Abstract
Background and Purpose
Emerging evidence suggests that exercise may improve cognitive function in older adults. The purpose of this pilot research project was to describe changes in measures of cognition and executive function in individuals with chronic stroke following participation in aerobic and strengthening exercise.
Methods
A single group pre-test post-test design was used. Nine individuals with chronic stroke (mean age 63.7±9.1, mean time since stroke 50.4±37.9 months) completed a 12-week program of aerobic and strengthening exercise, 3 days per week. The primary outcome measures examined executive function (digit span backwards and Flanker tests). Secondary measures examined various aspects of aerobic fitness (VO2peak, 6-minute walk distance) and function (Fugl-Meyer, 10-meter walk speed).
Results
Following the intervention significant improvements were found in the digit span backwards test (mean change = 0.56±0.9 digits; p=0.05), Fugl-Meyer score (mean change = 3.6±5.7; p=0.05), and SIS total score (mean change = 33.8±38.5; p =0.02). A significant correlation was found between improved aerobic capacity and improved performance on the Flanker test (r=0.74; p=0.02).
Discussion
The results of this pilot study indicate that a 12-week aerobic and strengthening exercise program improved selected measures of executive function and functional capacity in people with stroke. Limitations of this study include the small sample size and lack of a comparison group.
Conclusions
This pilot study contributes to the emerging evidence that exercise is associated with improved cognition in people with stroke. These benefits indicate the need for future study with a larger group to have sufficient power to further explore these relationships.
BACKGROUND AND PURPOSE
It has been hypothesized that aerobic exercise may improve cognition in older adults and promote neuroplasticity in people with neurologic pathology.1–6 Animal studies have demonstrated that exercise enhances neurogenesis specifically in the hippocampus,7 and that exercise prior to injury has a neuroprotective effect for both focal ischemic injury and slow degeneration models.8, 9 In humans, epidemiological research has suggested a strong relationship between higher levels of self-reported physical activity levels and reduced risk of cognitive decline.10–12
There is growing evidence that aerobic exercise interventions can improve cognition in healthy older adults, with several systematic reviews on the topic.5, 13 Aerobic exercise may improve brain blood flow and oxygen perfusion, which could lead to improved cognitive performance. Resistance exercise has also been found to improve performance on cognitive measures in healthy older women and men.14–16 Resistance training may be especially important for individuals with dementia, as resistance training is associated with increased insulin-like growth factor I (IGF-I) and decreased levels of homocystein.17 IGF-I is a neurotrophin that promotes neuronal survival and improves cognition, while homocystein may be neurotoxic and impairs neuropsychological function in older adults.
Few studies have investigated the result of exercise in people with dementia or other cognitive impairments.18 A meta-analysis that compared exercise outcomes in cognitively-impaired individuals suggested that they exhibit a response to exercise similar to cognitively-intact individuals.19 Further, a relationship between aerobic fitness, cognitive function, and brain volume measures has been identified in individuals with early Alzheimer’s Disease.20
In people with stroke, substantial evidence suggests that physical exercise is beneficial to improve aerobic fitness and muscle strength.21,22 However, the question of whether exercise can also have an effect on cognition in people with stroke has only recently begun to be addressed. A single session of 20 minutes of treadmill walking was found not to have an effect on cognitive tests,23 and no improvement on executive function measures were found following 8 weeks of aerobic exercise.24 However, previous research using regression modeling has provided evidence that cognition appears to influence functional performance in people with stroke. Various measures of mental status have been found to predict 6-minute walk distance in healthy elders, using the mini-mental status exam (MMSE), a depression symptom score, and measures of positive/negative affect and mood.25, 26 In people with chronic stroke, MMSE score has been found to correlate with balance scores,27 and we recently reported a significant predictive relationship between MMSE and gait speed.28 However, the MMSE was designed to be used as a screening tool for dementia.29–31 Therefore, it’s validity as a measure of overall mental status and sensitivity to change is limited.
The primary purpose of this pilot research project was to explore the relationship between improvement in aerobic fitness and change in measures of cognition and executive function in a small number of individuals with chronic stroke following participation in 12 weeks of aerobic and strengthening exercise. We also assessed the influence of this exercise on secondary measures of aerobic fitness and function.
METHODS
This study utilized a pre-test post-test design and was approved by the Human Subjects Committee of the University of Kansas Medical Center. All participants provided written informed consent prior to participation in this study.
