Abstract
Rationale
HIV-infected adults are at an increased risk of lower respiratory tract infections. HIV infection impairs systemic acquired immunity, but there is limited information in humans on HIV-related cell-mediated immune defects in the lung.
Objective
To investigate antigen-specific CD4+ T cell responses to influenza virus, Streptococcus pneumoniae and Mycobacterium tuberculosis antigens in bronchoalveolar lavage (BAL) and peripheral blood between HIV-infected individuals and HIV-uninfected Malawian adults.
Methods
We obtained BAL fluid and blood from HIV-infected individuals (n=21) and HIV-uninfected adults (n=24). We determined the proportion of T cell subsets including naive, memory and regulatory T cells using flow cytometry, and used intracellular cytokine staining to identify CD4+ T cells recognising influenza virus-, S pneumoniae- and M tuberculosis-antigens.
Main results
CD4+ T cells in BAL were predominantly of effector memory phenotype compared to blood, irrespective of HIV status (p<0.001). There was immune compartmentalisation with a higher frequency of antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis retained in BAL compared to blood in HIV-uninfected adults (p<0.001 in each case). Influenza virus- and M tuberculosis-specific CD4+ T cell responses in BAL were impaired in HIV-infected individuals: proportions of total antigen-specific CD4+ T cells and of polyfunctional IFN-γ and TNF-α-secreting cells were lower in HIV-infected individuals than in HIV-uninfected adults (p<0.05 in each case).
Conclusions
BAL antigen-specific CD4+ T cell responses against important viral and bacterial respiratory pathogens are impaired in HIV-infected adults. This might contribute to the susceptibility of HIV-infected adults to lower respiratory tract infections such as pneumonia and tuberculosis.
Keywords: HIV, human, lung, T cells, bronchoalveolar lavage, mucosa, bronchoscopy, immunodeficiency, lymphocyte biology, pneumonia, respiratory infection
Introduction
Respiratory infections are a leading cause of death in lower income countries, accounting for approximately three million deaths a year.1 Infants, the elderly and immunocompromised individuals are particularly susceptible to these infections. In particular, HIV-infected individuals are 30 times more likely than uninfected adults to suffer from bacterial pneumonia or active tuberculosis.2 3 Southern Africa is the region with the world's highest burden of HIV infection, with over nine countries, including Malawi, having an estimated HIV prevalence that is greater than 10%.4
Defence against respiratory infection involves mucosal and systemic immunity.5 Antigen-specific CD4+ T cells are important as they protect against respiratory infections.6–8 Cytokines secreted by CD4+ T cells, such as IFN-γ, TNF-α, IL-2, IL-17 and IL-22,9–12 are critical to the activation of macrophages9 and the recruitment of neutrophils,10 and enhance the magnitude and quality of CD8+ T cell responses.12 Immune protection against common viral and bacterial respiratory pathogens depends on the integrity of these effector responses. There is limited information about the phenotype and function of these CD4+ T cells within the human lung.
Studies of the human lung suggest that mechanisms of local intrapulmonary immunity may differ from those mediating systemic immunity. Influenza virus antigen-specific memory CD4+ T cells from lung tissue were present at higher frequencies and produced more IFN-γ than those from peripheral blood in patients undergoing lobectomy for a localised solitary peripheral lung carcinoma who had no symptoms of upper respiratory infection.13 In patients with tuberculosis, IFN-γ and TNF-α responses to Purified Protein Derivative (PPD) were stronger by CD4+ T cells from BAL fluid than by CD4+ T cells from peripheral blood.14
The decline in immunity caused by HIV is not equally distributed among immunological sites. In particular, the depletion of CD4+ T cells primarily occurs at sites of ‘persistent inflammation’ such as the mucosa, which may leave individuals vulnerable to acute infections. Brenchley et al showed that there was a rapid depletion of mucosal gut T cells during early HIV infection while pulmonary CD4+ T cell depletion was less acute.15 Clinical evidence indicates that there is a high burden of pneumococcal pneumonia and tuberculosis early on in HIV infection when peripheral CD4+ T cell counts are relatively stable.16 17 We hypothesised that HIV infection preferentially depletes antigen-specific T cells against common respiratory pathogens within the lung compartment, which predisposes individuals to respiratory infections.
