Abstract
Pneumococcal hemolytic uremic syndrome is recognized in a small portion of otherwise healthy children who have or have recently had Streptococcus pneumoniae infections, including severe pneumonia, meningitis, and bacteremia. As in other types of hemolytic uremic syndrome (HUS), pneumococcal HUS is characterized by microangiopathic hemolytic anemia, and thrombocytopenia, usually with extensive kidney damage. Although not demonstrated in vivo, the pathogenesis of pneumococcal HUS has been attributed to the action pneumococcal neuraminidase exposing the usually cryptic Thomsen-Friedenreich antigen (T-antigen) on red blood cells (RBC), and kidney glomeruli. We evaluated the effect of pneumococcal infection on desialylation of RBC and glomeruli during pneumococcal infections in mice. Following intravenous infection with capsular type 19F pneumococci, CFU levels exceeding 1000 CFU /mL blood by the third day were significantly more likely to result in exposed T-antigen on RBC than lower levels of bacteremia. In a pneumonia model, significantly more T-antigen was exposed on RBC in mice treated with penicillin than in those receiving mock treatment. Utilizing mutant pneumococci, we demonstrated that neuraminidase A but not neuraminidase B was necessary for exposure of T-antigen on RBC in vivo. Thus, pneumococcal neuraminidase A is necessary for the exposure of T-antigen in vivo and treatment with penicillin increases this effect. Interestingly, NanA− pneumococci were found in the blood in higher numbers and caused more deaths than wild type, NanB−, or the NanA−/NanB− pneumococci.
Keywords: Streptococcus pneumoniae, Hemolytic uremic syndrome, Thomsen-Friedenreich antigen, Neuraminidase
1. INTRODUCTION
Hemolytic uremic syndrome [1] is characterized by microangiopathic hemolytic anemia, acute renal failure, and thrombocytopenia [2–5]. Pneumococcal HUS is a relatively uncommon complication of invasive infection with Streptococcus pneumoniae and is classified as “infection-induced” HUS along with HUS caused by Escherichia coli O157:H7 and other bacteria producing shiga-like toxins [6]. The original case series describing HUS by Gasser et al. in 1955 included 2 infants with pneumonia, presumably pneumococcal pneumonia [7]. Pneumococcal HUS is most commonly seen in patients with pneumococcal pneumonia and/or meningitis and unlike HUS caused by gram-negative bacteria is not usually preceded by diarrhea [2]. Compared with HUS caused by E. coli, pneumococcal HUS occurs in younger age groups (most patients are less than 2 years), and is more likely to require renal dialysis and prolonged hospitalization. Pneumococcal HUS is estimated to comprise ~5 percent of all HUS cases in children and 0.4% – 0.6% of all invasive pneumococcal infections, but this figure is probably an underestimated because of lack of awareness [3, 4, 8].
A hypothesis for the pathogenesis of pneumococcal HUS was proposed by Klein in 1977, who found Thomsen-Friedenreich antigen (T-antigen) exposed on red blood cells (RBC) and in glomeruli of two children who died of pneumococcal pneumonia and sepsis [9]. The T-antigen is a disaccharide (Galβ1-3GalNAcα1-Ser/thr) that forms the core structure of O-linked mucin type glycans. The T-antigen is “cryptic”, normally being hidden by a terminal sialic acid [10] and is found on many cell types including RBC, platelets, glomerular capillary endothelium and renal tubular epithelium.
The neuraminidase from S. pneumoniae is hypothesized to expose T-antigen to naturally occurring IgM antibodies by the cleaving terminal sialic acid. Specifically these neuraminidases cleave sialic acid-containing substrates with α-2,6 and α-2,3 linkages of N-acetylneuraminic acid to galactose, and α-2,6 linkage to N-acetyl-galactosamine [11]. The Coomb’s test is usually positive in pneumococcal HUS patients, and T-antigen exposure on RBC and other cells can be detected in human and mouse samples using peanut lectin from Arachis hypogaea [12, 13]. The finding of neuraminidase activity in S. pneumoniae patient isolates is suggestive but not specific for pneumococcal HUS [3]. The neuraminidase activity has been assumed but not proven to be due to pneumococcal neuraminidase [14–17]. The involvement of antibody to T-antigen in pneumococcal HUS pathology is controversial [8]. Although some investigators speculate that a heavy bacterial load with pneumococcal may contribute to the development of HUS, it remains unclear why only a small percentage of invasive pneumococcal infections are complicated by HUS [14].
The S. pneumoniae genome encodes 3 neuraminidases: NanA, NanB and NanC [18]. The majority of isolates express NanA and NanB. NanC has high sequence identity to NanB but is not well characterized otherwise. Virtually all strains of S. pneumoniae possess the gene for neuraminidase A, and exhibit neuraminidase activity [18–21]. NanA has been shown to be important in the adherence to tracheal epithelium and nasopharyngeal colonization [22, 23] and binding/invasion of the blood-brain barrier [24]. NanA is thought to promote nasal colonization by removing sialic acid from cell surfaces thus exposing receptors to which pneumococci are able to bind [23, 25, 26]. Neuraminidase is also thought to act on mucin, glycolipids, and glycoproteins [27]. It can remove sialic acid from molecules such as IgA and lactoferrin and has been hypothesized to interfere with protection mediated by these molecules [28]. In most pneumococci, NanA is covalently attached to the peptidoglycan on the surface of S. pneumoniae by Sortase A, which recognizes the LPETG cell wall attachment motif of NanA [29–31]. TIGR4 is a commonly used strain in which a naturally-occurring mutation prevents cell wall attachment and the protein is released from the cell surface [31].