Participants
A convenience sample of individuals with a single stroke at least 6 months prior was recruited for this study. All participants were able to transfer from a sitting to a standing position and were able to walk 30 feet without assistance of another person. Medical clearance was received from their primary physicians for participation in an exercise program. Participants all had a baseline mini-mental status exam (MMSE)32 score of 23 or greater. Participants were excluded from the project if they had any of the following: (1) hospitalization for myocardial infarction, heart surgery, or congestive heart failure during the preceding 3 months, (2) significant cardiac arryhthmia, hypertrophic cardiomyopathy, severe aortic stenosis, or pulmonary embolus, (3) recent symptoms of chest discomfort, (4) currently smoking or significant pulmonary pathology, (5) musculoskeletal problems from conditions other than stroke that would limit ability to exercise; (6) were concurrently receiving physical therapy intervention outside of this study; or (7) other neurologic disorders in addition to the stroke.
Outcome Measures
Measures were assessed at baseline and after 12 weeks of exercise:
The primary outcome measures were tests of cognition/executive function, and included:
The Digit Span Backwards (DB) task from the Wechsler Adult Intelligence Scale (3rd edition; WAIS-III). This test is an index of working memory, which is a component of executive function. This task has been used previously as a measure of working memory in older adults with mild cognitive impairment,33 and has been found to be responsive to change with an exercise intervention.16 The test consists of seven pairs of random number sequences that are read aloud to the participant, and the task is to repeat the sequence in reverse order. The test is scored by identifying the number of digits that the individual can successfully repeat backwards.
- The Flanker test.34, 35 This is a test of attention and executive function that requires the individual to respond correctly to a cue embedded in an array of 5 arrows pointing in either the same “congruent” (e.g. <<<<<) or opposite “incongruent” direction (e.g. <<><<). This task has been used to examine the influence of cardiovascular fitness on cognitive performance in healthy elderly.2 Participants were asked to respond to the orientation of the central arrow by pressing a button on the computer with their less-involved hand. The index and middle fingers were positioned on a left-facing arrow and a right-facing arrow. They were tested in 6 blocks of 18 trials, with an equal number of congruent and incongruent trials presented in random order. The outcome variables for this task included 1) percent of correct trials with congruent stimuli, 2) percent of correct trials with incongruent stimuli, and 3) percent increase in reaction time with incongruent stimuli according to the following equation:
The Memory component of the Stroke Impact Scale (SIS version 2.0).36 The SIS memory component is a self-report measures ___
The secondary outcome measures were tests of aerobic fitness (peak aerobic capacity, 6-minute walk distance) and function (Fugl-Meyer, 10-meter walk speed):
-
Peak aerobic capacity. A total of 4 maximal graded exercise tests were conducted for each participant; 2 before the intervention and 2 after the intervention. The tests before and after the intervention were performed within 1 week of each other, one with a bicycle ergometer (Lode Corival, Groeningan Nederland) and one with a total body recumbent stepper (TBRS) (Nustep Inc, Ann Arbor MI). The protocol for the bicycle ergometer test was based on previous literature,37 and the protocol for the TBRS test was developed specifically by our lab for use in individuals with stroke (mTBRS-XT).38 The purpose of performing 2 exercise tests was to validate the mTBRS-XT protocol, and these results are reported elsewhere.38 For the purpose of this project, the data for each individual was from the testing protocol with the highest baseline fitness measures, as it was assumed that this would provide the closest approximation to maximal aerobic capacity for that participant.
Blood glucose level was checked prior to the exercise test, and participants were instructed in the testing procedure and oriented to a 15-point rating of perceived exertion (RPE).39 A metabolic cart (Parvo Medics TrueOne 2400) was used to collect expired gases with a 12-lead ECG to monitor heart rate and rhythm. The tests continued until maximal effort was achieved, defined as heart rate at 90% of predicted maximum unless one of the following occurred, which required early termination: angina, dyspnea, fatigue (voluntary exhaustion or inability to maintain cycling cadence > 40 rpm), hypertension (>250 mm Hg systolic or >115 mm Hg diastolic), hypotension, or ischemic ECG abnormalities.40 The peak heart rate, peak aerobic capacity (VO2peak), exercise duration, RER (respiratory equivalent ratio) and metabolic equivalent level (METs) were monitored and recorded during the exercise tests.