The authors have compared baseline T cell phenotypes and antigen-specific CD4+ T cells in BAL and peripheral blood, between HIV-infected individuals and HIV-uninfected adults. The aim of the authors was to compare T cell phenotypes in BAL and peripheral blood between the two groups of subjects; to assess antigen-specific CD4+ T cell responses to common respiratory antigens; and to investigate whether HIV infection differentially affects the lung and peripheral blood compartments.
Methods
Participants
Adult volunteers with no recent history of severe respiratory diseases and a normal chest x-ray were recruited by advertisement in the Queen Elizabeth Central Hospital, Blantyre, Malawi. All participants gave written-informed-consent to HIV testing, venesection and bronchoscopy. The authors enrolled HIV-uninfected adults and asymptomatic anti-retroviral therapy naive HIV-infected individuals (WHO stage 1) into the study. The exclusion criteria for the study were as follows: the presence of other immunocompromising illnesses such as diabetes and cancer, the use of immunosuppressive drugs, cigarette smoking, moderate or severe anaemia (HB<8 g/dl), and known or possible pregnancy. This study complies with local institutional guidelines and was approved by the College of Medicine Research Ethics Committee of the University of Malawi (COMREC P.01/09/717) and the Liverpool School of Tropical Medicine Research Ethics Committee (LSTM REC 08.61).
Sample collection and processing
Peripheral blood samples were collected on all subjects. Peripheral blood mononuclear cells (PBMCs) were isolated from blood by density centrifugation using Lymphoprep (Axis-shield, Norway) according to the manufacturer's instructions. Bronchoscopy and BAL collection was carried out as previously described.18 The BAL samples were filtered and spun to obtain a cell pellet. The cells were counted and re-suspended in complete cell culture media (containing RPMI, L-glutamine, penicillin/streptomycin and HEPES (all from Sigma-Aldrich, UK) with 2% (vol/vol) heat-inactivated human AB serum (National Blood Services, Blantyre)).
Phenotyping of T cell subsets
PBMCs and BAL cells were stained with fluorochrome conjugate antibodies when cell numbers were sufficient. Anti-CD3 fluorescein isothiocyanate (FITC), anti-CD4 Pacific blue, anti-CD8 allophycocyanin-H7 (APC-H7), anti-CD45RA phycoerythrin (PE), and anti-CCR7 allophycocyanin (APC) (all antibodies from BD Bioscience, UK) were used to characterise: naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−) and terminal effector T cells (CD45RA+CCR7−).19 Anti-CD4 pacific blue, anti-CD25 FITC (all antibodies from BD Bioscience, UK) and anti-FoxP3 PE (eBioscience, UK) were used to characterise regulatory T cells (CD4+CD25hiFoxP3+).20 The samples were acquired on CyAn ADP 9 Colour flow cytometer (Beckman Coulter, USA) and analysed using FlowJo (TreeStar, USA).
Intracellular cytokine staining
PBMCs and BAL cells re-suspended in complete cell culture media were cultured in a volume of 200 μl in a 96 well plate and stimulated with influenza vaccine (0.45 μg/ml), pneumococcal cell culture supernatant (8 μg/ml) or Purified Protein Derivative (PPD, 10 μg/ml) for 2 h. Brefeldin A (1 μl) (BD Bioscience, UK) was added at 2 h and the cells were cultured for a further 16 h. Cells were harvested and stained with Violet Viability dye (LIVE/DEAD® Fixable Dead Cell Stain kit, Invitrogen, UK) as per manufacturer's instructions. Cells were then surface stained with anti-CD4 FITC and CD8 PerCP (all BD Bioscience, UK). Next, cells were permeabilised and fixed using Cytofix/Cytoperm (BD Bioscience, UK) as per manufacturer's instructions. The cells were then stained with anti-interferon-gamma (IFN-γ) APC and anti-tumour necrosis factor-alpha (TNF-α) Alexa flour 488 antibodies (all BD Bioscience, UK) to detect intracellular cytokines. Lastly, cells were washed with 1x Perm Wash (BD Bioscience, UK), re-suspended in FACS flow and acquired on a flow on CyAn ADP 9 Colour flow cytometer (Beckman Coulter, USA). Flow cytometry analysis was done using FlowJo (TreeStar, USA).
