Abstract
In Xenopus, dorsal–ventral (D–V) patterning can self-regulate after embryo bisection. This is mediated by an extracellular network of proteins secreted by the dorsal and ventral centers of the gastrula. Different proteins of similar activity can be secreted at these two poles, but under opposite transcriptional control. Here we show that Crescent, a dorsal protein, can compensate for the loss of Sizzled, a ventral protein. Crescent is a secreted Frizzled-Related Protein (sFRP) known to regulate Wnt8 and Wnt11 activity. We now find that Crescent also regulates the BMP pathway. Crescent expression was increased by the BMP antagonist Chordin and repressed by BMP4, while the opposite was true for Sizzled. Crescent knock-down increased the expression of BMP target genes, and synergized with Sizzled morpholinos. Thus, Crescent loss-of-function is compensated by increased expression of its ventral counterpart Sizzled. Crescent overexpression dorsalized whole embryos but not ventral half-embryos, indicating that Crescent requires a dorsal component to exert its anti-BMP activity. Crescent protein lost its dorsalizing activity in Chordin-depleted embryos. When co-injected, Crescent and Chordin proteins greatly synergized in the dorsalization of Xenopus embryos. The molecular mechanism of these phenotypes is explained by the ability of Crescent to inhibit Tolloid metalloproteinases, which normally degrade Chordin. Enzyme kinetic studies showed that Crescent was a competitive inhibitor of Tolloid activity, which bound to Tolloid/BMP1 with a KD of 11 nM. In sum, Crescent is a new component of the D–V pathway, which functions as the dorsal counterpart of Sizzled, through the regulation of chordinases of the Tolloid family.
Keywords: BMP signaling, Wnt signaling, Tolloid, Chordin, sFRP Morphogenetic field, Sizzled, Ogon, Crossveinless-2
Introduction
The early embryo has the capacity of self-regulating pattern. For example, when an amphibian embryo is bisected in such a way that both halves contain a part of the dorsal Spemann organizer, each half can regenerate a complete embryo and give rise to identical twins. Similarly, transplantation of dorsal organizer tissue into the ventral side of the gastrula embryo can generate a second morphogenetic field, resulting in the formation of Siamese twins (Spemann, 1938; De Robertis, 2006). In Xenopus and zebrafish, this remarkable inductive capacity is mediated by a network of interacting secreted proteins that establishes a gradient of Bone Morphogenetic Protein (BMP) signaling along the D–V axis (Little and Mullins, 2006; De Robertis, 2009). The Spemann organizer, where BMP signaling level is lowest, secretes the BMP antagonists Chordin, Noggin and Follistatin along with two BMPs, ADMP and BMP2. In the ventral center, genes such as Crossveinless-2 (CV2), BMP4, BMP7 and Sizzled are expressed in regions of high BMP signaling. This reciprocal transcriptional control at opposite poles helps explain self-regulation (De Robertis, 2009).
Chordin is a key D–V regulator secreted in large amounts by dorsal organizer tissue (Lee et al., 2006). Chordin binds to BMPs in the extracellular space and prevents them from binding to their cognate receptors, thus preventing signaling. Chordin/BMP complexes formed in more dorsal regions of the embryo are transported to ventral regions, where BMP ligands are released from inactive Chordin/BMP complexes by the cleavage of Chordin at two specific sites by Tolloid proteinases (Piccolo et al., 1997). This cleavage is facilitated by Ont-1, a scaffold protein of the Olfactomedin family that brings together Tolloid and its substrate Chordin (Inomata et al., 2008). Mathematical modeling suggests that the dorsal to ventral flux of Chordin/BMP provides robustness to the system (Ben-Zvi et al., 2008; Plouhinec and De Robertis, 2009). The Chordin/BMP/Tolloid/CV2 network is an evolutionarily conserved biochemical pathway that regulates D–V patterning in many invertebrates, including Drosophila, and vertebrates (Little and Mullins, 2006; De Robertis, 2008; Umulis et al., 2009).
Extracellular regulation of growth factor signals is a common theme in embryonic patterning (Zakin and De Robertis, 2010). In addition to Chordin, many other growth factor inhibitors are produced in the Xenopus gastrula, such as the BMP inhibitors Noggin (Zimmerman et al., 1996), Follistatin (Hemmati-Brivanlou et al., 1994), and Gremlin (Hsu et al., 1998), and Wnt inhibitors such as Dickkopf (Dkk, an LRP6 inhibitor) (Glinka et al., 1998) and the sFRPs Frzb, sFRP2, Sizzled and Crescent (De Robertis and Kuroda, 2004). Multivalent inhibitors, such as Cerberus, which antagonizes Nodal, BMP and Wnt, and Coco/Cerl2, which inhibits Nodal, Activin and BMP, are also secreted (Belo et al., 2009; Schwickert et al., 2010). CV2 is expressed ventrally, where it avidly binds Chordin and Chordin/BMP complexes, serving as a sink for the continuous flow of dorsally secreted molecules towards the ventral center (Ambrosio et al., 2008; Kelley et al., 2009).
sFRPs contain Frizzled Wnt-binding domains and antagonize Wnt signaling by preventing their binding to Frizzled receptors (Leyns et al., 1997; Shibata et al., 2005). Structural predictions suggest that the Frizzled domains in sFRPs may recognize lipid modifications present in Wnts (Willert et al., 2003; Bazan and de Sauvage, 2009). Some sFRPs have also been shown to enhance Wnt signaling (Uren et al., 2000; Bovolenta et al., 2008). Importantly, Crescent and Frzb were recently found to greatly enhance the diffusion of Wnt in Xenopus embryos, transporting Wnts and allowing them to signal at considerable distances from where they are secreted (Mii and Taira, 2009).
Perhaps the most surprising function of any sFRP is that of the ventrally expressed sFRP Sizzled (Salic et al., 1997), also called Ogon/Mercedes in zebrafish (Hammerschmidt et al., 1996). Sizzled appears to have lost the Wnt inhibitory activity of its Frizzled domain (Collavin and Kirschner, 2003; Yabe et al., 2003). Importantly, Sizzled acts as a feedback inhibitor of BMP signaling by binding to and competitively inhibiting Tolloids, the metalloproteinases that cleave Chordin (Lee et al., 2006; Muraoka et al., 2006). Sizzled is a key player in D–V self-regulation: when BMP levels increase, sizzled expression in the ventral center increases, causing inhibition of Tolloid enzymes, preventing the release of BMP from Chordin/BMP complexes and, in this indirect way, decreasing BMP signaling (Lee et al., 2006).
Crescent is the closest relative of Sizzled, and was initially isolated in our laboratory as a cDNA expressed in the anterior endomesodermal crescent of the chick embryo (Pfeffer et al., 1997). In Xenopus, Crescent is expressed on the dorsal side of the gastrula in the deep anterior endoderm and later in the prechordal plate (Pera and De Robertis, 2000; Shibata et al., 2000). Crescent differs from Sizzled in that it is able to bind and inhibit Wnt8 and Wnt11 activity in Xenopus embryos (Shibata et al., 2005; Marvin et al., 2001; Schneider and Mercola, 2001; Dickinson and Sive, 2009).
An interesting feature of the D–V patterning pathway is that many of its components have counterparts of similar structure and biochemical activity in the dorsal and in the ventral center. For example, Chordin and CV2, as well as ADMP/BMP2 and BMP4/BMP7, are expressed on opposite sides of the gastrula embryo (Fig. 1A). Given the sequence similarity between Crescent and Sizzled, it seemed possible that these two sFRPs could constitute an additional pair of secreted molecules with similar functions, expressed at different poles of the embryo under opposite transcriptional control. If so, Crescent and Sizzled could provide a new layer of resilience to the D–V patterning pathway.