Walking endurance. The distance walked in six minutes (6-Minute Walk Test) was used as a measure of endurance. This measure has been found to be a reliable submaximal test of cardiovascular fitness in people with stroke.47, 48
The Fugl-Meyer test. This test was used as a measure of voluntary motor control of the upper and lower extremity.41 The motor score includes upper and lower extremity components for a total of 100 possible points. Reliability, validity, and responsiveness of this measure in people with stroke has been reported previously.42–44
Self-selected gait speed. The time required to walk a distance of 10 meters was measured (10-meter walk test), and the average of 2 trials was used for analysis. This measure has been found to be a determinant of functional mobility in people with stroke.45, 46
-
SIS total score and mobility subscale score. The non-memory domains of the SIS (strength, hand function, mobility, activities of daily living, communication, emotion, participation, physical function, and percent recovery) were measured. Reliability, validity, and responsiveness of the SIS is well established in people with stroke.36, 49, 50 Raw scores for each domain were transformed into a score ranging from 0–100 using the equation:36
A total SIS score was computed that included each of the domains plus memory, and a mobility subscale was determined by adding the domain scores for strength, ADLs, mobility, and hand function.36
Intervention
The intervention consisted of a 12-week program of aerobic exercise and lower extremity muscle strengthening exercise, 3 times each week for 1 hour sessions. Heart rate and blood pressure were monitored during each session. For the participants who were diagnosed with diabetes, blood glucose levels were measured prior to each exercise session; exercise was not permitted if the blood glucose values were below 70 or above 300.51 The sessions started with aerobic exercise that incorporated upper and lower extremity reciprocal movements on a TBRS. The initial intensity of exercise for each individual was determined by the heart rate during the exercise test at 50% peak oxygen uptake and/or RPE 11–14, which indicates a light-to-moderate level of intensity for older adults.40 The 30-minute session included a 5-minute warm up period, maintenance of target heart rate for 20 minutes, and a 5-minute cool down period. Heart rate and RPE were monitored every 5 minutes during exercise to ensure that the target intensity was reached, and resistance and speed were gradually progressed to maintain training heart rate intensity. Strengthening exercises for the lower extremities were performed in a sitting position using resistive bands [Theraband, Hygenic Corporation, Akron OH] for bilateral knee flexors, knee extensors, ankle dorsiflexors, and ankle plantar flexors. These muscles were targeted because they are important for transfers and gait. Participants started with 1 set of 10 repetitions of each exercise, focusing on technique, with slow movements and minimal compensations. These exercises were gradually progressed by adding repetitions and increasing resistance by changing elastic bands.
Data Analysis
Analysis was performed using SPSS 16.0 for Windows. For each of the outcome measurements, descriptive statistics (mean, standard deviation) were calculated and scatter plots were examined visually to find outliers caused by data entry or other errors. Normal distribution of variables was confirmed through visual analysis of histograms, calculations of skewness indices,52 and the Kolmogorov-Smirnov Z statistic. The use of parametric statistical tests were justified for this small data set, as no significant difference was found between a normal distribution and the distribution of each variable, with a probability of the Z statistic above 0.05 in all cases (range of 0.2 to 0.9). The pre-intervention and post-intervention scores were compared using a one-tailed paired t-test, with significance set at α = 0.05. The relationships among change in cognitive measures and change in aerobic fitness were determined using Pearson’s correlation coefficient.
RESULTS
Twenty-four people were screened for the project, 11 people initiated the study, and 9 people completed all testing and at least 27 of the 36 sessions (75% adherence rate). One participant withdrew after 1 week because of transportation and schedule conflicts. The other participant withdrew after 4 weeks of exercise after experiencing a fall at home. No other adverse events were reported by the participants in this study. Demographics for the 9 individuals who completed the study are provided in Table 1.
Table 1.
Participant demographics.
| Subject ID | Age | Sex | Months since stroke | Side of stroke | MMSE | Diabetes |
|---|---|---|---|---|---|---|
| 1 | 64 | M | 39 | L | 29 | Yes |
| 2 | 75 | F | 118 | L | 26 | Yes |
| 3 | 45 | F | 99 | L | 28 | No |
| 4 | 62 | M | 25 | R | 29 | No |
| 5 | 60 | F | 12 | L | 27 | No |
| 6 | 62 | F | 22 | R | 29 | No |
| 7 | 76 | M | 17 | L | 23 | Yes |
| 8 | 62 | M | 70 | L | 29 | Yes |
| 9 | 67 | M | 52 | R | 30 | No |
| Mean (SD) | 63.7 (9.1) | 50.4 (37.9) | 27.8 (2.2) |
MMSE = Mini Mental Status Exam
Following the intervention, scores on the digit span backwards test (working memory) were significantly improved, with trends for improvement in the Flanker test scores for both the congruent and incongruent items (Table 2). Significant improvements were noted in the SIS total score and FM total score following the intervention (Table 3). Changes in aerobic fitness were not significant following the intervention as determined by both VO2peak and 6-minute walk distance, however there were strong trends for these measures as well as for the SIS mobility subscale and the 10-meter walk speed.