Statistical analysis
Statistical analyses and graphical presentation were carried out using Graphpad Prism 5 (GraphPad Software, USA). Student t tests were used for the volunteer demographic data with the exception of gender, where a χ2 test was used instead. For the experimental data, Mann–Whitney U test was used for non-paired data and Wilcoxon sign ranked test for paired data. Results are given as mean with ranges or medians with IQRs. Differences were considered statistically significant if p values were less than 0.05.
Results
Demographic characteristics of study population
Basic demographics are shown in table 1. HIV-uninfected Malawian adults ((n=24, females 11) mean age 38 years)) and asymptomatic HIV-infected adults ((n=21, females 11) mean age 40 years)) participated in the study. The mean CD4 count for HIV-infected individuals was 375 cells/μl. All participants were asymptomatic and had no recent history of respiratory infection or tuberculosis. The mean BAL cell concentration was comparable between HIV-infected individuals and HIV-uninfected adults (16.2×106cells/100 ml vs 20.5×106cells/100 ml respectively; p=0.1134), but the proportion of lymphocytes in the BAL cells was higher in HIV-infected individuals than HIV-uninfected adults (17.8% vs 9.0%; p=0.0106).
Table 1.
Characteristics of subjects enrolled in the study
| HIV-uninfected adults n=24 | HIV-infected adults n=21 | p Value | |
| Sex: female/male | 11/13 | 11/10 | 0.6611 |
| Age, mean yr±SD | 38±17 | 40±18 | 0.7733 |
| Systemic mean CD4+ cells/μl±SD | 767±208 | 375±172 | <0.0001 |
| BAL cells/100ml BALF, mean±SD | 16.2×106±8.2×106 | 20.5×106±9.5×106 | 0.1134 |
| BAL lymphocyte %, mean±SD | 9.0±7.3 | 17.8±14.1 | 0.0106 |
| WHO Stage | NA | Stage 1 |
BAL, bronchoalveolar lavage; BALF, BAL fluid; N/A, not applicable.
Proportions of naive, memory and regulatory T cells in BAL and peripheral blood
CD4 and CD8 T cells
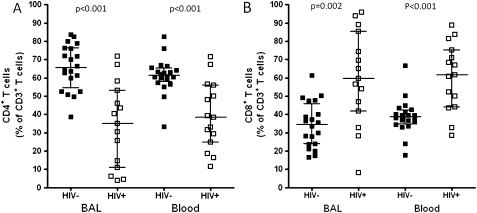
There was a lower proportion of CD4+ T cells in the total CD3+ T cell population in HIV-infected individuals compared to HIV-uninfected adults in both BAL (median 35.0% vs 65.5%, p<0.001) and peripheral blood (median 38.5% vs 61.2%, p<0.001). The proportion of CD8+ T cells in the total CD3+ T cell population was higher in HIV-infected individuals compared to HIV-uninfected adults in both BAL (median 59.7% vs 34.5%, p<0.05) and peripheral blood (median 61.5% vs 38.8%, p<0.05) (figure 1A).
Figure 1.
Lower proportion of CD4+ T cells in BAL and peripheral blood of HIV-infected individuals compared to HIV-uninfected adults. T lymphocytes obtained from BAL and peripheral blood were stained with anti-CD3 FITC, anti-CD4 Pacific blue and anti-CD8 APC-H7 fluorochrome conjugated antibodies. (A) The data shows a lower proportion of CD4+ T cells in BAL and peripheral blood of HIV-infected individuals compared to HIV-uninfected adults. (B) The data shows a higher proportion of CD8+ T cells in BAL and peripheral blood of HIV-infected individuals compared to HIV-uninfected adults. Significance was assessed using Mann–Whitney U test. Black horizontal bars represent median and IQRs.