Unlike NanA, NanB has no attachment motif and is released into the environment [32]. NanB acts as an intramolecular trans-sialidase specifically cleaving α2–3 linked sialic acid substrates [33]. Both NanA and NanB have been reported to play roles in the development of upper and lower respiratory tract infection and sepsis [34]. In animal studies, neuraminidase has been reported to play a role in virulence at the mucosal surface [23] and in systemic disease [34]. Although evidence suggests that neuraminidase is not well expressed by pneumococci during systemic infection, immunization with NanA does offer some protection against sepsis following pneumococcal challenge [28, 35, 36].
We used animal models and in vitro assays to evaluate the ability of NanA and NanB to act in vivo in mice to expose T-antigen. We studied capsular type 19 and 14 strains because they are among the most common capsular types reported in cases of pneumococcal HUS [5]. Our findings demonstrated that during invasive infection in mice enough neuraminidase is made to expose T-antigen on host surfaces of RBC and glomeruli. By using mutant pneumococci we were able to show that NanA, but not NanB, was required for in vivo desialation of host cells in vivo. We also examined the possibility that penicillin, which results in the lytic death of pneumococci, may release enough neuraminidase from the cell wall to enhance desialation of host RBC.
2. RESULTS
2.1. Kidney tissue in CBA/N mice is sensitive to the neuraminidase activity of S. pneumoniae
In the present study we found that intravenous infection of CBA/N mice with EF3030, HUS05, TJ0893, HUS03 and BG8090 strains of S. pneumoniae resulted in the exposure of T-antigen in the kidney along capillaries in the glomeruli, as demonstrated histologically using peroxidase labeled peanut lectin (Figure 1). Glomeruli with exposed T-antigen were located throughout the kidney. Using this qualitative assay differences were not observed in the ability of different strains to expose T-antigen in glomeruli. As expected, infection with NanA− mutants of EF3030 did not expose T-antigen on glomeruli (data not shown). The negative control for this study was examination of glomeruli of mice injected with lactated ringers instead of pneumococci. Tantigen was not exposed on the glomeruli of these “mock infected” mice (data not shown).
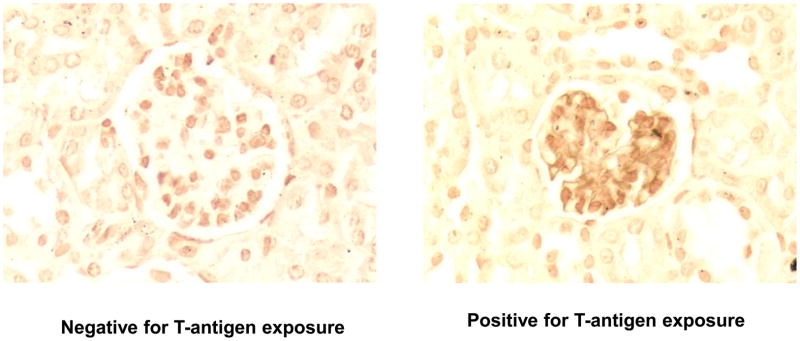
Figure 1.
Exposure of T-antigen on glomeruli during infection with S. pneumoniae. Mice were infected intravenously with S. pneumoniae 1×107 CFU of EF3030. Fixed kidneys were reacted with HRP-labeled peanut lectin. Control kidneys from mice injected with ringers but no bacteria did not expose T-antigen and no-exposure was seen in kidneys from mice infected with NanA− EF3030 (data not shown).
2.2. Bacteremia is sufficient to cause exposure of T-antigen on mouse RBC
Preliminary studies showed that intravenous infection with ~107 CFU of the strains used in the study above generally led to self-resolving bacteremia in CBA/N mice. Peak levels of bacteremia generally occurred by day 3, and by day 5, when the mice were euthanized, bacteremia was ≤ 100 CFUs/mL in all mice. Three to five-fold higher numbers of CFU invariably lead to instant sepsis and death within 24 hours. As a result we only monitored T-antigen exposure the infections with 107 CFU.
The maximum CFUs observed for each mouse during the first 72 hours following infection is shown in figure 2. For 23 of the 26 mice infected with these 5 strains that had bacteremia of ≥1000 CFUs/mL exposure of T-antigen was observed; none of the 23 mice with <1000 CFU/mL exhibited T-antigen on their RBC by our assay (P<0.0001; Fisher exact test). Of the 5 strains examined HUS03 appeared to differ from the group as a whole however (P <0.04; Fisher exact test), in that ≥200,000 CFU/mL appeared to be its threshold for exposure of T-antigen.
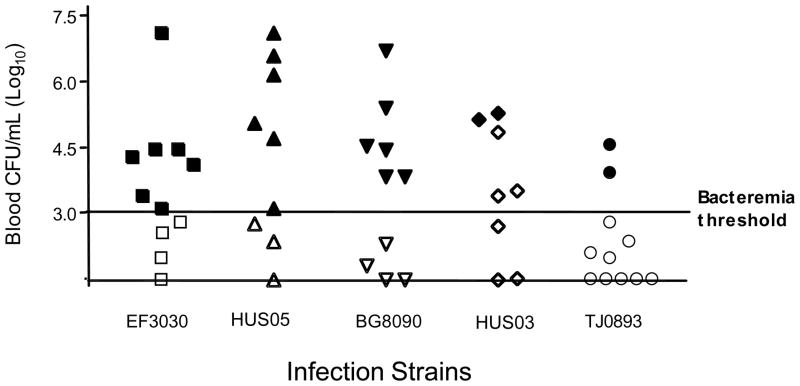
Figure 2.