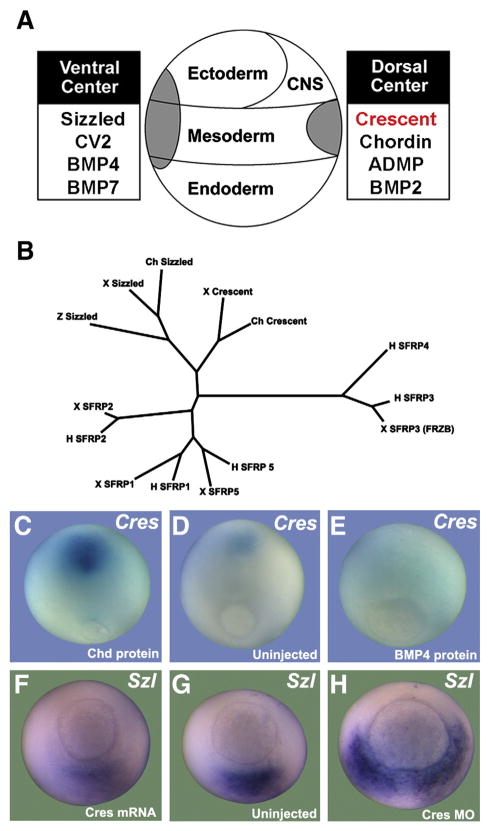
Fig. 1.
Xenopus crescent is expressed dorsally and repressed by BMP signaling. (A) D–V Patterning is regulated by proteins secreted by the dorsal and ventral signaling centers. For the proteins listed, proteins of similar function are secreted by the two sides, but under opposite transcriptional control. (B) sFRPs of Xenopus (x), human (h), zebrafish (z) and chicken (ch) origin were compared using Molecular Evolutionary Genetics Analysis (MEGA) software (Tamura et al., 2007). Crescent and Sizzled are philogenetically related, and distant from the other sFRPs. (C–E) Crescent expression is under negative transcriptional control by BMP4 signaling. Microinjection of Chordin (Chd) protein increases crescent transcripts, while microinjection of BMP4 protein decreases crescent expression in stage 12 gastrulae. (F–H) sizzled expression is inhibited by injection of crescent mRNA and markedly expanded upon depletion of Crescent (Cres MO); an uninjected sibling at stage 11 is shown for comparison.
In this paper we show that Crescent inhibits the activity of Tolloid proteinases and is under the opposite transcriptional regulation from that of Sizzled. Crescent bound to the Tolloid enzyme BMP1 with affinities within the physiological range. Enzyme kinetic analyses showed that Crescent inhibited the cleavage of a fluorogenic peptide substrate mimicking the Chordin cleavage site. Since Crescent itself was not cleaved, it acts as a competitive inhibitor of Tolloid proteinases. This novel function of Crescent in the Chordin/BMP pathway was supported by embryological experiments in which the anti-BMP phenotypes caused by Crescent overexpression were shown to require Chordin. Co-injections of Crescent and Chordin protein into the blastula cavity had synergistic dorsalizing effects. A point mutation mimicking the zebrafish ogon mutation eliminated Tolloid inhibition in biochemical assays and greatly reduced the anti-BMP effects of Crescent protein in Xenopus embryos. We propose that Crescent is a competitive inhibitor of Tolloid proteinases, and a novel component of the extracellular Chordin/BMP biochemical pathway that regulates D–V patterning.
Materials and methods
Morpholino oligos and embryonic manipulations
Antisense Morpholinos (Gene Tools) were as described: Chordin MO (Oelgeschläger et al., 2003), Sizzled MO (Collavin and Kirschner, 2003), and Crescent MO (5′-CTCTGACACACCTGAGGGCCATT-3′). Each MO was microinjected four times radially into 4-cell embryos (34 ng total). Bisection experiments were performed by cutting stage 9 embryos across their prospective D–V axis into two equal halves, using a surgical blade. Embryos were bisected and cultured in 0.3 x Modified Barth solution (Gurdon, 1976; Reversade and De Robertis, 2005). For mRNA microinjection, 200 pg of Xenopus crescent (Pera and De Robertis, 2000) or crescentWobble were injected four times radially into 2- or 4-cell embryos. CrescentWobble (…5′ ATG GCT CCA CAA CTG TGC CAA 3′…) was generated by introducing 5 synonymous mutations (underlined nucleotides) in the wild type Xenopus laevis Crescent sequence targeted by our Crescent Morpholino. Double axes were induced by microinjections of 2.5 or 5 pg of Wnt8 mRNA and inhibited with 200 pg of Crescent or CrescentD103N mRNA. For protein microinjections, affinity-purified Crescent-Flag or CrescentD103N-Flag (both at 5 μM, 60 nl), and recombinant mouse Chordin (2.5 μM, 60 nl) or human BMP4 (0.4 μM, 60 nl, R&D Systems) were microinjected into the blastocoele at mid-blastula (stage 8.5). Detailed procedures for whole-mount in situ hybridization are available at http://www.hhmi.ucla.edu/derobertis/protocol_page/protocol.html.
Biochemical methods
Xenopus Chordin-Myc was produced in baculovirus (Piccolo et al., 1996) and subsequently affinity-purified or used directly as substrate. Xenopus Crescent-HA and Crescent-Flag were tagged at the C-terminus by PCR. To generate CrescentD103N, the site-directed mutagenesis Quikchange kit (Stratagene) was used. These proteins, plus Xlr-PC and Szl-Fc (Lee et al., 2006), were produced by transient transfection (Fugene, Roche) of HEK 293 T cells. Conditioned medium containing secreted proteins was affinity-purified using PC (Roche), Protein A (Sigma), Flag (Sigma), or HA beads (Covance) according to manufacturer instructions. For BMP1 enzymatic assays, commercial recombinant human BMP1 protein (R&D Systems) and a fluorogenic substrate synthesized based on the sequence of the main cleavage site of Tolloid on Chordin, Mca-SMQSDGAK-Dnp (Bachem), were used (Lee et al., 2009). Reactions were performed with 25 μM fluorogenic Chordin-peptide substrate in Xld Buffer (Piccolo et al., 1997) with the addition of 0.01% Brij 35.
Enzymatic activities were measured in a fluorescent plate reader (excitation=320 nm, emission=405 nm) and initial velocities calculated from the rate of fluorescence increase in 60 minute reactions. For enzyme kinetics studies, Lineweaver-Burk plots were constructed using initial velocities (vi) obtained from fluorometric enzyme assays at different Chd-peptide and Crescent-Flag concentrations. Dixon plots were generated by modifying the concentration of inhibitor for two different substrate concentrations, and plotting the inverse of the initial velocity versus the concentration of inhibitor. The kinetic constants Km (Michaelis constant), Vmax (maximal velocity) and Ki (inhibition constant) were calculated as described in Dixon and Webb (1979). For Xlr and BMP1 in vitro enzymatic digestion assays, 30 nM baculovirus Chd-Myc was incubated in Xld Buffer with affinity-purified Xlr-Flag or human BMP1 (R&D Systems) containing the indicated concentrations of Szl-Fc, Crescent-Flag or Crescent-HA at 25 °C (or 37° in a few instances) for 2 hr. Western blots were visualized using pico chemiluminescent substrate (Pierce) or the LiCOR Odyssey infrared imager system.