Naive and memory T cells
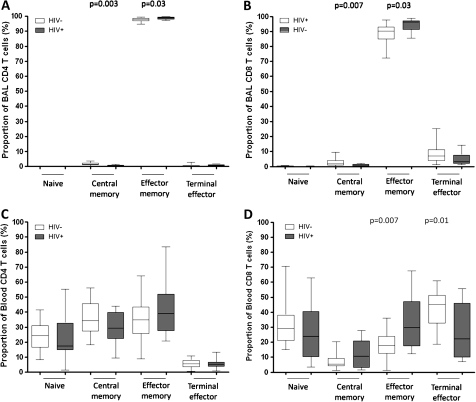
To examine whether the proportion of T cell subsets was similar between compartments and whether they were altered by HIV infection, the authors measured expression of CD45RA and CCR7 on CD4+ and CD8+ T cells—as described in the materials and methods section. BAL CD4+ and CD8+ T cells were predominantly of effector memory phenotype (CD4+CD45RA−CCR7−, median 98%; CD8+CD45RA−CCR7−, median 90%) compared to peripheral blood CD4+ and CD8+ T cells which were distributed among naive (CD4+CD45RA+CCR7+, median 25%; CD8+CD45RA+CCR7+, median 30%), central memory (CD4+CD45RA−CCR7+, median 37%; CD8+CD45RA−CCR7+, median 5%), effector memory (CD4+ CD45RA−CCR7−, median 37%; CD8+ CD45RA−CCR7−, median 20%) and terminal effector phenotypes (CD4+CD45RA+CCR7−, median 6%; CD8+ CD45RA+CCR7−, median 45%) (figure 2). In BAL, there was a higher proportion of effector memory cells in HIV-infected individuals compared to HIV-uninfected adults (CD4, median 98.9% vs 98.1% p=0.03; CD8, median 96.3% vs 90.3% p=0.03) and a lower proportion of central memory T cells (CD4, median 0.37% vs 1.42% p=0.003; CD8, median 0.44% vs 1.88% p=0.007) (figure 2A,B). In peripheral blood, there was no difference in peripheral blood CD4+ T cell subsets between HIV-infected and HIV-uninfected groups. However, the HIV-infected group had a higher proportion of CD8+ effector memory T cells (median 29.6% vs 17.8%, p=0.007) and a lower proportion of CD8+ terminal effector T cells (median 22.2% vs 45.0%, p=0.01) than the HIV-uninfected group (figure 2C,D).
Figure 2.
The proportions of naive and memory T cell subsets are different between BAL and peripheral blood, and are altered during HIV infection. T lymphocytes obtained from BAL and peripheral blood were stained with anti-CD3 FITC, anti-CD4 Pacific blue, anti-CD8 APC-H7, anti-CD45RA PE and anti-CCR7 APC fluorochrome conjugated antibodies. The proportion of naïve (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−) and terminal effector (CD45RA+CCR7−) were defined. (A, B, C, D) The data shows that BAL T cells (upper) were predominantly of effector memory phenotype compared to peripheral blood (lower), in which T cells were distributed among naive, central memory, effector memory and terminal effector phenotypes. (A, B) The data shows a higher proportion of effector memory and lower proportion of central memory BAL CD4+ (left) and CD8+ (right) T cells in HIV-infected individuals compared to HIV-uninfected adults. (C, D) The data shows no difference in peripheral blood CD4+ T cell subsets between HIV-infected individuals compared to HIV-uninfected adults (left), but there was a higher proportion of CD8+ effector memory and a lower proportion of CD8+ terminal effector in HIV-infected individuals compared to HIV-uninfected adults (right). Black horizontal bars represent median and IQRs. Statistical significance was analysed by the Mann–Whitney U test. p value <0.05 was used to determine statistical significance.