Table 2.
Change in cognitive measures following the intervention.
| Pre- intervention |
Post- intervention |
Change | P value |
|
|---|---|---|---|---|
| Digit Span Backwards | 3.22 (0.7) digits | 3.78 (0.8) digits | 0.56 (0.9) digits | 0.05† |
| Flanker % Correct (congruent) | 92.4 (3.7) | 94.6 (3.1) | 2.2 (4.3) | 0.08 |
| Flanker % Correct (incongruent) | 71.8 (18.2) | 83.3 (17.1) | 11.4 (24.9) | 0.1 |
| Flanker % Increase RT | 29 (35.9) | 21.3 (23.4) | −7.7 (21.9) | 0.16 |
| SIS-memory score | 73.3 (18.7) | 76.7 (17.4) | 3.4 (15.5) | 0.26 |
p≤ 0.05 with one-tailed, paired t test
RT = Reaction time, SIS = Stroke Impact Scale
Table 3.
Change in measures of functional capacity following the intervention
| Pre-intervention | Post-intervention | Change | P- value |
|
|---|---|---|---|---|
| VO2peak(ml/kg/m) | 13.9 (4.5) | 15.2 (4.3) | 1.4 (2.4) | 0.06 |
| 10-meter walk speed (m/s) | 0.6 (0.5) | 0.7 (0.6) | −0.08 (0.1) | 0.08 |
| 6-minute walk (m) | 760 (696.3) | 826.3 (661) | 66.3 (117.2) | 0.06 |
| Fugl-Meyer | 87.7 (29.1) | 91.2 (26.4) | 3.6 (5.7) | 0.05† |
| SIS Total | 526.4 (101.1) | 560.2 (100.5) | 33.8 (38.5) | 0.02† |
| SIS Mobility Subscale | 252.1 (83) | 266.4 (67.6) | 14.2 (25) | 0.06 |
p≤ 0.05 with one-tailed, paired t test
VO2peak = peak oxygen uptake, SIS = Stroke Impact Scale, s = seconds, m = meters
The relationships among change in aerobic fitness as determined by VO2peak and change in cognitive measures were explored further (Table 4 and Figure 1). A significant relationship was found between improved aerobic fitness and improved identification of incongruent arrangements on the Flanker test. The relationship between improved aerobic fitness and improved correct responses on the congruent Flanker items and self-report of memory function on the SIS subscale were moderately strong but did not reach significance.
Table 4.
Correlation between change in aerobic fitness and cognitive measures.
| Pearson Correlation Coefficient (r) with Change in VO2peak |
p value | |
|---|---|---|
| Change in SIS-memory score | 0.567 | 0.11 |
| Change in Flanker (congruent) | 0.588 | 0.1 |
| Change in Flanker (incongruent) | 0.74 | 0.02† |
| Change in Flanker RT Cost | −0.231 | 0.55 |
| Change in Digit Span Backwards | −0.485 | 0.19 |
p≤ 0.05 with one-tailed, paired t test
SIS = Stroke Impact Scale, RT = reaction time
Figure 1.