Bacteremia is sufficient to cause T-antigen exposure during infection. Mice were infected intravenously with ~107 CFUs of S. pneumoniae. Symbols denote the highest number of CFUs obtained for an individual animal at their 24, 48, and 72 h bleeds following infection. Closed and open symbols indicate animals that become positive or remained negative, respectively, for T-antigen exposure on RBC during five-day infection period. Animals with no detectable bacteremia were assigned a value of 101.48, the limit of detection of CFUs in the blood. Bacteremia was self-resolving and no mice died following infection. For each strain of bacteria we used the Fisher exact test to look for an association between CFUs greater than 103 CFUs/mL and T-antigen exposure. The association was significant for all strains at P < 0.02 except for HUS03. Using a multi-row Chi-square contingency table the ability of mice with >103 CFUs to exhibit T-antigen on RBC was found to vary among the strains at P < 0.02.
These results raised the possibility that differences in NanA levels produced by the different bacteria were responsible for the difference in the threshold of bacteremia associated with T-antigen exposure between HUS03 and the other strains examined. To examine this possibility growth curves were conducted with the strains in our standard THY broth and levels of neuraminidase activity at various time points. The neuraminidase activity observed for HUS03 in vitro at all phases of growth was indistinguishable from that of the other strains (data not shown). These findings, however, do not rule out the possibility that the strains still might differ in neuraminidase production during an in vivo during an infection.
2.3. Treatment with penicillin increased the chance of T-antigen exposure
Since, neuraminidase A is cell surface attached it was possible that the lysis of pneumococci caused by treatment with antibiotics could release neuraminidase and make it more able to pass from the lung into the blood. To test this possibility mice were infected with EF3030 via nasal aspiration under conditions where the pneumococci were not detected in the blood [37]. After 5 days of antibiotic treatment, the bacterial load in the lungs was almost 100-fold lower in penicillin treated mice than in Ringer’s solution treated mice (P=0.0001, Mann-Whitney test) (Figure 3). The bacterial load in the untreated group of mice euthanized at 24 hours was higher than that of Ringer’s-treated mice on day 6 (Figure 3) indicating that the EF3030 infection in mice was self resolving as expected [37].
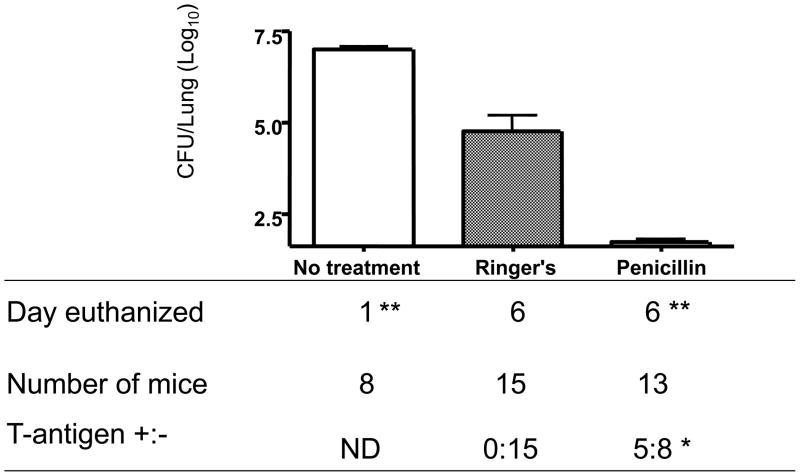
Figure 3.
Clearance of S. pneumoniae following penicillin treatment increases T-antigen exposure. Mice received lung infections with 107 CFUs EF3030 given IN. At 24 hours post infection, animals received penicillin or lactated Ringers treatment every 24 hours until euthanized on day 6, at which time the numbers of CFUs in their lungs were determined. *Effect of penicillin on exposure of T-antigen in mice, P=0.0131 versus Ringer's solution control by the Fisher’s exact test. **Differences in the numbers of CFUs in the lungs of penicillin-treatment versus Ringer's solution control was significant at P≤0.0001, by the Mann-Whitney test.
However, the infected mice receiving 6000U of penicillin were statistically more likely to have T-antigen exposed on their RBC (P=0.0131, Fisher’s exact test) than infected mice receiving only Ringer’s solution. Of the 39 infected mice only 3 were found to be bacteremic (CFUs > 101.49 CFU/mL). These three mice were excluded from statistical analysis of T-antigen exposure. Our results suggest that the penicillin induced autolysis of pneumococci [38, 39] resulted in neuraminidase release from the bacteria in the lungs, which reached the circulation where it was able expose T-antigen exposure on RBC.
2.4. Neuraminidase deficient strains of S. pneumoniae
Parent and neuraminidase deficient strains were grown in THY to O.D. 0.5 at 600 nm. Mutations were verified using PCR, Western blotting and neuraminidase activity measurements (Data not shown).