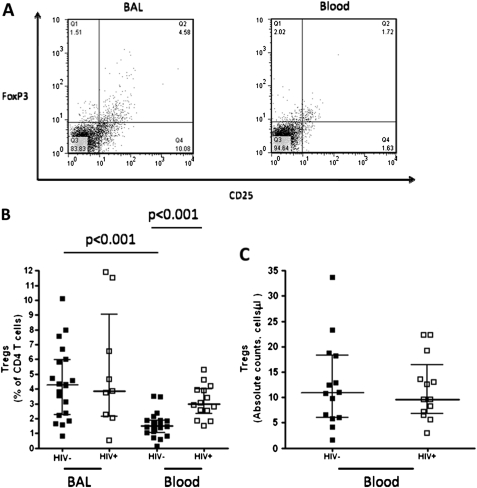
Regulatory T cells (Tregs)
We investigated the hypothesis that in HIV-infected individuals, persistent immune activation by HIV results in a higher frequency of regulatory T cells. Blood and BAL Tregs were defined as CD4+CD25hiFoxP3+ as described in the materials and methods (figure 3A). The proportions of Tregs in HIV-infected individuals were similar in BAL and peripheral blood (median 3.7% vs 3%, p>0.01), but were higher in BAL compared to peripheral blood in HIV-uninfected adults (median 4.3% vs 1.5%, p<0.001) (figure 3B). There was a higher proportion of Tregs in the peripheral blood of HIV-infected individuals than in the HIV-uninfected adults (median 3% vs 1.5%, p<0.001). However, in BAL the proportions were similar between the groups (median 3.7% vs 4.3%, p>0.01) (figure 3B). Absolute counts, however, of Tregs in peripheral blood were similar between HIV-uninfected adults and HIV-infected individuals (median 11 cells/μl vs 9 cells/μl, p>0.01) (figure 3C).
Figure 3.
Higher frequency of regulatory T cells in BAL compared to peripheral blood, but altered in HIV-infected individuals. T lymphocytes obtained from BAL and peripheral blood were stained with anti-CD4 Pacific blue, anti-CD25 FITC and anti-Foxp3 PE fluorochrome conjugated antibodies. Regulatory T cells (Tregs) were defined as CD4+ T cells expressing CD25hi and FoxP3+. (A) A flow cytometry representative dot plot showing Tregs in BAL and peripheral blood from a healthy control. (B) The data shows a higher frequency of Tregs in BAL than peripheral blood in HIV-uninfected adults. It also shows a higher frequency of peripheral blood Tregs in HIV-infected individuals compared to HIV-uninfected adults. (C) The data shows no difference in the absolute counts of Tregs in peripheral blood between HIV-infected individuals and HIV-uninfected adults. Black horizontal bars represent median and IQRs. Statistical significance was analysed by the Mann–Whitney U test in the HIV-uninfected versus HIV-infected comparison, and Wilcoxon Signed rank test in the BAL versus peripheral blood comparison. p value <0.05 was used to determine statistical significance.
Antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis in BAL and peripheral blood
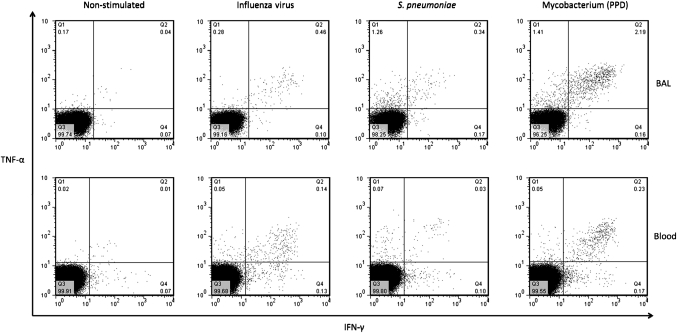
To investigate respiratory pathogen antigen-specific T cell responses in BAL and peripheral blood, we measured the quality and magnitude of the CD4+ T cell response to influenza virus, S pneumoniae and M tuberculosis antigens using intracellular cytokine staining. We detected antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis in BAL and peripheral blood (figure 4).
Figure 4.
Representative flow cytometry dot flow from an HIV-uninfected adult showing multiple subsets of antigen-specific CD4+ T cells in BAL and peripheral blood. BAL and peripheral blood lymphocytes were stimulated with antigens and T cell responses were measured by intracellular cytokine staining. Representative flow cytometry dot plots from an HIV-uninfected adult showing interferon-γ (IFN-γ) and TNF-alpha (TNF-α) responses in BAL (top) and peripheral blood (bottom) cells, in an unstimulated negative control and cells stimulated with influenza virus, S. pneumoniae and M tuberculosis antigens.