Surface plasmon resonance analyses
Surface plasmon resonance measurements were performed in a BIAcore 3000 system. Affinity purified Crescent-Flag protein, diluted to 20 μg/ml in 10 mM sodium acetate (pH 5.0), was bound on a carboxymethylated dextran (CM5) sensor chip using amine coupling to a level of approximately 6000 response units. Binding of recombinant human BMP1 protein (carrier free, R&D Systems) and washes were performed in Xld Buffer. Each experimental cycle consisted of a flow Crescent-Flag at various concentrations. After each cycle, non-crosslinked proteins were removed by a flow of 10 mM HCl in order to regenerate the chip surface. Data were analyzed with BIAevaluation 4.1 software and curve-fitting was done with the assumption of one-to-one binding (Wang et al., 2003).
Quantitative RT-PCR
Total RNA from 10 whole embryos (at stage 11) per sample was extracted with the Absolutely RNA Microprep kit (Stratagene). Synthesis of cDNA was done using random hexamer primers and the StrataScript Reverse Transcriptase (Stratagene). Quantitative RT–PCR was performed on the Mx3000P (Stratagene) apparatus using the Brilliant SYBR Green QPCR Master Mix (Stratagene). Measurements were performed in quadruplicates and normalized to the expression levels of ODC (Ornithine decarboxylase). The formula 2−ΔΔCt was used to calculate fold induction values. Bars indicate standard deviations. RT-PCR conditions and primers can be found at http://www.hhmi.ucla.edu/derobertis/protocol_page/protocol.html.
Results
Crescent regulates BMP signaling
The Xenopus gastrula contains a dorsal and a ventral signaling center under opposite transcriptional regulation by BMP signaling (Fig. 1A). From a molecular standpoint, Crescent resembles Sizzled, Chordin is like CV2, and ADMP/BMP2 and BMP4/BMP7 are all BMPs. Within the sFRP family, Crescent is most similar to Sizzled (Fig. 1B). This prompted us to investigate whether Crescent had a similar biochemical activity to that of Sizzled (Lee et al., 2006).
To determine whether the expression of Crescent on the dorsal region of the embryo was transcriptionally repressed by BMP signals, we microinjected BMP4 or Chordin proteins into the blastocoele of stage 8.5 Xenopus blastulae (Figs. 1C–E). Embryos injected with the BMP antagonist Chordin showed increased levels of crescent transcripts (n=9/9) (Fig. 1C), while embryos injected with BMP4 showed decreased transcripts (n=11/14) (Fig. 1E) when compared to uninjected controls (n=9/10) (Fig. 1D) at late gastrula. This indicated that Crescent is normally repressed by BMPs, explaining its preferential expression on the dorsal side of the embryo, where BMP signaling is low.
To study the function of Crescent during gastrulation, an antisense morpholino (Cres MO) was designed. Crescent mRNA or Crescent MO were injected into each blastomere of 4-cell stage Xenopus embryos. Sizzled expression was used as the readout for regions of high BMP signaling, since its transcription is activated by BMPs (Collavin and Kirschner, 2003). Embryos overexpressing Crescent showed reduced sizzled expression in the ventral side (n=14/16) (Fig. 1F), while depletion of Crescent with MO expanded the ventral sizzled expression (n=9/10) (Fig. 1H) compared to uninjected controls (n=10/10) (Fig. 1G). Moreover, Crescent overexpression increased Chordin transcripts (Figs. 2A and B), which are normally repressed by BMP signaling (n=27/30). These results indicated that Crescent regulates the BMP signaling gradient and came as a surprise, since Crescent had previously only been implicated in inhibition of Wnt signals (Pera and De Robertis, 2000; Shibata et al., 2005).
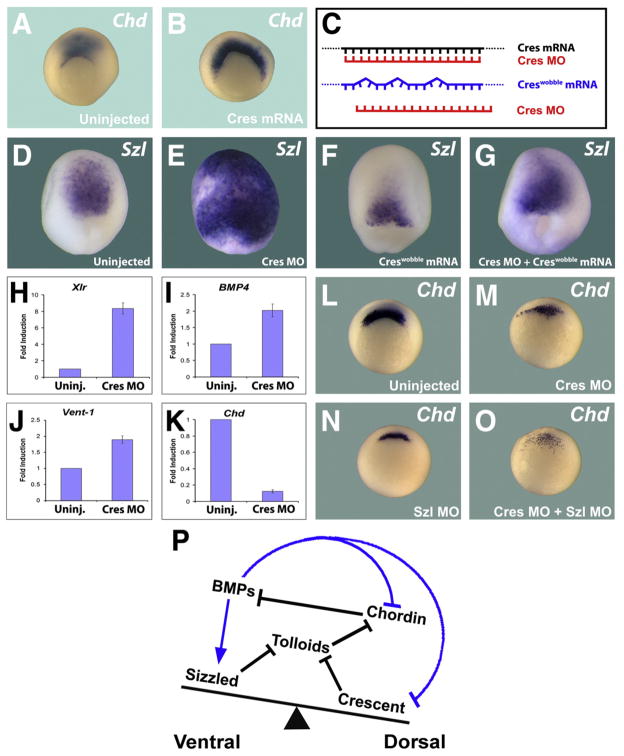
Fig. 2.
Crescent regulates BMP signaling. (A,B) Microinjection of crescent mRNA expands the expression of chordin, a gene that is negatively regulated by BMP signaling. (C) Diagram showing a control crescent mRNA containing wobble position mutations is no longer targeted by Crescent MO. (D–G) Crescent MO greatly expands expression of the ventral (high-BMP) marker sizzled, while crescentWobble mRNA reduces Sizzled expression and rescues the effects of Crescent MO. (H–K) Knockdown of Crescent increases the transcript levels of BMP induced genes (Xlr, BMP4, Vent-1), while reducing the levels of chordin, a gene repressed by BMP signaling. (L–O) chordin mRNA expression is reduced by Crescent MO or Sizzled MO. Note that simultaneous depletion of both sFRPs causes a synergistic ventralizing effect, greatly reducing chordin transcripts at early gastrula. (P) Model in which the BMP gradient is represented by a see-saw in which dorsal and ventral inhibitors of Tolloid metalloproteinases adjust the D–V gradient through the proteolytic degradation of Chordin. Blue arrows symbolize transcriptional regulation by BMPs, black arrows indicate direct protein–protein interactions.
To assess the specificity of the Crescent MO, we designed a mutant crescent mRNA, crescentWobble, which contains several mismatches with the antisense morpholino but retains the identical amino acid coding sequence (Fig. 2C). Depletion of Crescent (Cres MO) greatly increased sizzled transcripts (Fig. 2E), while overexpression of crescentWobble mRNA reduced them (Fig. 2F) compared to control embryos (Fig. 2D). Co-injection of Cres MO and crescentWobble mRNA rescued the Crescent MO phenotype (compare Figs. 2D–G), indicating that the phenotype of Cres MO was specific. To further evaluate the ventralizing phenotype displayed upon knockdown of Crescent, total mRNA was collected from embryos injected with Cres MO and uninjected controls, and quantitative RT-PCR were performed. Knockdown of Crescent (Cres MO) led to an increase in the BMP induced genes Xolloid related (Xlr) (2 H) (Dale et al., 2002), BMP4 (2I), and Vent-1 (2 J), and to a decrease in the BMP repressed gene Chordin (2 K) (Reversade and De Robertis, 2005).
The results presented so far indicate that Crescent normally reduces BMP signaling. Our hypothesis is that Crescent and Sizzled might control Tolloid activity from opposite poles of the embryo. When Crescent is depleted, increased expression of Sizzled may compensate in part for the lack of Crescent. If this were the case, simultaneous depletion of both Crescent and Sizzled should better relieve Tolloid proteinases from inhibition and cause increased ventralization of the gastrula. Chordin expression was used to probe for BMP signaling levels, since Chordin is transcriptionally inhibited by BMP signals (Reversade and De Robertis, 2005). Embryos depleted of either Crescent (n=24/28) or Sizzled (32/35) showed reduced chordin transcripts (Figs. 2I–K). Indeed, embryos doubly depleted of Crescent and Sizzled showed even more severely reduced chordin levels (n=29/31) (Fig. 2L). We propose that Crescent and Sizzled cooperate in vivo, from opposite poles of the embryo, to fine tune the D–V BMP signaling gradient during development (2P).