2.5. NanA exposes T-antigen on RBC in vivo
Intravenous infection with EF3030 resulted in the exposure of T-antigen on circulating RBC (solid symbols, Figure 4). However, infection with strains deficient in NanA alone (Sam11 and Sam1) or deficient in both NanA and NanB (Sam30 and Sam3) did not result in exposure of T-antigen on circulating RBC (open symbols, Figure 4) following intravenous infection (P=0.002, chi-square analysis). The two strains with mutations in nanB but not nanA (Sam 20 and Sam 2) exposed T-antigen as efficiently as the wild type strain EF3030. Thus, the expression of NanA but not NanB was required for T-antigen exposure in vivo.
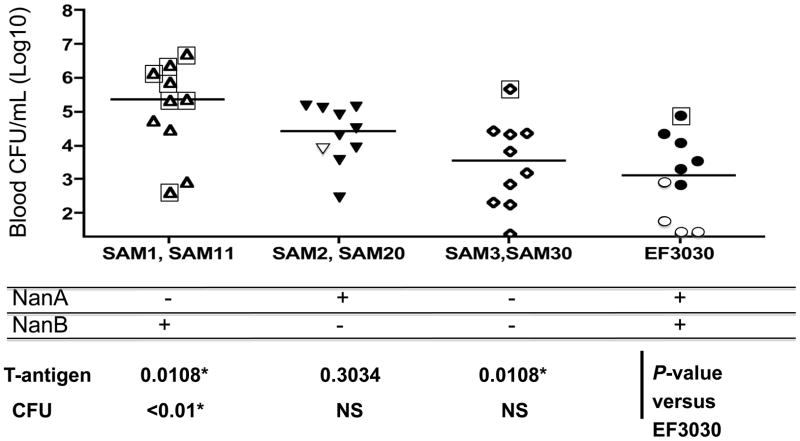
Figure 4.
NanA was required for T-antigen exposure. Groups of 10 mice were infected intravenously with EF3030 or neuraminidase deficient derivatives of EF3030. The mice were bled every 24 hours, until death. Each symbol represents the highest number of CFU observed for each mouse in the first 72 hours. Closed and open symbols indicate animals that were positive or negative for T-antigen on their RBCs during the 5-day period of observation. For each NanA/NanB genotype two independent mutants were examined and the pooled data are shown. Overall the numbers of T antigen positive and negative mice of the different genotypes were found to differ among the groups using a 2×4 chi-square analysis (P < 0.0001). The differences in CFUs in the blood for the 4 genotypes was significant at P=0.012 by Kruskal-Wallis. With the Duns multiple comparison test only the difference between NanA−/NanB+ and EF3030 was statistically significant (P < 0.05). Mice that died are marked with □. All deaths occurred only on days 2, 3, or 4. The median day of death for mice infected with NanA− EF3030 was 3. The single deaths in groups infected with NanA−/NanB− and EF303 were on days 4 and 3 respectively. By a 2×4 Chi-Square analysis the distributions of deaths in the four groups was significant by P<0.0005. The NanA−/NanB+ group differed from each of the other groups by Fisher exact test at P<0.02.
2.6. Effects of NanA and NanB on bacteremia and mortality
Intravenous infections with the nanA and nanB mutant strains resulted in somewhat higher levels of bacteria in the blood for the mutant strains than for the wild type EF3030. This difference was only statistically significant, for the comparison of the NanA deficient strain with the EF3030 parent (Figure 4). It was also observed that 7 of 10 mice infected with NanA− pneumococci died following lung infection whereas only 0 to 1 of 10 mice infected with either wild type, NanB− or NanA /NanB pneumococci died (Figure 4). An earlier study by Manco et al. using the much more virulent D39 strain background observed that both mutations in both nanA and nanB decreased blood CFUs [34]. The difference in results between their study and ours may be caused by the much greater virulence of D39 versus EF3030 pneumococci, which may affect the relative role of NanA on virulence of the different strains.
3. DISCUSSION AND CONCLUSION
HUS is an unpredictable consequence of S. pneumoniae infection of infants and young children. While the incidence of diarrhea associated with Gram-negative HUS is higher than with pneumococcal HUS, patients with pneumococcal HUS require a longer average hospital stay, have a higher mortality, and more frequently require renal dialysis [2, 8]. It has long been assumed that the neuraminidase detected in the blood of patients with pneumococcal HUS is produced by the pneumococci and is responsible for exposing T-antigen in the kidney and on RBC of HUS patients [9]. Our findings provide experimental support for this earlier prediction and demonstrate that NanA, but not NanB is necessary for T-antigen exposure.
Gene activation studies indicate that nanA is more strongly expressed in the transparent phase of pneumococci that colonizes the surface of the nasal mucosa than in opaque phase pneumococci responsible for invasive disease [28]. However, NanA is sufficiently expressed during invasive disease that immunity to NanA offers some protection in mice against intraperitoneal-sepsis and intranasal-sepsis following inoculation of S. pneumoniae [34, 36].
This study demonstrated that infection of mice with wild-type pneumococci, but not NanA-deficient pneumococci resulted in exposure of T-antigen in vivo. Since the expression of NanA increased the numbers of pneumococci in circulation during the first 72 hours following infection, it was clear that exposure of T-antigen required NanA and not pneumococcal infection per se. This conclusion is further supported by the fact that in the study depicted in figure 4 the mice with the highest levels of CFU in the blood were those infected with the NanA pneumococci, and none of them exhibited any exposure of T-antigen on their RBC. NanB, however, had no effect on the exposure of T-antigen in vivo. The activity of NanA but not NanB in the exposure of T-antigen on RBC in vivo was consistent with NanB’s reported low enzymatic activity and the fact that the pH optima of NanB (pH 4.5) is well below the pH of blood [32].