Differences between BAL and peripheral blood compartments
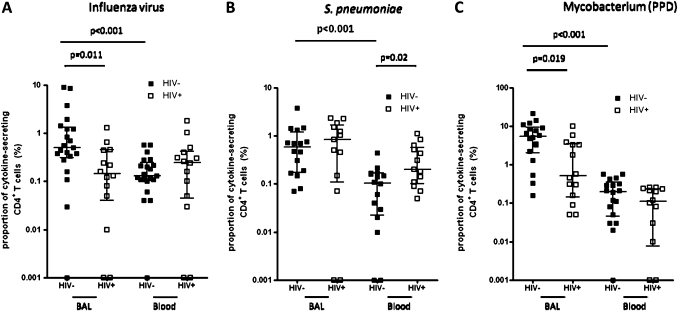
There was a higher percentage frequency of antigen-specific CD4+ T cells against influenza virus (median 0.51% vs 0.13%, p<0.001), S pneumoniae (median 0.59% vs 0.11%, p<0.001) and M tuberculosis (median 5.53% vs 0.20%, p<0.001) in BAL compared to peripheral blood (figure 5A–C). The proportion of antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis producing either IFN-γ alone, TNF-α alone or both cytokines were different between BAL and peripheral blood (figure 6A–C). Background responses were subtracted from all the antigen-specific CD4+ T cell responses.
Figure 5.
Lower frequency of antigen-specific BAL CD4+ T cells in HIV-infected individuals compared to HIV-uninfected adults. BAL and peripheral blood lymphocytes were stimulated with antigens and the magnitude of the antigen-specific T cell response was measured by intracellular cytokine staining. The total of all cytokine-secreting CD4+ T cells was used to represent the percentage frequency of antigen-specific cells. (A) The data shows a higher percentage frequency of influenza virus antigen-specific CD4+ T cells in BAL compared to peripheral blood in HIV-uninfected adults. It also shows a lower percentage frequency of BAL influenza virus antigen-specific CD4+ T cells in HIV-infected individuals compared to HIV-uninfected adults. (B) The data shows a higher percentage frequency of S pneumoniae antigen-specific CD4+ T cells in BAL compared to peripheral blood in HIV-uninfected adults. It also shows a higher percentage frequency of S pneumoniae antigen-specific peripheral blood CD4+ T cells in HIV-infected individuals compared to HIV-uninfected adults. (C) The data shows a higher percentage frequency of M tuberculosis antigen-specific CD4+ T cells in BAL compared to peripheral blood in HIV-uninfected adults. It also shows a lower percentage frequency of BAL M tuberculosis antigen-specific CD4+ T cells in HIV-infected individuals compared to HIV-uninfected adults. Black horizontal bars represent median and IQRs after background responses were subtracted from all the antigen-specific CD4 T cell responses. Statistical significance was analysed by the Mann–Whitney U test in the HIV-uninfected versus HIV-infected comparison, and Wilcoxon Signed rank test in the BAL versus peripheral blood comparison. p value <0.05 was used to determine statistical significance.
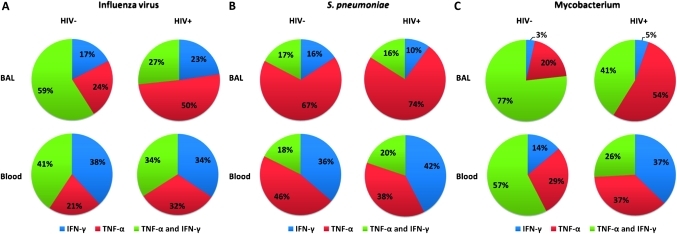
Figure 6.
Lower proportion of polyfunctional antigen-specific CD4+ T cells in BAL and peripheral blood of HIV-infected individuals compared to HIV-uninfected adults. BAL and peripheral blood lymphocytes were stimulated with antigens and the quality of the antigen-specific T cell response was measured by intracellular cytokine staining. The proportion of single producers (IFN-γ alone or TNF-α alone) and double producers (IFN-γ and TNF-α) in the antigen-specific CD4+ T cell population was defined. (A) The data shows that in both BAL (upper) and peripheral blood (lower), the proportion of double producers (green) in the influenza virus antigen-specific CD4+ T cell population was lower in HIV-infected individuals (right) than HIV-uninfected adults (left). It also shows that the proportion of subsets of antigen-specific CD4+ T cells against influenza virus including IFN-γ single producers (blue), TNF-α single producers (red), and IFN-γ/TNF-α double producers (green) were different between BAL (upper) and peripheral blood (lower) in HIV-uninfected adults. (B) The data shows that the proportion of subsets of antigen-specific CD4+ T cells against S pneumoniae (including single producers and double producers), were different between BAL (upper) and peripheral blood (lower) in HIV-uninfected adults. (C) The data shows that in BAL (upper) and peripheral blood (lower) the proportion of double producers (green) in the M tuberculosis antigen-specific CD4 T cell population was lower in HIV-infected individuals (right) than HIV-uninfected adults (left). It also shows that the proportion of subsets of antigen-specific CD4+ T cells against M tuberculosis (including single producers, and double producers), were different between BAL (upper) and peripheral blood (lower) in HIV-uninfected adults.