Crescent inhibits the degradation of chordin by Tolloid metalloproteinases
We next asked whether the mechanism by which Crescent reduced BMP signaling was through inhibition of the Tolloid proteinases that cleave the BMP antagonist Chordin (Fig. 2M). To test this hypothesis, we conducted biochemical studies measuring the digestion of Chordin by Tolloid in the presence of Crescent (Figs. 3A and B). The reactions were performed using affinity-purified Crescent protein (Cres-Flag or Cres-HA) as the inhibitor, Xenopus Chordin (Chd-myc) produced in baculovirus as the substrate, and either commercial hBMP1 or affinity-purified Xolloid-related-Flag (Xlr) as the proteinase. The digestion reactions were performed as described in Piccolo et al. (1997), and analyzed by Western blots using antibodies against the Chd-Myc tag.
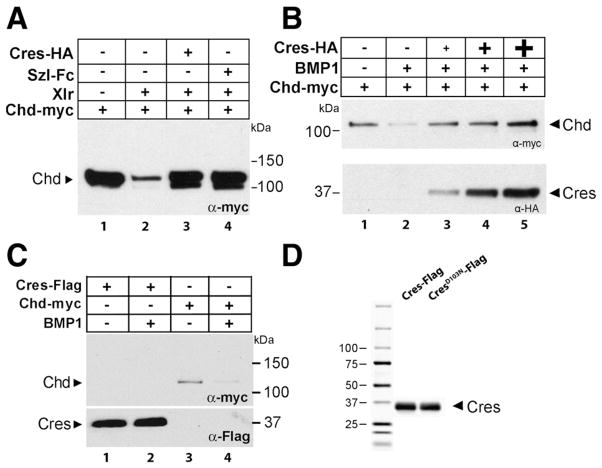
Fig. 3.
Crescent inhibits the proteolytic activity of Xlr or BMP1 on Chordin. (A) Purified Crescent-HA (150 nM) inhibited the proteolysis of Chordin-myc by Xlr to the same extent as Sizzled-Fc at the same concentration. (B) Crescent-HA inhibited in a dose-dependent manner (16, 50, 150 nM) the cleavage of Chordin (30 nM) by BMP1 (10 nM). (C) Crescent-Flag (150 nM) was not cleaved by BMP1 (10 nM) (compare lane 1 and 2), while Chordin-myc (30 nM) was cleaved in the same experiment (lanes 3 and 4). (D) Crescent proteins affinity-purified over an anti-flag matrix stained with Coomassie Brilliant Blue to indicate their purity.
Chd-myc was digested by purified Xlr protein (Fig. 3A, compare lanes 1 and 2). Addition of Crescent protein inhibited the proteolytic cleavage of Chordin by Xlr (compare lanes 2 and 3); the level of inhibition was comparable to that achieved by the same concentration of Sizzled (Fig. 3A, lane 4), a bona fide Tolloid inhibitor (Lee et al., 2006). This result could also be reproduced using another Tolloid proteinase, hBMP1 (data not shown). Furthermore, the inhibitory effect of Crescent on Tolloid enzymes was dose-dependent (Fig. 3B).
One possible explanation for the inhibition of Tolloid activity could be that Crescent itself might be a substrate for Tolloid proteinases. This possibility could be ruled out, for Crescent-Flag protein was not digested by BMP1 (Fig. 3C, lanes 1 and 2), while Chordin was degraded in the same experiment (Fig. 3C, lanes 3 and 4). The purity of our Crescent-flag proteins was verified through Coomassie Brilliant Blue staining (Fig. 3D). These results show that Crescent protein is an inhibitor of Tolloid proteinases.
Crescent binds and competitively inhibits Tolloid proteinases
To analyze the mechanism by which Crescent inhibits Tolloids, a Chordin-mimicking fluorogenic substrate was used (Chd-peptide). This fluorogenic substrate is an octapeptide containing target amino acids of Chordin recognized by Tolloid metalloproteinases. The octapeptide is flanked by a fluorophore on one side and its quencher on the other (Lee et al., 2009). Upon cleavage by Tolloids, the fluorophore is released from quenching and emits fluorescence at 405 nm. When the kinetics of the enzyme reaction was analyzed at increasing concentrations of the substrate (fluorogenic Chd-peptide), double reciprocal Lineweaver-Burk plots (Fig. 4A) showed that Crescent displayed typical competitive inhibitor kinetics (Dixon and Webb, 1979): at two concentrations of Crescent-Flag, the apparent Km for the substrate changed, while the maximal velocity (Vmax) did not.
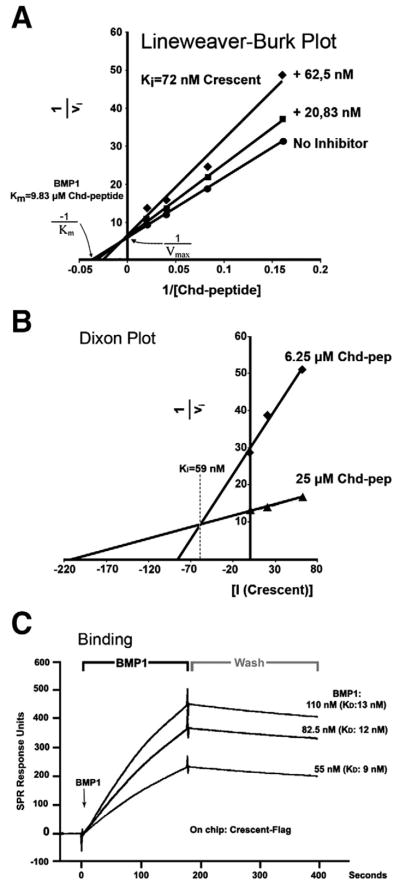
Fig. 4.
Crescent binds and competitively inhibits Tolloid Proteinases. (A) Lineweaver-Burk plot (1/vi over 1/[S]) showing that Crescent is a competitive inhibitor of Tolloid proteinases. (B) Dixon Plot (1/vi over [I]) from which the Ki (59 nM) could be obtained directly. (C) BIAcore surface Plasmon resonance sensograms for the Crescent-BMP1 interaction in real time, showing an average KD of 11 nM. The points in time when BMP1 binding starts, and when washing with buffer starts, are indicated.
The inhibition constant (Ki) is defined as the concentration of inhibitor at which half of the enzyme is in complex with the inhibitor. The Ki for Crescent inhibition was obtained using a Dixon Plot (Fig. 4B), a graphical method which yields the Ki directly without calculation. The initial velocities (vi) were determined at a series of inhibitor concentrations, and then plotted as 1/vi against the concentration of inhibitor [I]. These reactions were performed at two substrate concentrations, producing two slopes. The point at which both these lines intersect is equal to −Ki in the case of a competitive inhibitor (Dixon and Webb, 1979). The Ki of Crescent determined by this method was 59 nM (Fig. 4B).
After determining that Crescent is a competitive inhibitor of Tolloids, we analyzed the physical properties of their interaction. The binding affinity between Crescent and Tolloid was measured by surface plasmon resonance (BIAcore) analyses (Fig. 4C). Crescent-Flag proteins were covalently crosslinked to the surface of a sensor chip. hBMP1 was passed over this chip at constant flow, and changes in the refractive index caused by associations and dissociations recorded for different concentrations of hBMP1 (Fig. 4C). The affinity of the binding, expressed by the dissociation constant (KD), was calculated from the quotient between the kinetic rates of association and dissociation. The KD for the interaction between Crescent and BMP1 was on average 11 nM, which corresponds to a binding of high affinity within the physiological range (Hojima et al., 1985).