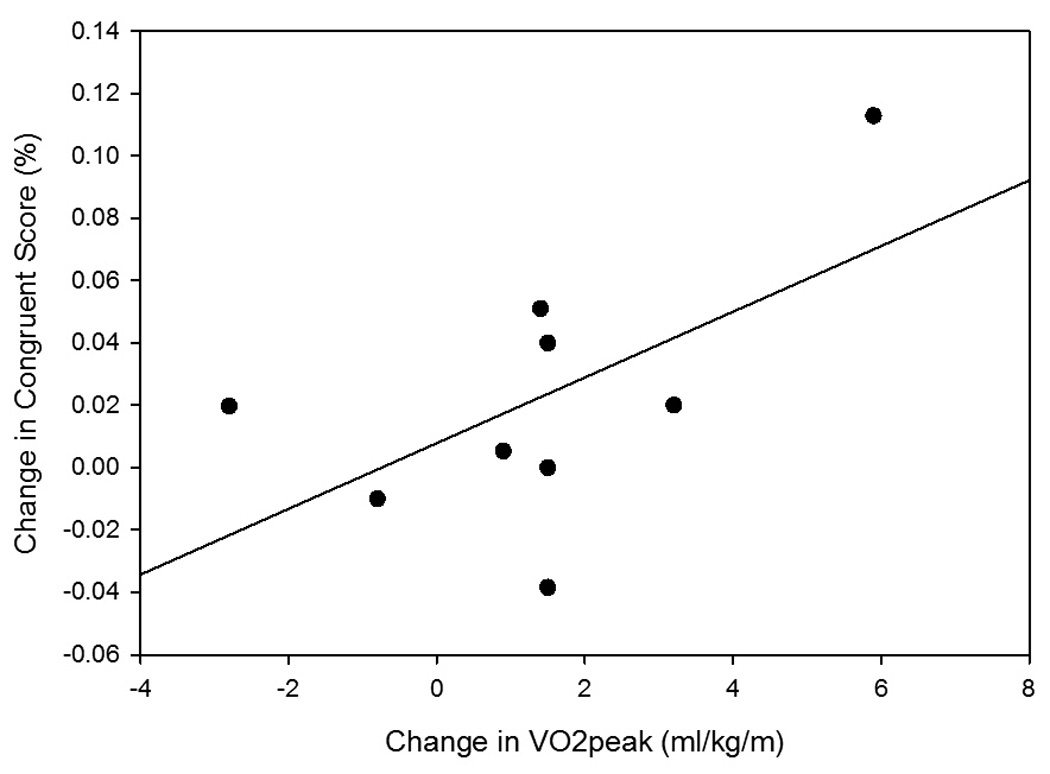
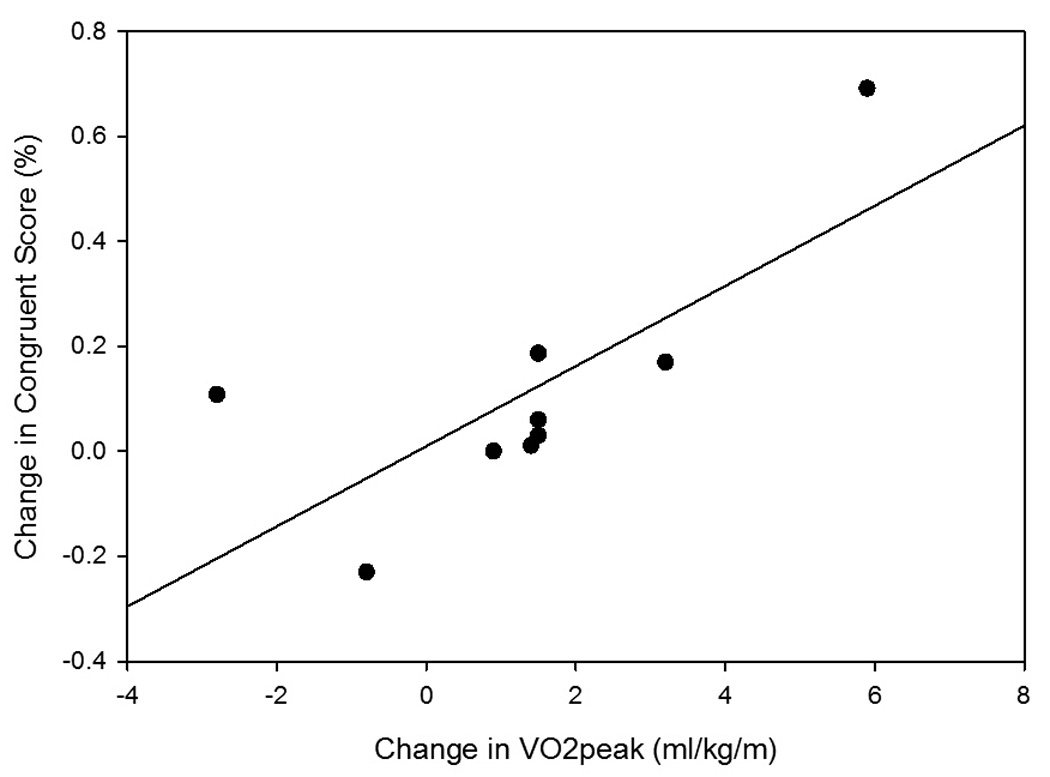
Scatterplot with trend line for relationship between change in aerobic fitness and change in cognitive tests: (A) relationship between VO2peak and percent correct on Flanker congruent items (changecon), and (B) relationship between VO2peak and percent correct on Flanker incongruent items (changeinc).
