in DRO-81-1 cells.18 These results confirm that the drug is acting through similar pathway mechanisms in vivo as it is in vitro.
Evaluation of in vivo serum calcitonin levels with treatment
Finally with evidence of tumor regression in vivo and downregulation of RET and reduction of uncleaved caspase 3 with drug treatment, we analyzed mouse serum for biochemical evidence of tumor functionality by calcitonin levels using a serum-based ELISA assay. Figure 6 demonstrates a significant decrease in calcitonin levels (depicted as the average with standard error bars) sampled from treated animals (n=3; mean=79.7 pg/mL) after 21 days of treatment compared to controls (n=3; mean=149 pg/mL), p<0.05.
Figure 6. Withaferin A treatment significantly reduces xenograft MTC tumor calcitonin production in vivo.
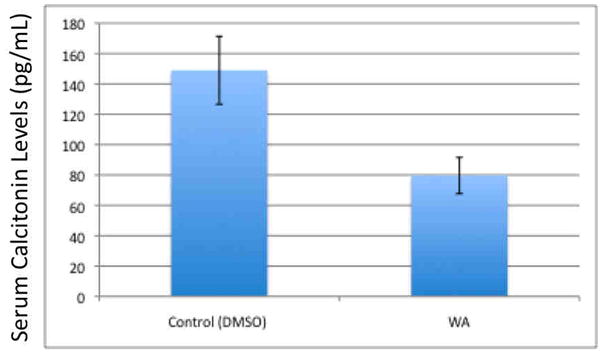
DRO 81-1 xenograft nu/nu mice were treated with withaferin A (5.0 mg/Kg/day) or with vehicle. Twenty-one days after treatment, animals were sacrificed to collect tissue samples and blood. Mouse serum calcitonin levels were measured using The Calcitonin Immunoassay from ALPCO Diagnostics (Salem, NH) using manufacturer controls. Graph is average of 3 animal samples with standard deviation error bars. WA treated animals had a significantly lower calcitonin (79 pg/mL) compared to controls (149 pg/mL; p<0.05) indicating the drug’s effect on tumor biochemical functionality.
DISCUSSION
In the search for improved targeted therapies, a single-agent cure for a disease such as medullary thyroid cancer which has multiple phenotypes and RET mutations is unlikely, however identifying drugs with better safety profiles which are selective in down-regulating RET activation as well as downstream cell-signalling pathways normally upregulated in these tumors presents a reasonable strategy for combination therapy. In previous work we demonstrated that Withaferin A is a novel natural product withanolide which has potent, selective anticancer activity in both well-differentiated and poorly differentiated thyroid cancers including medullary cancers in vitro. In DRO 81-1 medullary cells WA demonstrated increased potency compared to 17-AAG (IC50WA=0.69 μM; IC5017AAG=2.41 μM; p<0.05), a drug that has been evaluated in MTC in clinical studies.18
In this study the efficacy of this drug was evaluated in a preclinical animal model using a xenograft of DRO 81-1 cells. DRO81-1 cells were chosen over TT cells (which have been reported more extensively in the literature14–15, 19, 21–24) mainly because they are faster-growing tumors in vivo and yet demonstrate all the important qualities of medullary tumors, including development of visceral metastases to the liver, lungs and spleen as well as calcitonin production. This reproducibility of growth (100% tumor development) and log-phase growth pattern with visible animal morbidity and mortality in a 4 to 8 week period depending on the number of cells injected, make this tumor model attractive from a preclinical design approach and allow this tumor model to be a reasonable alternative to the TT cell model.
The animal in vivo data demonstrate a significant survival advantage with drug treatment compared to controls even using a frugal sample size of 5 animals per treatment group. Since this was an initial pilot evaluation of efficacy, these data provide a reasonable foundation to justify a larger efficacy study looking at long-term survival and recurrence rates in treated animals as well as toxicity with long-term treatment beyond 3 weeks. Drug treatment in vivo also demonstrated an initial regression of tumor followed by eventual tumor growth and progression, although it was delayed compared to control animals and had a lower growth-rate trajectory at the time of progression. Future studies are planned to address if this same pattern of response would occur with prolonged drug treatment beyond 3 weeks. Several mechanisms could account for this phenomenon including development of drug-resistance, lack of potency in completely inhibiting kinase phosphorylation, induction of alternative prosurvival pathways, or even adaptive immune modulation to treatment.