We conclude from these biochemical studies that Crescent competes for the binding of Chordin substrates to the BMP1/Tolloid catalytic site, and that this binding has a dissociation constant in the 10−9 Molar range.
Chordin is required for the dorsalizing activity of crescent
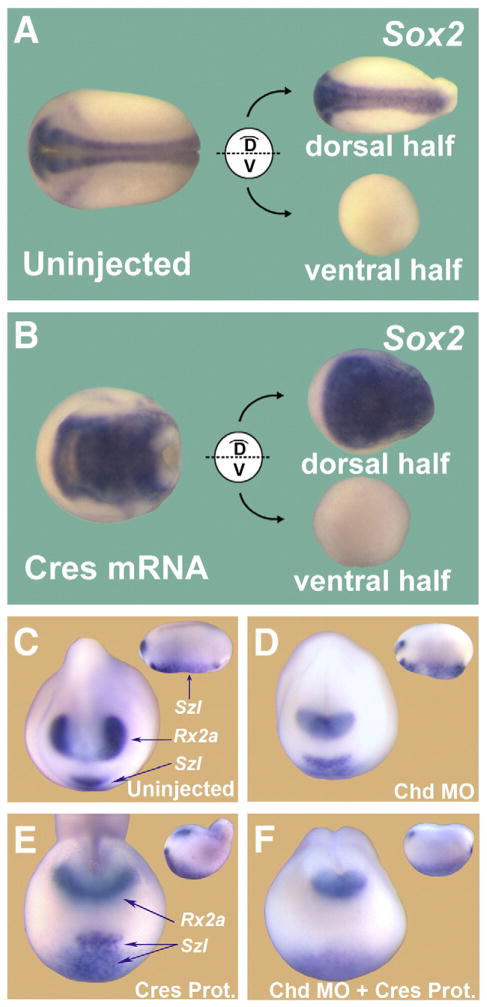
In previous work, the clue that led to the discovery of the mechanism of action of Sizzled was the observation that microinjections of sizzled mRNA had no effect on ventral half-embryos bisected at blastula. This indicated that Sizzled required a dorsal component in order to mediate its dorsalizing (anti-BMP) effects (Lee et al., 2006). A similar approach was taken here for Crescent. Microinjections of Crescent mRNA dorsalized whole Xenopus embryos, as indicated by the expansion of the pan-neural marker SOX2 (Figs. 5A and B). To determine whether dorsal components were required for this anti-BMP activity, bisection experiments were carried out (Reversade and De Robertis, 2005). Only the dorsal halves of Crescent-injected embryos showed an expansion of SOX2 (n=16/16), while ventral halves were unaffected by Crescent overexpression (Fig. 5B). Control experiments with chordin mRNA showed that this BMP inhibitor very effectively dorsalized ventral half-embryos (Oelgeschläger et al., 2003 and data not shown). These results show that Crescent is incapable of affecting ventral tissues directly, and requires dorsal components in order to elicit its effects.
Fig. 5.
Crescent requires Chordin in order to dorsalize the embryo. (A) Embryos bisected along their D–V axis. The dorsal half self-regulates, forming a well-proportioned embryo, while the ventral half forms a belly piece consisting of ventral tissues. (B) Crescent mRNA microinjection increases SOX2 expression in dorsal halves, but has no effect on ventral half-embryos. Thus, the dorsalizing activity of Crescent requires a dorsal component. (C) Uninjected control embryos showing normal Rx2a and Sizzled transcript levels at stage 20. (D) Embryos injected with Chordin MO showing a ventralized phenotype consisting of reduced Rx2a and expanded posterior Sizzled transcripts (inset). (E) Injection of Crescent protein into the blastocoele dorsalizes embryos, expanding Rx2a expression, decreasing Sizzled expression in the posterior-ventral region and increasing Sizzled in the anterior-ventral region (where BMP2 is expressed). (F) Injections of Crescent protein into Chordin-depleted embryos are without dorsalizing effects; this result indicates that Crescent protein requires Chordin to dorsalize Xenopus embryos. Insets show lateral views.
Our biochemical experiments indicated that Chordin was the likely dorsal component required for the dorsalizing activity of Crescent. To test this hypothesis, we injected Crescent protein into blastula embryos that had been previously depleted of Chordin by antisense Chd morpholino oligos (Oelgeschläger et al., 2003). As expected, Chordin-depleted embryos showed classical ventralized features (n=15/16), with reduction of Rx2a and an increase of posterior Sizzled (Szl), which marks ventral BMP4/7 signaling (Figs. 5C and D). Crescent protein injected into the blastocoele caused dorsalization with an expansion of the forebrain and eye marker Rx2a (n=36/39) (Fig. 5E, compare to C). (The anterior and ventral expression of Sizzled also observed in 5E is due to the upregulation of BMP2, in dorsalized embryos; BMP2 is expressed in this region when BMP signaling is lowered, Inomata et al., 2008). However, in embryos that had been previously depleted of Chordin, Crescent protein was devoid of dorsalizing (anti-BMP) activity, as indicated by the reduction of Rx2a and residual posterior Szl expression (n=29/34) (Fig. 5F). These results indicate that Crescent requires Chordin in order to dorsalize the Xenopus embryo.
A crescent mutant mimicking an ogon mutation has impaired anti-BMP activity
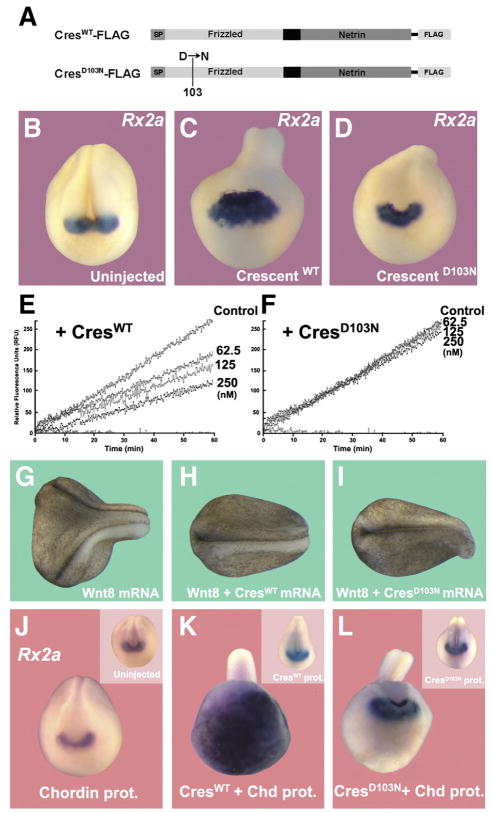
The isolation of the ogon/mercedes mutation in zebrafish opened the way to the discovery of the role of Sizzled in BMP signaling. This point mutation is located in the cysteine-rich frizzled domain. SizzledOGON, in which an Aspartic acid is replaced by an Asparagine, renders Ogon/Sizzled biologically inactive (Yabe et al., 2003). Introducing this mutation into Xenopus sizzled (sizzledOGON) causes it to lose its ability to inhibit Tolloid Proteinases (Lee et al., 2006). Taking advantage of the sequence conservation between Crescent and Sizzled, we made a construct mimicking the ogon mutation in Crescent, CrescentD103N (Fig. 6A). When microinjected into the blastocele of Xenopus embryos, affinity-purified CrescentD103N protein (n=18/21) was unable to expand the dorsal marker Rx2a when compared to the same amount of microinjected CrescentWT protein (18/18) (Figs. 6B–D). In biochemical experiments, CrescentWT inhibited cleavage of the fluorogenic Chd-peptide by BMP1 enzyme in a dose-dependent manner (Fig. 6E), while the point mutant CrescentD103N failed to inhibit the reaction at the same concentrations (Fig. 6F). Moreover, embryos injected with CrescentD103N were less dorsalized than those injected with CrescentWT protein (Supplemental Fig. S1). The CrescentD103N mutant loses Tolloid-inhibiting activity, but retains its ability to inhibit Wnt signaling. This was ascertained by coinjecting Wnt8 mRNA together with crescentWT or crescentD103N mRNA (Figs. 6G–I). Axis induction by xWnt8 mRNA (n=39/47, 83% with secondary axes) was blocked both by crescentWT (n=55/55) or crescentD103N (n=52/52). We note that at 5 pg of xWnt8 mRNA, although secondary axes were eliminated, some dorsalizing activity persisted (although at 2.5 pg all effects of xWnt8 were eliminated), as shown in Fig. 6I. This indicates that a small amount of anti-Wnt potency might be affected in the mutant. We conclude that the two functions of Crescent are separable, with CrescentD103N losing its Tolloid-inhibiting activity but retaining its function as a Wnt inhibitor. The dorsalizing phenotypic activity of Crescent can be mostly ascribed to its effects on BMP signaling through Tolloid inhibition.