DISCUSSION
We have described the changes in aerobic fitness, cognitive function, and functional measures in a small group of individuals with chronic stroke following participation in 12 weeks of aerobic and strengthening exercise in this pilot study. Improvements in a measure of working memory (digit span backwards test) were noted following exercise. However, the magnitude of this change was small (mean 0.56 digits) and may not be clinically significant. Other researchers found a 1.0 digit change in healthy older adults after 4 weeks of strengthening exercise.16 Although this measure has not been used extensively in people with stroke, previous researchers have described differences in the digit span backwards test between older adults with and without mild cognitive impairment (range of 3.3–4.4 digits and 4.6–5.6 digits, respectively).33 The post-intervention scores for our participants are within the range reported for adults with mild cognitive impairment.
A significant correlation between change in aerobic fitness and improved score on an executive function test (the Flanker-incongruent score) was found following the intervention. The incongruent condition was more challenging than the congruent condition, as indicated by the higher error rate. Executive function as measured with the Flanker test has been previously described with very low error rates in healthy adults, with correct scores in ~99% of congruent trials and ~97% of incongruent trials.2, 35 Our participants scored lower, with 92.4% correct responses in the congruent condition and 71.8% correct responses in the incongruent condition at baseline. We found that our participants had a 29% increase in reaction time with incongruent stimuli compared to responses to congruent stimuli. This level of interference is comparable to the 26% increase found in older adults with low levels of physical fitness.2 Our participants showed a 7.7% improvement in their ability to overcome interference following participation in exercise. Although the clinical significance of this change is unknown, it is less than the 11% improvement reported following a 6-month exercise intervention in older adults.2
A meta-analysis of the effects of exercise and cognition in older adults found strong support for the hypothesis that aerobic fitness training improves cognitive performance on a variety of tasks.5 The strongest benefit was noted for executive-control processes, as compared to other measures of cognition (speed, visuospatial, or controlled- processing). Both of our cognitive outcome measures (digit span backwards and Flanker tests) could be considered to be in the executive-control domain. Further, the meta-analysis found that combined aerobic and strengthening exercise programs have a greater influence on cognitive measures compared to aerobic exercise alone.5 Although our subjects underwent both strength and aerobic training, our analysis focused on changes in aerobic fitness. It would be interesting to assess the relationships between changes in motor strength or fat-free mass (e.g. with dual-x-ray absorptiometry measures) with measures of cognition in future studies.
The effect of exercise on cognition in people with stroke is only beginning to be explored. A randomized controlled trial in people with chronic stroke examined the effect on executive function, motor learning, and mobility of 8 weeks of aerobic-only exercise compared to a control intervention (home stretching program).24 Greater improvements in information processing speed and motor learning were found in the aerobic intervention group. However, no differences were noted between the groups in tests of executive function (e.g. Trail Making B-A, Stroop, and Wisconsin Card Sorting tests). Direct comparison between studies is limited by the different cognitive outcome measures and the overlap between different cognitive domains assessed by these measures. These early results are encouraging and examination of the dose and type of exercise on cognition in people with stroke seems important for further study.
Most of our participants improved aerobic fitness following the intervention, and these changes showed a trend toward statistical significance. Other authors have found improved aerobic fitness and motor function following exercise in people with chronic stroke.24, 53–56 The baseline VO2peak values in our participants are comparable to those in other studies, and illustrate the poor cardiorespiratory fitness of people poststroke. We may have found greater changes in aerobic fitness with a longer intervention, or with a higher level of training intensity.
The FM motor score improved significantly in our subjects following the intervention. It has been suggested that a change of 6–7 points in the FM score indicates clinical significance.57 Our participants had a smaller change (mean 3.6 points) although their baseline score was >80 which indicates a relatively mild severity of motor deficits.58 Total SIS scores also improved significantly, even though the intervention did not include functional task practice. The SIS score change of 33.8 noted in our participants reflects change over 8 different domains, and interpretation of changes in this self-report measure must be interpreted with caution in the absence of a control group that received a comparable amount of attention, as it is possible that the subjects improved simply by virtue of participation in a study.59 SIS total scores have been reported to distinguish between mild and moderate stroke (605.3 and 512.5 respectively, at 6 months following stroke),36 and the total SIS scores of our subjects fell within this range.
One interesting factor is that 4 of our 9 participants were diagnosed with diabetes mellitus, and older adults with type 2 diabetes may have deficits in memory and learning (specifically list learning tasks).60 These deficits may be secondary to a chronic hyperglycemia-induced ‘central neuropathy’,61 cortical atrophy,62, 63 or atrophy specifically in the hippocampus and amygdala.64, 65 The possible confounding influence of the dual diagnosis of diabetes and stroke on executive function has not received attention in the literature but would be another important area of future study.