The exposure of T-antigen by infecting pneumococci was a general effect of pneumococcal infection, since it was observed with all 5 wild-type strains examined. Several capsular polysaccharide serotypes have been reported in cases of pneumococcal HUS, and case reports suggest many of the patients had a heavy bacterial load, e.g., empyema or more than one site of infection (e.g., pneumonia and meningitis) [4, 8]. We observed that levels of bacteremia exceeding 103/mL in mice were associated with the in vivo exposure of T-antigen on RBC. For one of our strains, however, more CFUs/mL of blood was required to expose T-antigen than for the other four strains. Since we observed no less NanA produced in vitro by this strain, this result suggests that the threshold of bacteremia required for T-antigen exposure might be affected by differences in the activity or amount of NanA produced during infections.
Interestingly, the absence of NanA from EF3030 resulted in higher blood levels of CFU following lung infection, and lead to much higher death rate (Figure 4). While this may seem surprising for an antigen thought of as a virulence antigen, it must be remembered that selection for invasive disease is probably not necessarily important in the evolution of pneumococci, which are through to be spread largely spread and acquisition of colonization [40]. The greater virulence of the NanA− than wild-type EF3030 may be because the NanA− bacteria are less adhesive to the host gangliosides and thus more able to spread through tissues [23, 26]. The fact that NanA pneumococci were significantly more virulent than mice lacking both NanA or NanB (Figure 4) is consistent with the claim by Manco et al that NanB has an effect on invasive pneumococcal disease [34].
Our observation that penicillin treatment during lung infection of mice enhanced T-antigen exposure on circulating RBC suggested that neuraminidase released by the autolysis of the pneumococci following antibiotic treatment could reach the circulatory system. Withholding antibiotics during patient treatment is not a viable option in pneumococcal infection. But, one possible approach to minimize T-antigen exposure and resultant risk of HUS might be to give passive antibody to NanA or a neuraminidase inhibitor to infants suffering from sever pneumococcal infections.
A difference in T-antigen exposure, by itself, is probably not the only predictor in the development of HUS [3]. Antibody to neuraminidase develops in most individuals by 2 years of age [41] and antibody titers to T-antigen can be detected in most infants by 6 months of age [8]. Thus, major additional factors predisposing infants to HUS may be variable levels of sensitizing maternal antibodies to T-antigen and the presence of neutralizing maternal antibodies to pneumococcal neuraminidase.
4. METHODS
4.1. S. pneumoniae
Wild-type pneumococcal strains were all clinical isolates (Table 1). Strains EF3030, BG8090, and TJ0893 were capsular types 19F, 19, and 14 [37, 42, 43]. Strains HUS03 (19A) and HUS05 (19F) were collected from cases of pneumococcal HUS [44] in Dallas, Texas, 1997–1998 with IRB approval. Pneumococci were grown to exponential phase in Todd Hewitt broth containing 0.5% yeast extract [45], concentrated by centrifugation when necessary, and were aliquoted and stored in THY containing 10% glycerol at −80°C until use. Strains carrying antibiotic markers were plated on selective blood agar containing appropriate antibiotics.
Table 1.
Strains included in this study
| Strain | Capsule type | Phenotype | Derivation | Reference |
|---|---|---|---|---|
| EF3030 | 19F | NanA+, NanB+ | Clinical isolate | [37] Otitis Media ~1980 |
| HUS05 | 19F | NanA+, NanB+ | Clinical isolate | [44] HUS isolate, 1995 |
| BG8090 | 19 | NanA+, NanB+ | Clinical isolate | Meningitis isolate, 1986 |
| HUS03 | 19A | NanA+, NanB+ | Clinical isolate | [44] HUS isolate, 1995 |
| TJ0893 | 14 | NanA+, NanB+ | Clinical isolate | Pneumonia isolate (AIDS patient), 1993 |
| Sam1 | 19 | NanA−, NanB+ | EF3030 | This study |
| Sam11 | 19 | NanA−, NanB+ | EF3030 | This study |
| Sam2 | 19 | NanA+, NanB− | EF3030 | This study |
| Sam20 | 19 | NanA+, NanB− | EF3030 | This study |
| Sam3 | 19 | NanA−, NanB− | EF3030 | This study |
| Sam30 | 19 | NanA−, NanB− | EF3030 | This study |
A NanA deficient strain in the D39 background was made by insertion-duplication mutagenesis and has been described previously [46]. Chromosomal DNA was isolated from this strain and transformed into strain EF3030 to produce a nanA mutant of EF3030. Transformations were carried out as described previously [47] and genes were backcrossed into recipient strains three times to eliminate co-transformation with non-contiguous DNA (strains Sam1, Sam11). Transformants were selected based on erythromycin resistance and verified with PCR. EF3030 NanB deficient strains were also constructed using the insertion-duplication mutagenesis technique (Sam2, Sam20). EF3030 derivatives lacking NanA and NanB were constructed by transforming EF3030 with DNA from an existing nanA/nanB double mutant into EF3030 to produce two independent strains containing both the NanA and NanB mutations (Sam3, Sam30) [34]. The double transformants were selected using erythromycin alone; as nanA and nanB are closely linked on the chromosome [29] and co-transformants containing both mutants could easily be recovered. The presence of both mutations in the new strains was confirmed by PCR analysis.