Fig. 6.
A Crescent mutant mimicking the Ogon mutation lacks Tolloid inhibitory activity and has less anti-BMP activity in the Xenopus embryo. (A) Flag-tagged affinity-purified protein CrescentWT and CrescentD103N. (B–D) Microinjection of CrescentWT protein (2.5 μM) into the blastocele dorsalizes embryos and expands Rx2a expression, while microinjection of the same concentration of CrescentD103N had reduced dorsalizing ability and was unable to expand Rx2a expression. (E, F) CrescentWT inhibited cleavage of a fluorogenic Chordin peptide by BMP1 enzyme in a dose-dependent manner, whereas CrescentD103N was unable to inhibit this reaction. (G–I) CrescentD103N is able to inhibit the induction of secondary axes by xWnt8 mRNA, indicating that the Wnt-inhibiting and Tolloid-inhibiting activities of CrescentWT are separable. (J–L) A sub-threshold amount of Chordin protein injected into the blastocoele has very limited effect. If, in addition to this amount of Chordin, embryos also received a modest amount of CrescentWT, synergetic cooperation between Crescent and Chordin proteins was observed, manifested as an extreme increase in Rx2a expression in ectoderm. Embryos injected with CrescentD103N, although retaining a dorsally “kinked” phenotype (probably caused by inhibition of convergence and extension movements that require Wnt signaling), did not exhibit this increase in the Rx2a forebrain marker, when co-injected with Chordin protein. Insets show frontal views of embryos without injection of Chordin protein.
We next tested the effect of Crescent and its Ogon-like mutant in embryos sensitized by microinjection of a low amount of Chordin protein that does not change D–V patterning on its own (Fig. 6J, compare with its inset). CrescentWT protein was injected into the blastocoele in amounts that only slightly increased dorsalization (n=8/8) (Fig. 6J, inset). Interestingly, when Chordin and Crescent proteins were co-injected, a massive expansion of the forebrain marker Rx2a was induced (n=9/12) (Fig. 6K). This strong synergy between Chordin and Crescent on Rx2a expression was not observed when the same amount of CrescentD103N protein was co-injected (n=6/6) (compare Figs. 6K–L). The equivalent point mutations in the frizzled domain of Crescent (this work) or of Sizzled (Lee et al., 2006) caused both proteins to lose their metalloproteinase inhibiting activity. The remarkable synergistic interaction between microinjected Chordin and Crescent is explained by the inhibition of endogenous Tolloid enzymes, which normally degrade Chordin, by Crescent protein.
Discussion
Crescent is a Tolloid inhibitor
An intriguing question is why certain putative Wnt antagonists bearing frizzled domains, such as Crescent or Sizzled, and BMP antagonists such as Chordin or Noggin, cause very similar over-expression phenotypes. For example, in zebrafish overexpression of Sizzled or Chordin causes embryos to become dorso-anteriorized and lose ventral BMP-dependent structures such as the ventral fin (Yabe et al., 2003). This puzzle started to be answered when the surprising molecular mechanism of Sizzled action was discovered. Sizzled plays a critical role in the BMP pathway as a competitive inhibitor of the Tolloid proteinases that degrade the BMP antagonist Chordin, rather than through the modulation of Wnt signaling (Lee et al., 2006; Muraoka et al., 2006).
This unexpected function of Sizzled in Chordin proteolysis led us to test here whether the sFRP Crescent, expressed in the opposite pole of the developing gastrula, might have a similar function. A few hints pointed in this direction: within the sFRP family, Crescent is closest to Sizzled in terms of primary structure (44% identity, 63% similarity) (Collavin and Kirschner, 2003), and is expressed at the time and place in the developing gastrula at which Tolloid inhibition is likely to be important (Pera and De Robertis, 2000; Shibata et al., 2000). Crescent has well-documented effects on Wnt8 and Wnt11 activity in Xenopus embryos (Shibata et al., 2005; Mii and Taira, 2009; Dickinson and Sive, 2009). By regulating the cleavage of Chordin by Tolloids, Crescent is now shown to be able to regulate both the BMP and Wnt pathways in the developing embryo.
Crescent inhibits Tolloid proteinases, which are known to provide the rate-limiting step in the Chordin/BMP pathway (Lee et al., 2009). Using biochemical and embryological approaches, we found that Crescent is a competitive inhibitor of Tolloids, which enhance BMP signaling by inhibiting the cleavage of the BMP antagonist Chordin in the extracellular space. Crescent protein inhibited the proteolytic cleavage of Chordin by Xlr or BMP1 in vitro, without itself being cleaved by the proteases. Crescent functions as a competitive inhibitor of Tolloid metalloproteinases. We also found that Crescent protein was able to bind BMP1 with high affinity, in the physiologically relevant nanomolar range (Hojima et al., 1985; Lee et al., 2006). Tolloid inhibition explains the dorsalized phenotypes caused by Crescent protein microinjections. Crescent was unable to dorsalize ventral half-embryos and required endogenous Chordin to dorsalize intact Xenopus embryos. CrescentD103N is a point mutant mimicking the Ogon mutation in Sizzled, which loses its Tolloid inhibiting activity in vitro while retaining its Wnt-inhibiting properties. Interestingly, this mutant displayed a much reduced dorsalizing capacity and was ineffective in cooperating with Chordin protein when coinjected into Xenopus embryos (Fig. 6). Thus, the Tolloid inhibiting activity of Crescent appears to be responsible for a significant part of its phenotypic effects in Xenopus embryos.
Crescent is at the intersection of Wnt and BMP signaling
Xenopus Crescent was isolated in a screen for proteins secreted in gastrulating Xenopus embryos and was found to be expressed in Spemann’s organizer, in particular at the leading edge of the endomesoderm (Pera and De Robertis, 2000). Like all sFRPs, Crescent contains a cysteine-rich domain (CRD) homologous to the extracellular domain present in Frizzled receptors (Pera and De Robertis, 2000; Shibata et al., 2000). This is why most sFRPs, with the exception of Sizzled, are thought to bind Wnt ligands and regulate Wnt signaling (Leyns et al., 1997; De Robertis and Kuroda, 2004; Bovolenta et al., 2008).