One of the primary limitations of this pilot project was the difficulty with recruitment and retention during this 12-week project. The intensity of this project (3 times each week for 12 weeks) and the requirement that participants come to an urban academic medical center for the intervention may have contributed to the difficulty with recruitment and retention. The small number of subjects increased the possibility of a Type II error, when the sample size has insufficient power to detect a statistically significance difference.59
In summary, the findings of this pilot study indicate that participation in a 12-week aerobic and strengthening exercise program improved selected measures of executive function in people with stroke. These results contribute to the emerging literature on improvements in cognition following exercise in people with stroke. These benefits indicate the need for future study with a larger group to further explore these relationships.
Acknowledgements
We would like to acknowledge the valuable assistance of Eric Vidoni PT PhD who assisted with programming the Flanker test, and Anne Neuer DPT who assisted with scoring the Flanker test on this project.
This project was funded by National Institute of Disability and Rehabilitation Research grant H133F05006 and supported, in part, by the University of Kansas Medical Center General Clinical Research Center grant M01 RR 02394, National Center for Research Resources/National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflict of interest to disclose.
A component of this project was presented in a platform at the World Conference of Physical Therapy conference in Vancouver Canada in 2007.
References
- 1.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 2.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Nat Acad Sci. 2004;101:3316. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: A brief review. Brain Behav Immun. 2004;18:214. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Churchill JD, Galvez R, Colcombe SJ, Swain RA, Kramer AF, Greenough WT. Exercise, experience, and the aging brain. Neurobiol Aging. 2002;23:941. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psych Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 6.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague-dawley rats in vivo. Neurosci. 2004;124:71. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Endres M, Gertz K, Lindauer U, et al. Mechanism of stroke protection by physical activity. Ann Neurol. 2003;54:582. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 9.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28:1757. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intl Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 11.Pignatti F, Rozzini R, Trabucchi M, Yaffe K. Physical activity and cognitive decline in elderly persons. Arch Int Med. 2002;162:361–362. doi: 10.1001/archinte.162.3.361. [DOI] [PubMed] [Google Scholar]
- 12.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 13.Angevaren M, Aufdemakmpe G, Verhaar H, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD005381.pub3. Art. No.: CD005381. [DOI] [PubMed] [Google Scholar]
- 14.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Int Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Hanley C, Nimon JP, Westen SC. Cognitive health benefits of strengthening exercise for community-dwelling older adults. J Clin Exp Neuropsychol. 2010:1–6. doi: 10.1080/13803391003662702. [DOI] [PubMed] [Google Scholar]
- 17.Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–27. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes D, Forbes S, Morgan D, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD006489.pub2. Art. No.: CD006489. [DOI] [PubMed] [Google Scholar]
- 19.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurol. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluding P, Billinger SA. Exercise-induced changes of the upper extremity in chronic stroke survivors. Top Stroke Rehabil. 2005;12:58–68. doi: 10.1310/LET5-XNBY-98Q6-Q8TG. [DOI] [PubMed] [Google Scholar]
- 22.Saunders D, Greig C, Young A, Mead G. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD003316.pub2. Art. No.: CD003316. [DOI] [PubMed] [Google Scholar]
- 23.Ploughman M, McCarthy J, Bossé M, Sullivan HJ, Corbett D. Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? a randomized cross-over trial. Arch Phys Med Rehabil. 2008;89:2041–2047. doi: 10.1016/j.apmr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Quaney B, Boyd LA, McDowd JM, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord SR, Menz HB. Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil. 2002;83:907–911. doi: 10.1053/apmr.2002.33227. [DOI] [PubMed] [Google Scholar]
- 26.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: A quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 27.Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Phys Ther. 2005;85:150–158. [PubMed] [Google Scholar]
- 28.Kluding P, Gajewski BJ. Lower extremity strength differences predict activity limitations in people with chronic stroke. Phys Ther. 2009;89:73–81. doi: 10.2522/ptj.20070234. [DOI] [PubMed] [Google Scholar]