Differences between HIV-infected and HIV-uninfected adults
The percentage frequency of antigen-specific CD4+ T cells against influenza virus (median 0.15% vs 0.51%, p<0.05) and M tuberculosis (median 0.5% vs 5.53%, p<0.05) antigens were lower in HIV-infected individuals compared to HIV-uninfected adults in BAL (figure 5A,C). However, this was not the case in peripheral blood where the percentage frequencies were comparable (Influenza virus, median 0.3% vs 0.13%, p>0.05; M tuberculosis, median 0.12% vs 0.19% p>0.05)(figure 5A,C). The percentage frequency of antigen-specific CD4+ T cells against S pneumoniae were similar in HIV-infected compared to HIV-uninfected adults in BAL (median 0.84% vs 0.59%, p>0.05). However, in peripheral blood the percentage frequency was higher in HIV-infected compared to HIV-uninfected adults (median 0.2% vs 0.1%, p=0.02) (figure 5B). Background responses were subtracted from all the antigen-specific CD4+ T cell responses.
Further, in BAL and peripheral blood, there was a lower proportion of multiple cytokine-producing (polyfunctional) antigen-specific CD4+ T cells against influenza virus (BAL, 59% vs 27%; Blood, 41% vs 34%) and M tuberculosis (BAL, median 77% vs 41%; Blood, median 57% vs 26%) in HIV-infected individuals compared to HIV-uninfected adults (figure 6A,C).
Discussion
The authors have demonstrated compartment differences between T cell immunity in the bronchoalveolar space and the periphery. These include a predominant presence of effector memory T cells and regulatory CD4+ T cells in BAL, and a higher percentage frequency of antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis in BAL compared to peripheral blood. Our data has also demonstrated that HIV-infected individuals have impaired pulmonary CD4+ T cell immunity, which is characterised by lower proportions of total CD4+ T cells and impaired antigen-specific BAL CD4+ T cell response to influenza virus and M tuberculosis antigens.
Consistent with previous observations,21 we noticed that BAL CD4+ and CD8+ T cells were predominantly of effector memory phenotype irrespective of HIV status, while peripheral blood T cell phenotypes were distributed among naive, central memory, effector memory and terminal effector. Effector memory T cells migrate to the lung following antigen presentation by antigen presenting cells in the local draining lymph nodes.22 In the lung, effector memory T cells may be involved in mediating host defence against pathogens through macrophage activation and neutrophil recruitment.9 10 Formulation of vaccines which can induce effector memory T cells in the lung, mimicking the natural route of exposure, might improve the efficacy of future vaccines against pulmonary mucosal infections such as pneumococcal pneumonia.
The authors observed a higher proportion of regulatory T cells in BAL fluid compared to peripheral blood in HIV-uninfected adults. Immunity in the lung is well regulated. A high degree of regulation is necessary because an excessive response may lead to destructive immunopathology, while a weak response may lead to prolonged infection. The higher proportion of Tregs in BAL, compared to peripheral blood in HIV-uninfected adults, may reflect the capacity for highly regulated immunity in the lung. While beneficial to the host, this sophisticated level of pulmonary immunocompetence poses a challenge to the potential success of mucosally-administered vaccines against respiratory pathogens.