Crescent binds to with Wnt11 and Wnt5a and has a role in the regulation of convergent extension movements during gastrulation and neurulation, via modulation of the non-canonical Wnt pathway (Pera and De Robertis, 2000; Shibata et al., 2005). The role of Crescent as a Wnt inhibitor is displayed during the development of the stomodeum or mouth, in which Crescent and Frzb are required to antagonize Wnt signaling; low levels of Wnt are essential for perforation of the buccopharyngeal membrane (Dickinson and Sive, 2009). Wnt signaling inhibition by Crescent is also critical for heart development (Marvin et al., 2001; Schneider and Mercola, 2001).
Recently, a new twist in the Wnt-regulating ability of Crescent has been discovered. Experiments in Xenopus have demonstrated that Crescent and Frzb enhance the diffusion of Wnts in the gastrulating embryo (Mii and Taira, 2009). Crescent increased the diffusion of Venus-tagged Wnt8 and Wnt11. Crescent is thought to achieve this by binding and transporting Wnts, improving their diffusibility. Wnt ligands transported by Crescent retain signaling capacity and Crescent has a biphasic effect: at low concentrations the interaction between Crescent and Wnt8 causes an increase in canonical Wnt signaling at a distance, while at high concentrations Crescent acts as a Wnt antagonist. It is conceivable that the increased diffusibility of Wnt ligands when co-injected with Crescent might be related to the inhibition of Tolloid metalloproteinases. Tolloid proteinases digest a diverse set of precursors into mature functional proteins. Among these substrates are many proteins important in the formation of the extracellular matrix, such as fibrillar procollagens (BMP1 is also known as Procollagen C peptidase), Latent TGF-β-binding protein (LTBP), Lysyl oxidase, Promyostatin, Osteoglycin and Biglycan (Hopkins et al., 2007). Tolloids play fundamental roles in development, and it is thought that many of these involve remodeling of the extracellular matrix (Hopkins et al., 2007). Inhibition of Tolloids, causing failure to properly process precursors of the extracellular matrix, might modify the landscape through which morphogens need to diffuse.
One important question that we were unable to address experimentally is whether the binding of Wnt to Crescent modified its ability to inhibit Tolloid protease activity. This experiment was attempted, but failed because the detergent-containing buffer required to maintain purified Wnt in solution, as well as conditioned medium from cell cultures, are incompatible with the biochemical assays of Tolloid activity. It is, however, an interesting question to contemplate, for if Wnt binding to Crescent modulated its inhibition of Tolloid, Crescent might behave as a receptor of a Wnt activity that would function entirely in the extracellular space.
sFRP constitute the largest family of Wnt inhibitors, for which a growing number of novel Wnt-independent functions are being discovered (Bovolenta et al., 2008). Of these alternative Wnt-independent roles for sFRPs, the best established one is that of Sizzled as a competitive inhibitor of Tolloid Proteinases (Lee et al., 2006; Muraoka et al., 2006). Sizzled does not antagonize Wnt signaling in vivo (Collavin and Kirschner, 2003; Yabe et al., 2003), but instead acts as a feedback inhibitor of BMP signaling by competitively inhibiting the cleavage of the BMP antagonist Chordin (Lee et al., 2006; Muraoka et al., 2006). In addition, sFRP2 has been recently shown to act as an enhancer of the procollagen C proteinase activity (Kobayashi et al., 2009). It seems likely that additional functions for sFRPs will continue to be uncovered.
Crescent is part of the D–V patterning pathway
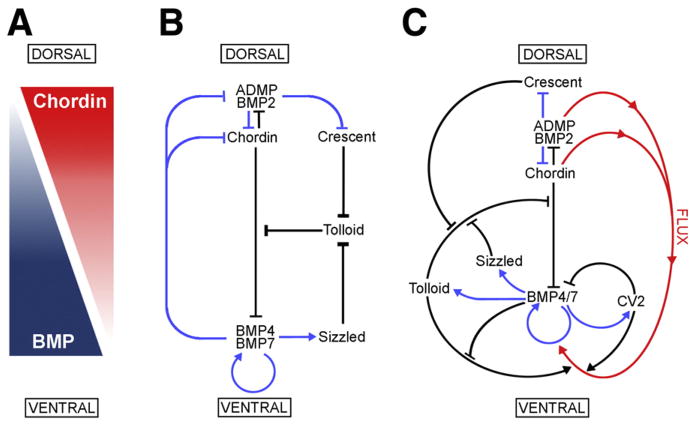
As shown in Fig. 7, patterning of the D–V axis is mediated by an extracellular network of interacting proteins. At the heart of this network is the signaling gradient generated by BMP-Chordin antagonism (Fig. 7A). BMPs (BMP2/4/7 and ADMP) are secreted both ventrally and dorsally, and their signaling causes cell differentiation towards ventral fates. The dorsally secreted antagonist Chordin binds BMPs and impedes the binding of BMPs to their receptors, ensuring that the dorsal side of the embryo is kept free of BMP signaling (Piccolo et al., 1996). The Chordin/BMP antagonism creates a gradient of BMP signaling along the D–V axis, which is reinforced by an array of proteins that help keep BMP signaling high on the ventral and low in the dorsal side, either by sequestering, transporting or solubilizing BMP ligands (Zakin and De Robertis, 2010).
Fig. 7.
Model of the extracellular network of proteins that control D–V patterning. Crescent was shown here to be a component of the extracellular biochemical network that controls D–V patterning. Arrows in blue represent transcriptional regulation by BMPs, arrows in black symbolize direct protein–protein interactions, and the red arrows indicate flux of Chordin/ADMP/BMP from the dorsal toward the ventral side of the embryo. Each protein–protein interaction indicated here is supported by biochemical and embryological studies in Xenopus. (A) The BMP/Chordin gradient plays a central role in D–V patterning. (B) Tolloid metalloproteinases are inhibited by Crescent from the dorsal and Sizzled from the ventral side. (C) The extracellular biochemical pathway of D–V development in Xenopus embryos, including the flux of Chordin/BMP complexes from dorsal to ventral. Two important regulators of the Chordin/BMP pathway have been omitted for simplicity in this diagram. One is Ont-1, an adaptor Olfactomedin-related protein that binds both Chordin and Tolloid, which facilitates Chordin proteolysis (Inomata et al., 2008). The other regulator is Twisted-gastrulation (Tsg), a protein that binds both BMP and Chordin, facilitating BMP signaling, the binding of BMP to Chordin, and the cleavage of Chordin by Tolloids (De Robertis and Kuroda, 2004; Little and Mullins, 2006).
Many components of this extracellular network that regulates D–V patterning have counterparts of similar structure and function secreted under opposite transcriptional control on the other side of the embryo. Examples of this are BMP2 and ADMP on the dorsal and BMP4 and BMP7 on the ventral side (Reversade and De Robertis, 2005; Inomata et al., 2008), as well as Chordin dorsally and the structurally related protein CV2 on the ventral side (Ambrosio et al., 2008). The experiments presented here indicate that Crescent and Sizzled constitute a new pair of proteins expressed at opposite poles of the embryo and having similar functions (Fig. 7B). Their opposing transcriptional regulation should confer additional resilience and robustness to the regulatory network. For example, in loss of function experiments when Crescent expression is knocked down by morpholinos, the transcriptional upregulation of Sizzled is able to compensate in part, explaining why the double knockdown of Crescent and Sizzled causes a much severe high-BMP phenotype (Fig. 2).
BMP antagonism by Chordin is not irreversible. BMPs can be liberated from this inhibition by Tolloid metalloproteinases that cleave Chordin, enabling BMPs to signal again (Piccolo et al., 1997). In the Xenopus embryo, 3 Tolloid enzymes are expressed: BMP1, Xolloid (Xld) and Xolloid Related (Xlr). While the first two are expressed ubiquitously in the early embryo, Xlr is transcriptionally upregulated in more ventral regions by BMP4/7 signaling (Goodman et al., 1998; Dale et al., 2002). These metalloproteinases release BMPs from the Chordin-BMP complex, allowing BMPs to signal (Fig. 7C). The regulation of Tolloid activity is crucial to D–V patterning.