4.2. Mice
Female CBA/CaHN-Btkxid/J (CBA/N) mice were purchased for Jackson Laboratory, Bar Harbor, Maine. Mice were 8–12 weeks old at the time of the experiments. Animal studies were done under approval from University of Alabama at Birmingham IACUC 041206026.
4.3. Intravenous infection of CBA/N mice with S. pneumoniae
Bacteremia/sepsis was induced by intravenous infection ~107 CFUs of the indicated strains as described [48]. Eight to 11 mice were used in each group. Blood (0.07 ml diluted immediately with 500μl of 1% BSA in PBS) was collected every 24 hours by retro-orbital puncture under isoflurane (Minrad Inc. Buffalo, NY) for the first 3 days post infection, and when indicated on day 5. Serial dilutions of the blood were plated on blood agar plates and CFUs enumerated [48]. Mice were euthanized 5 or 7-days post infection as indicated and kidneys were harvested where indicated.
4.4. Detection of T-antigen exposure on RBC
To detect T-antigen exposure on RBC, diluted blood was collected as described and the RBC pelleted. The pelleted RBC were suspended to 2% in physiologic saline. One volume of RBC was incubated with 2 volumes of Arachis hypogea lectin, peanut lectin, (Gamma Biologicals, Houston, TX) for 5 minutes at room temperature. Peanut lectin binds Galβ-1-3GalNAc. The mixture was centrifuged to pellet the RBC. RBC were resuspended and read macroscopically for agglutination. Detectable RBC agglutination following centrifugation was indicative of T-antigen exposure (T-antigen+). RBC that failed to show detectable agglutination were considered negative for T-antigen exposure (T-antigen−). Negative agglutination was verified using a 1.5X magnifying glass.
4.5. T-antigen exposure in the kidney
For histology, organs from infected mice were fixed in 10% formalin, dehydrated, and embedded in paraffin. Four-micron thick sections were cut. Sections were deparaffinized in 2 changes of xylene and rehydrated through graded ethanol solutions ending in deionized water. Exposed T-antigen was detected by incubating the sections with 1mg/mL horse radish peroxidase (HRP)-labeled peanut lectin (Sigma, St. Louis, Missouri) for 1 hour at room temperature. Following incubation slides were incubated with 3-3’ diaminobenzidine tetrahydrochloride (DAB) (Sigma, St. Louis, Missouri) at room temperature for 30 minutes. Slides were dehydrated using ethanol, mounted and observed.
4.6. The effect of penicillin on the exposure of T-antigen by infecting pneumococci
A mouse model of pneumococcal pneumonia was used [47]. Mice were anesthetized to promote aspiration prior to being inoculated intranasally with 107 CFUs of EF3030 in 40 μl Ringer’s Injection solution (Abbott labs, Chicago). Beginning 24 hours after infection, mice were treated intraperitoneally with 6000U penicillin G or Ringer’s solution every 24 hours and were euthanized 6 days post inoculation. A third group of mice received neither penicillin nor Ringer’s solution and were euthanized 24 hours after infection. The purpose of the third group was to quantitate the levels of bacteria in the lungs prior to any treatment. All groups of mice were monitored for survival every 8 hours until euthanized. When each mouse was euthanized lungs, nasal wash, and blood were collected. Organs and blood were plated on blood agar plates containing gentamicin (4μg/mL).
4.7. Neuraminidase activity
Neuraminidase activity was detected on logarithmic phase cultures in a quantitative assay using the substrate, 2’-(4-methylumbelliferyl)-a-D-N-acetylneuraminic acid (MUAN) (Sigma Chemical) as described by Lock et al [20]. The substrate, 2’-(4-methylumbelliferyl)-a-D-N-acetylneuraminic acid (MUAN) (Sigma Chemical), is prepared as a 0.35% solution in water. Bacteria were grown to an optical density of 0.5 at 600nm. Cultures contained approximately 1×106 CFUs/mL bacteria. Cells were centrifuged pelleted. Cell pellets and culture supernatants were collected. Cell pellets were lysed using a solution containing 0.1% sodium deoxycholate, 0.01% sodium dodecyl sulfate, and 0.15M sodium citrate) at stored at −20°C until time of assay. For neuraminidase activity measurements, equal volumes of substrate and cell lysate or supernatant were mixed. Mixtures were incubated for 5 minutes at 37°C at pH 7.0. The reaction was stopped by the addition of 10 volumes of 0.5 M sodium carbonate buffer (pH 10.5). The amount of 4-methyl-umbelliferone (MU) released was measured (excitation wavelength of 355 nm and emission wavelength of 460 nM) [49]. Measurements were made and quantitated by comparison to the optical density of a known concentration of MU.
4.8. Statistical analysis
For statistical analysis, Mann Whitney, chi square analysis, Kruskal-Wallis, and Duns multiple comparison were used as indicated.
Acknowledgments
We would like to thank Alexis Brooks-Walter for her contributions in initial studies with our HUS strains. Janet Yother helped with the fluorimetric studies. Special thanks to Trenton R. Schoeb, Eugene Rivers, II, Roslyn Crowder, J. Yvette Hale, James Watt and Tamela Thomas.