In terms of mechanism of action of the drug in vivo, it appears that tumor inhibition both at the proliferative/growth level as well as at the regulatory pathway phosphorylation level are similar to in vitro results. Both in vitro and in vivo tumor cells demonstrate inhibition of RET activation and phosphorylation as well as inhibition of total RET expression. Additionally, prosurvival proteins such as ERK and Akt are inhibited in vitro both functionally and in terms of total protein expression. In vivo tumors also demonstrated cleavage of caspase 3 indicating probable induction of apoptotic cell death. There was good correlation of in vivo and in vitro drug effects at similar concentration levels. While a majority of inhibition of RET, Akt, and ERK phosphorylation was achieved at 3–5μM WA concentrations, this inhibition was not complete, allowing for some level of continued protein expression which, over time, could account in part for tumor progression that was observed in the animals after completion of the treatment period. Since no significant toxicity was observed clinically in the treated animals, it would be important in the future to determine maximum tolerated dose-levels and repeat efficacy and toxicity studies at higher drug doses to determine if greater levels of tumor inhibition can be achieved safely. While it has promising efficacy without significant toxicity thusfar, formal toxicology testing in the future will be necessary to better evaluate its safety profile in vivo. Overall these early in vivo data provide another level of evidence in support of further development and translation of this compound toward a potential clinical application in the future for patients with metastatic medullary thyroid carcinoma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Riordain DS, O’Brien T, Weaver AL, et al. Medullary thyroid carcinoma in multiple endocrine neoplasia types 2A and 2B. Surgery. 1994;116:1017–23. [PubMed] [Google Scholar]
- 2.Cohen MS, Moley JF. Surgical Treatment of Medullary Thyroid Carcinoma. Journal of Internal Medicine. 2003;253:1–11. doi: 10.1046/j.1365-2796.2003.01166.x. [DOI] [PubMed] [Google Scholar]
- 3.Brierley J, Tsang R, Simpson WJ, et al. Medullary thyroid cancer: analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid. 1996;6:305–10. doi: 10.1089/thy.1996.6.305. [DOI] [PubMed] [Google Scholar]
- 4.Tisell LE, Dilley WG, Wells SA., Jr Progression of postoperative residual medullary thyroid carcinoma as monitored by plasma calcitonin levels. Surgery. 1996;119:34–9. doi: 10.1016/s0039-6060(96)80210-8. [DOI] [PubMed] [Google Scholar]
- 5.Martins RG, Rajendran JG, Capell P, et al. Medullary Thyroid Cancer: Options for Systemic Therapy of Metastatic Disease? J Clin Oncol. 2006;24(11):1653–1655. doi: 10.1200/JCO.2005.05.4106. [DOI] [PubMed] [Google Scholar]
- 6.Cakir M, Grossman AB. Medullary thyroid cancer: molecular biology and novel molecular therapies. Neuroendocrinology. 2009;90:323–348. doi: 10.1159/000220827. [DOI] [PubMed] [Google Scholar]
- 7.Zatelli MC, Luchin A, Piccin D, et al. Cyclooxygenase-2 inhibitors reverse chemoresistance phenotype in medullary thyroid carcinoma by a permeability glycoprotein-mediated mechanism. J Clin Endocrinol Metab. 2005;90:5754–5760. doi: 10.1210/jc.2005-1362. [DOI] [PubMed] [Google Scholar]
- 8.Wells SA, Jr, Santoro M. Targeting the RET Pathway in Thyroid Cancer. Clin Cancer Res. 2009;15(23):7119–23. doi: 10.1158/1078-0432.CCR-08-2742. [DOI] [PubMed] [Google Scholar]
- 9.de Groot JW, Links TP, Plukker JT, et al. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27:535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 10.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 11.Santoro M, Carlomagno F. Drug insight: Small-molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat Clin Pract Endocrinol Metab. 2006;2:42–52. doi: 10.1038/ncpendmet0073. [DOI] [PubMed] [Google Scholar]
- 12.Banerji U, Judson I, Workman P. The clinical applications of heat shock protein inhibitors in cancer - present and future. Curr Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- 13.Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17- demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 14.Ball DW. Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opin Oncol. 2007;19:18–23. doi: 10.1097/CCO.0b013e32801173ea. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MS, Hussain HB, Moley JF. Inhibition of medullary thyroid carcinoma cell proliferation and RET phosphorylation by tyrosine kinase inhibitors. Surgery. 2002 Dec;132(6):960–6. doi: 10.1067/msy.2002.128562. discussion 966–7. [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Heymach JV, O’Reilly MS, et al. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumour growth and angiogenesis. Expert Opin Investig Drugs. 2007;16:239–249. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 17.Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib in metastatic hereditary medullary thyroid carcinoma: Follow-up results of an openlabel phase II trial. J Clin Oncol. 2007;25:6018. [Google Scholar]
- 18.Samadi A, Loo P, Mukerji R, et al. A novel HSP90 modulator with selective activity against thyroid cancers in vitro. Surgery. 2009 Dec;146(6):1196–207. doi: 10.1016/j.surg.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic Acid Profiling Analysis of 40 Human Thyroid Cancer Cell Lines Reveals Cross-Contamination Resulting in Cell Line Redundancy and Misidentification. J Clin Endocrinol Metab. 2008;93(11):4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Z, Eisenberg DP, Singh B, et al. Treatment of aggressive thyroid cancer with an oncolytic herpes virus. Int J Cancer. 2004;112:525–532. doi: 10.1002/ijc.20421. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Hattori Y, Komori M, et al. Combination of RET siRNA and irinotecan inhibited the growth of medullary thyroid carcinoma TT cells and xenografts via apoptosis. Cancer Sci. 2010 Feb 2; doi: 10.1111/j.1349-7006.2009.01484.x. [Epub ahead of print] PMID: 20132218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Hai T, Ye L, et al. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab. 2010;95(1):439–44. doi: 10.1210/jc.2009-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quidville V, Segond N, Tebbi A, et al. Anti-tumoral effect of a celecoxib low dose on a model of human medullary thyroid cancer in nude mice. Thyroid. 2009;19(6):613–21. doi: 10.1089/thy.2008.0194. [DOI] [PubMed] [Google Scholar]
- 24.Ning L, Kunnimalaiyaan M, Chen H. Regulation of cell-cell contact molecules and the metastatic phenotype of medullary thyroid carcinoma by the Raf-1/MEK/ERK pathway. Surgery. 2008;144(6):920–4. doi: 10.1016/j.surg.2008.07.020. discussion 924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]