CV2 is a secreted molecule that contains Chordin-like domains that bind BMPs, but remains localized at its site of synthesis on the ventral side (Rentzsch et al., 2006; Serpe et al., 2008; Ambrosio et al., 2008; Zakin et al., 2010). Acting as an anti-BMP, CV2 binds BMPs and promotes their endocytosis and destruction, serving as a feedback inhibitor (Zhang et al., 2008; Kelley et al., 2009). CV2 also acts as a pro-BMP. Since it remains near the cells where it was produced, CV2 also functions as a molecular sink, concentrating Chordin/BMP complexes diffusing from more dorsal regions on the ventral side (Ambrosio et al., 2008; Blair, 2007). Once concentrated on the ventral region, Chordin/BMP complexes can be cleaved by Tolloids (with the help of the extracelluar Ont-1 scaffold protein, Inomata et al., 2008), liberating active BMPs (Fig. 7C) which allows the embryo to reach peak BMP signaling levels in the ventral center (reviewed in Zakin and De Robertis (2010)).
Tolloid metalloproteinases play a crucial role in the D–V patterning pathway, and the ventral and dorsal signaling centers regulate their proteolytic activity by secreting proteins that can inhibit these enzymes. An additional inhibitory feedback loop is provided by the recently discovered ability of BMP4 to inhibit Tolloids (Fig. 7C) when its levels become high (Lee et al., 2009). Since Tolloid proteinases constitute the rate-limiting step in the D–V Chordin/BMP pathway, it is not surprising that these enzymes are highly regulated. Sizzled, produced abundantly on the ventral side (Lee et al., 2006), serves as an important negative feedback inhibitor. On the opposite side of the gastrulating embryo, Crescent similarly protects Chordin from Tolloid metalloproteinases by competitively inhibiting them, ensuring optimal levels of Chordin on the dorsal side. Self-regulation of D–V pattern results from this opposition between dorsal and ventral secreted BMP signals and their extracellular regulators.
Crescent in evolution
From an evolutionary standpoint, the Chordin/BMP/Tolloid pathway represents the ancestral D–V patterning system, which has been conserved in the early embryos of Drosophila, beetles, spiders, hemichordates, amphioxus, fish, amphibians and birds (reviewed in De Robertis, 2008; Umulis et al., 2009). Drosophila has retained many components of the system (Short-gastrulation/Chordin, Dpp/BMP, Tolloid, Tsg and CV2), but its genome does not contain even a single sFRP. Therefore, the D–V system of secreted proteins can generate pattern without Crescent or Sizzled. However, sFRPs are found in some invertebrates such as nematodes and annelids, so insects must have undergone gene loss. Like Xenopus, the chick embryo expresses Crescent and Sizzled abundantly in dorsal and ventral regions, respectively (Pfeffer et al., 1997; Wittler et al., 2008). It is therefore very surprising that a functional Crescent is not present in the genome of placental mammals. The platypus (Ornithorhynchus anatinus), contains a perfect copy of Crescent (Warren et al., 2008). An intact crescent gene is also found in the opossum (Monodelphis domestica), a marsupial. However, in the genome of placental mammals Crescent can no longer be identified, except in the dog genome (Canis lupus familiaris) (Lindblad-Toh et al., 2005), in which a pseudogene containing multiple inactivating mutations and deletions in Crescent is still recognizable (J.L. Plouhinec and E.M.D.R., unpublished observations). Perhaps the fine regulation of D–V pattern was no longer needed after the mammalian egg lost its yolk (which is still present in the platypus) and consequently the need for self-regulation of pattern during the epiboly movements by which the embryo surrounds the yolk. In evolution, once a gene is no longer needed, it is rapidly lost (De Robertis, 2008). Although no longer required for patterning mice and men, Crescent is a conserved gene important in the regulative development of lower vertebrates, which functions in both the Wnt and the BMP signaling pathways.
Supplementary Material
Acknowledgments
The authors thank Jack Greenan and D. Geissert for technical assistance, members of our laboratory for discussions and comments on the manuscript, and Drs. R. Lehrer and Grace Jung for invaluable help with the BIAcore analyses. Doctoral studies by D.P. are supported by a Fulbright Science and Technology Award. This work was supported by the NIH (HD21502-24). E.M.D.R. is a Howard Hughes Medical Institute investigator.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.ydbio.2011.01.029.
References
- Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 is a BMP feedback inhibitor that binds Chd/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, de Sauvage FJ. Structural ties between cholesterol transport and morphogen signaling. Cell. 2009;138:1055–1056. doi: 10.1016/j.cell.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Belo JA, Silva AC, Borges AC, Filipe M, Bento M, Gonçalves L, Virotino M, Salgueiro AM, Texeira V, Tavares AT, Marques S. Generating asymmetries in the early vertebrate embryo: the role of the Cerberus-like family. Int J Dev Biol. 2009;53:1399–1407. doi: 10.1387/ijdb.072297jb. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo B, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;4:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Evo-Devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M, Webb EC. Enzymes. Academic Press; New York: 1979. [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976;36:523–540. [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJM, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nüsslein-Volhard C. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann Organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hojima Y, van der Rest M, Prockop DJ. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. J Biol Chem. 1985;260:15996–16003. [PubMed] [Google Scholar]
- Hopkins DR, Keles S, Greenspan D. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell. 2008;134:854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal–ventral signaling: secreted frizzled-related proteins as inhibitors of Tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 2009;23:2551–2562. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann Organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad-Toh K, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. Sizzled controls dorso–ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Pera E, De Robertis EM. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech Dev. 2000;96:183–195. doi: 10.1016/s0925-4773(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, De Robertis EM, Izpisúa-Belmonte JC. Crescent, a novel chick gene encoding a frizzled-like cysteine-rich domain, is expressed in anterior regions during early embryogenesis. Int J Dev Biol. 1997;41:449–458. [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, De Robertis EM. Systems biology of the self-regulating morphogenetic gradient of the Xenopus gastrula. Cold Spring Harb Perspect Biol. 2009;1:a001701. doi: 10.1101/cshperspect.a001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–4748. doi: 10.1242/dev.124.23.4739. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert A, Vick P, Getwan M, Weber T, Schneider I, Eberhardt M, Beyer T, Pachur A, Blum M. The Nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr Biol. 2010;20:738–743. doi: 10.1016/j.cub.2010.02.061. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-binding protein crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ono H, Hikasa H, Shinga J, Taira M. Xenopus crescent encoding a Frizzled-like domain is expressed in the Spemann organizer and pronephros. Mech Dev. 2000;96:243–246. doi: 10.1016/s0925-4773(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Shibata M, Itoh M, Hikasa H, Taira S, Taira M. Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech Dev. 2005;122:1322–1339. doi: 10.1016/j.mod.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Spemann H. Embryonic development and induction. Yale University Press; New Haven, Conn: 1938. reprinted by Hafner Publishing Company, 1962. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Umulis D, Michael, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- Warren, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wittler L, Saborowski M, Kessel M. Expression of the chick Sizzled gene in progenitors of the cardiac outflow tract. Gene Expr Patterns. 2008;8:471–476. doi: 10.1016/j.gep.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. Extracellular regulation of BMP signaling. Curr Biol. 2010;20:R89–R92. doi: 10.1016/j.cub.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Chang EY, Plouhinec JL, De Robertis EM. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev Biol. 2010;347:204–215. doi: 10.1016/j.ydbio.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.