Although Trudy Murphy is employed at the Centers for Disease Control and Prevention, her scientific contribution to the study was performed prior to her employment at the CDC. The findings and conclusions in this article have neither been formally disseminated by the CDC, nor should they be construed to represent the determination or policy of any federal Agency.
This work was supported by NIH grant AI21548 to D.E.B. Mamie T. Coats was supported by the Ruth L. Kirschstein National Research Service Award.
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the CDC (Centers for Disease Control and Prevention).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandt C, Power UF, Plotnicky-Gilquin H, Huss T, Nguyen T, Lambert PH, et al. Protective immunity against respiratory syncytial virus in early life after murine maternal or neonatal vaccination with the recombinant G fusion protein BBG2Na. J Infect Dis. 1997 Oct;176(4):884–91. doi: 10.1086/516503. [DOI] [PubMed] [Google Scholar]
- 2.Constantinescu AR, Bitzan M, Weiss LS, Christen E, Kaplan BS, Cnaan A, et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am J Kidney Dis. 2004 Jun;43(6):976–82. doi: 10.1053/j.ajkd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Copelovitch L, Kaplan BS. Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Nephrol. 2008 Nov;23(11):1951–6. doi: 10.1007/s00467-007-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray B. Is pneumococcal hemolytic-uremic syndrome a new disease? Infections in Medicine. 2001;18(5):251–8. [Google Scholar]
- 5.Waters AM, Kerecuk L, Luk D, Haq MR, Fitzpatrick MM, Gilbert RD, et al. Hemolytic uremic syndrome associated with invasive pneumococcal disease: the United kingdom experience. J Pediatr. 2007 Aug;151(2):140–4. doi: 10.1016/j.jpeds.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 6.Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006 Aug;70(3):423–31. doi: 10.1038/sj.ki.5001581. [DOI] [PubMed] [Google Scholar]
- 7.Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R. Hämolytich-urämiche Syndrome: bilaterale Nierenrindennekrosen bei akuten erworbenen hämolytischen Anämien [Hemolytic-uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia.] Schweiz Med Wochenschr. 1955;85:905–9. [PubMed] [Google Scholar]
- 8.Cabrera GR, Fortenberry JD, Warshaw BL, Chambliss CR, Butler JC, Cooperstone BG. Hemolytic uremic syndrome associated with invasive Streptococcus pneumoniae infection. Pediatrics. 1998 Apr;101(4 Pt 1):699–703. doi: 10.1542/peds.101.4.699. [DOI] [PubMed] [Google Scholar]
- 9.Klein PJ, Bulla M, Newman RA, Muller P, Uhlenbruck G, Schaefer HE, et al. Thomsen- Friedenreich antigen in haemolytic-uraemic syndrome. Lancet. 1977 Nov 12;2(8046):1024–5. doi: 10.1016/s0140-6736(77)92915-4. [DOI] [PubMed] [Google Scholar]
- 10.Hanisch FG, Baldus SE. The Thomsen-Friedenreich (TF) antigen: a critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen. Histol Histopathol. 1997 Jan;12(1):263–81. [PubMed] [Google Scholar]
- 11.Scanlon KL, Diven WF, Glew RH. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme. 1989;41(3):143–50. doi: 10.1159/000469069. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins CK, Yuan S, Lu Q, Ziman A, Goldfinger D. A severe case of atypical hemolytic uremic syndrome associated with pneumococcal infection and T activation treated successfully with plasma exchange. Transfusion. 2008 Jul 30; doi: 10.1111/j.1537-2995.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 13.Zabel PL, Noujaim AA, Shysh A, Bray J. Radioiodinated peanut lectin: a potential radiopharmaceutical for immunodetection of carcinoma expressing the T antigen. Eur J Nucl Med. 1983;8(6):250–4. doi: 10.1007/BF00522515. [DOI] [PubMed] [Google Scholar]
- 14.Brandt J, Wong C, Mihm S, Roberts J, Smith J, Brewer E, et al. Invasive pneumococcal disease and hemolytic uremic syndrome. Pediatrics. 2002 Aug;110(2 Pt 1):371–6. doi: 10.1542/peds.110.2.371. [DOI] [PubMed] [Google Scholar]
- 15.Cochran JB, Panzarino VM, Maes LY, Tecklenburg FW. Pneumococcus-induced T-antigen activation in hemolytic uremic syndrome and anemia. Pediatr Nephrol. 2004 Jan 9;19(3):317–21. doi: 10.1007/s00467-003-1382-z. [DOI] [PubMed] [Google Scholar]
- 16.McGraw ME, Lendon M, Stevens RF, Postlethwaite RJ, Taylor CM. Haemolytic uraemic syndrome and the Thomsen Friedenreich antigen. Pediatr Nephrol. 1989 Apr;3(2):135–9. doi: 10.1007/BF00852894. [DOI] [PubMed] [Google Scholar]
- 17.Novak RW, Martin CR, Orsini EN. Hemolytic-uremic syndrome and T-cryptantigen exposure by neuraminidase-producing pneumococci: an emerging problem? Pediatr Pathol. 1983 Oct–Dec;1(4):409–13. doi: 10.3109/15513818309025872. [DOI] [PubMed] [Google Scholar]
- 18.Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun. 2006 Jun;74(6):3360–5. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King SJ, Whatmore AM, Dowson CG. NanA, a neuraminidase from Streptococcus pneumoniae, shows high levels of sequence diversity, at least in part through recombination with Streptococcus oralis. J Bacteriol. 2005 Aug;187(15):5376–86. doi: 10.1128/JB.187.15.5376-5386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock RA, Paton JC, Hansman D. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog. 1988 Jan;4(1):33–43. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- 21.Shakhnovich EA, King SJ, Weiser JN. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect Immun. 2002 Dec;70(12):7161–4. doi: 10.1128/IAI.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong HH, Liu X, Chen Y, James M, Demaria T. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta Otolaryngol. 2002 Jun;122(4):413–9. doi: 10.1080/00016480260000111. [DOI] [PubMed] [Google Scholar]