- 29.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and education level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 30.Bour A, Rasquin S, Boreas A, M L, F V. How predictive is the MMSE for cognitive performance after stroke? J Neurol. 2010;257:630–637. doi: 10.1007/s00415-009-5387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geri Psych. 2006;14:391–400. doi: 10.1097/01.JGP.0000216181.20416.b2. [DOI] [PubMed] [Google Scholar]
- 32.Folstein M, Folstein S, McHugh P. 'Mini-mental state' A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Liu-Ambrose TY, Ashe MC, Graf P, Beattie BL, Khan KM. Increased risk of falling in older community-dwelling women with mild cognitive impairment. Phys Ther. 2008;88:1482–1491. doi: 10.2522/ptj.20080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botvinick M, Nystron LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cog Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 36.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale Version 2.0: evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 37.Yates JS, Studenski S, Gollub S, et al. Bicycle ergometry in subacute-stroke survivors: Feasibility, safety, and exercise performance. J Aging Phys Activity. 2004;11:64–74. doi: 10.1123/japa.12.1.64. [DOI] [PubMed] [Google Scholar]
- 38.Billinger SA, Kluding P, Tseng BY. Modified total body recumbent stepper exercise test (mTBRS-XT) to obtain VO2 peak in people with chronic stroke. Phys Ther. 2008;88:1188–1195. doi: 10.2522/ptj.20080072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colberg SR, Swain DP, Vinik AI. Use of heart rate reserve and rating of perceived exertion to prescribe exercise intensity in diabetic autonomic neuropathy. Diabetes Care. 2003;26:986. doi: 10.2337/diacare.26.4.986. [DOI] [PubMed] [Google Scholar]
- 40.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 41.Fugl-Myer A, Jaasko L, Leyman I, S O, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 42.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1607–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Hsu M, Sheu C, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 44.Hsueh IP, Hsu M-J, Sheu C-F, Lee S, Hsieh C-L, Lin J-H. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the stroke rehabilitation assessment of movement. Neurorehabil Neural Repair. 2008;22:737–738. doi: 10.1177/1545968308315999. [DOI] [PubMed] [Google Scholar]
- 45.Perry J, Garrett M, Gronley JK, Mulroy S. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 46.Mulroy S, Gronley JK, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18:125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 47.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke: Relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–761. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 48.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: Test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin KC, Fu T, Wu CY, Hsieh YW, Chen CL, Lee PC. Psychometric comparisons of the Stroke Impace Scale 3.0 and Stroke-Specific Quality of Life Scale. Quality of Life Res. 2010;19:435–443. doi: 10.1007/s11136-010-9597-5. [DOI] [PubMed] [Google Scholar]
- 50.Duncan PW, Bode RK, Min Lai S, Perera S. Rasch analysis of a new stroke-specific outcome scale: the stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 51.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2009;32:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandrekar SJ, Mandrekar JN. Are our data symmetric? Stat Methods Med Res. 2003;12:505–513. doi: 10.1191/0962280203sm346oa. [DOI] [PubMed] [Google Scholar]
- 53.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Trincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 54.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African-American group of stroke survivors. Med Sci Sports Exerc. 2000;32:1990–1996. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Car M, Jones J. Physiological effects of exercise on stroke survivors. Top Stroke Rehabil. 2003;9:57–64. doi: 10.1310/0J2K-MDNX-1Q0L-8LX6. [DOI] [PubMed] [Google Scholar]
- 56.Duncan PW, Studenski S, Richards L, Gollub S, Lai SM, Reker D. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 57.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke: Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 59.Portney L, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall Health; 2000. [Google Scholar]
- 60.Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes/Metab Res Rev. 2000;16:308. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Ryan CM, Geckle M. Circumscribed cognitive dysfunction in middle-aged adults with Type 2 diabetes. Diabetes Care. 2000;23:1486. doi: 10.2337/diacare.23.10.1486. [DOI] [PubMed] [Google Scholar]
- 62.Pirttila T, Jarvenpaa R, Laippala P, Frey H. Brain atrophy on computerized axial tomography scans: Interaction of age, diabetes, and general morbidity. Gerontol. 1992;38:285. doi: 10.1159/000213342. [DOI] [PubMed] [Google Scholar]
- 63.Araki Y, Nomura M, Tanaka H, et al. MRI of the brain in diabetes mellitus. Neuroradiol. 1994;36:101. doi: 10.1007/BF00588069. [DOI] [PubMed] [Google Scholar]
- 64.Soininen H, Puranen M, Helkala EL, Laakso M, Riekkinen PJ. Diabetes mellitus and brain atrophy: A computed tomography study in an elderly population. Neurobiol Aging. 1992;13:717. doi: 10.1016/0197-4580(92)90095-f. [DOI] [PubMed] [Google Scholar]
- 65.den Heijer T, Vermeer SE, SEvan Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]