- 23.Tong HH, Blue LE, James MA, DeMaria TF. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun. 2000 Feb;68(2):921–4. doi: 10.1128/iai.68.2.921-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med. 2009 Aug 31;206(9):1845–52. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong HH, James M, Grants I, Liu X, Shi G, DeMaria TF. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchillas infected with a Streptococcus pneumoniae neuraminidase-deficient mutant or its isogenic parent strain. Microb Pathog. 2001 Dec;31(6):309–17. doi: 10.1006/mpat.2001.0473. [DOI] [PubMed] [Google Scholar]
- 26.Van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA, Briles DE. Pneumococcal carriage results in ganglioside- mediated olfactory tissue infection. Proc Natl Acad Sci U S A. 2003 Nov 10;24:14363–7. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001 Jun;65(2):187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King SJ, Hippe KR, Gould JM, Bae D, Peterson S, Cline RT, et al. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol Microbiol. 2004 Oct;54(1):159–71. doi: 10.1111/j.1365-2958.2004.04252.x. [DOI] [PubMed] [Google Scholar]
- 29.Kharat AS, Tomasz A. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect Immun. 2003 May;71(5):2758–65. doi: 10.1128/IAI.71.5.2758-2765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camara M, Boulnois GJ, Andrew PW, Mitchell TJ. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect Immun. 1994 Sep;62(9):3688–95. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001 Jul 20;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 32.Berry AM, Lock RA, Paton JC. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J Bacteriol. 1996 Aug;178(16):4854–60. doi: 10.1128/jb.178.16.4854-4860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gut H, King SJ, Walsh MA. Structural and functional studies of Streptococcus pneumoniae neuraminidase B: An intramolecular trans-sialidase. FEBS Lett. 2008 Oct 15;582(23–24):3348–52. doi: 10.1016/j.febslet.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Manco S, Hernon F, Yesilkaya H, Paton JC, Andrew PW, Kadioglu A. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect Immun. 2006 Jul;74(7):4014–20. doi: 10.1128/IAI.01237-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock RA, Paton JC, Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988 Dec;5(6):461–7. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 36.Yesilkaya H, Soma-Haddrick S, Crennell SJ, Andrew PW. Identification of amino acids essential for catalytic activity of pneumococcal neuraminidase A. Res Microbiol. 2006 Jul–Aug;157(6):569–74. doi: 10.1016/j.resmic.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Briles DE, Hollingshead SK, Paton JC, Ades EW, Novak L, Van Ginkel FW, et al. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J Infect Dis. 2003 Aug 1;188(3):339–48. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- 38.Hakenbeck R, Martin C, Morelli G. Streptococcus pneumoniae proteins released into medium upon inhibition of cell wall biosynthesis. J Bacteriol. 1983 Sep;155(3):1372–81. doi: 10.1128/jb.155.3.1372-1381.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queener S, Webber J. Beta-lactam Antibiotics for Clinical Use. Informa Health Care; 1986. [Google Scholar]
- 40.Bogaert D, Veenhoven RH, Sluijter M, Wannet WJ, Rijkers GT, Mitchell TJ, et al. Molecular epidemiology of pneumococcal colonization in response to pneumococcal conjugate vaccination in children with recurrent acute otitis media. J Clin Microbiol. 2005 Jan;43(1):74–83. doi: 10.1128/JCM.43.1.74-83.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simell B, Korkeila M, Pursiainen H, Kilpi TM, Kayhty H. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin A, pneumolysin, and pneumococcal surface protein A in children. J Infect Dis. 2001;183:887–96. doi: 10.1086/319246. [DOI] [PubMed] [Google Scholar]
- 42.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 Jan;60(1):111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997 Sep;23(3):127–37. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 44.Pastor P, Medley F, Murphy TV. Invasive Pneumococcal Disease in Dallas County, Texas: Results from Population-Based Surveillance in 1995. Clinical Infectious Diseases. 1998;26:590–5. doi: 10.1086/514589. [DOI] [PubMed] [Google Scholar]
- 45.Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon WJ, et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008;8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry AM, Paton JC. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000 Jan;68(1):133–40. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balachandran P, Brooks-Walter A, Virolainen-Julkunen A, Hollingshead SK, Briles DE. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect Immun. 2002 May;70(5):2526–34. doi: 10.1128/IAI.70.5.2526-2534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182:1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 49.Berry AM, Paton JC, Glare EM, Hansman D, Catcheside DE. Cloning and expression of the pneumococcal neuraminidase gene in Escherichia coli. Gene. 1988 Nov 30;71(2):299–305. doi: 10.1016/0378-1119(88)90046-7. [DOI] [PubMed] [Google Scholar]