In addition to the overall phenotypic differences between BAL and peripheral blood T cells seen in this study, the authors observed a higher percentage frequency of antigen-specific CD4+ T cells against influenza virus, S pneumoniae and M tuberculosis in BAL, when compared to the peripheral blood of HIV-uninfected adults. The authors investigated a variety of respiratory antigens in this study and demonstrated that each pathogen has its unique cytokine secreting profile. The influenza virus antigen-specific CD4+ T cells were likely induced through previous infection or exposure. The pneumococcal antigen-specific CD4+ T cells were probably induced through previous exposure following contact, colonisation in the nasopharynx or disease. The M tuberculosis antigen-specific CD4+ T cells may have been induced by previous BCG vaccination, latent infection or exposure. Despite differences in kinetics of exposure, our data suggest that antigen-specific CD4+ T cell responses against common respiratory pathogens are compartmentalised in the lung, where they may confer protection at the site of antigen entry. Therefore, the induction of pulmonary antigen-specific CD4+ T cells through vaccination may provide a useful strategy for preventing respiratory infections in the lung.
In the context of HIV, first, the authors observed a higher proportion of BAL and peripheral blood effector memory CD4+ and CD8+ T cells in HIV-infected individuals than in HIV-uninfected adults. This may be attributed to the high level of hyper-activation associated with HIV infection,23 whereby naive T cells are driven along their differentiation pathway to become effector memory T cells.
Second, the authors observed a higher proportion of Tregs in the blood of HIV-infected individuals compared to HIV-uninfected adults. This observation is consistent with other studies on Tregs in peripheral blood during HIV infection.24 Expressing Tregs as absolute counts, however, showed that there was no difference between HIV-uninfected adults and HIV-infected individuals. This suggests that, during HIV infection, non-Tregs are preferentially depleted compared to Tregs. In contrast, the authors did not observe a difference in proportion of BAL Tregs between HIV-infected individuals and HIV-uninfected adults. However, the proportion of BAL CD8+ T cells was higher in HIV-infected compared to HIV-uninfected adults, suggesting that there is either a depletion of CD4+ T cells or an infiltration of CD8+ T cells. Taking these observations together, there is likely to be an altered ratio of Tregs to effector CD4+ and CD8+ T cells in BAL. This factor may either tip the balance to over-regulation of CD4 responses or lack of control of CD8 infiltrates in HIV-infected adults.
Third, consistent with the earlier observation on altered proportions of pulmonary T cell subsets in HIV-infected individuals, the percentage frequencies of influenza virus and M tuberculosis antigen-specific BAL CD4+ T cells were lower in HIV-infected individuals compared to HIV-uninfected adults. This is in line with a recent observation that there was depletion of BAL M tuberculosis antigen-specific CD4+ T cells in HIV-infected individuals.21 Recent work from other investigators has shown a preferential depletion of peripheral blood M tuberculosis antigen-specific but not cytomegalovirus (CMV) antigen-specific CD4+ T cells in HIV-infected adults.25 They concluded that M tuberculosis antigen-specific adaptive immunity is particularly vulnerable to HIV-associated immune damage. This study has shown that adaptive immunity to other respiratory antigens such influenza virus and not only M tuberculosis is also impaired in HIV-infected individuals. The impaired antigen-specific BAL CD4+ T cell response observed in this study might help to explain the increased susceptibility of HIV-infected individuals to influenza virus and M tuberculosis infections.17 26 The impaired BAL CD4+ T cell immunity to influenza virus in HIV-infected adults may result in increased risk to secondary bacterial infection such as pneumococcal pneumonia. The authors observation that there were detectable antigen-specific CD4+ T cell defects in BAL, but not in peripheral blood, has implications for clinical practice and for vaccine development. Most available assays, being based on peripheral blood, do not detect local responses in the lung and may therefore give misleading results when used for diagnosis or for estimations of vaccine efficacy.
Lastly, the authors demonstrated that there was a lower proportion of polyfunctional CD4+ T cells in BAL and peripheral blood of HIV-infected individuals compared to HIV-uninfected adults. This observation is consistent with others that have showed impaired polyfunctionality in M tuberculosis antigen-specific CD4+ T cells in BAL from HIV-infected adults.21 Recently, it has been shown that during HIV infection, M tuberculosis antigen-specific T cells in blood change from polyfunctional CD4+ T cells to a more predominant monofunctional CD8+ T cell phenotype.27 The authors speculate that owing to hyperactivation induced by HIV,23 T cells are driven towards the terminal differentiation stages of their life cycle, where there is loss of the ability to secrete more than one cytokine.28 It has been reported that polyfunctional T cells may be functionally superior to monofunctional T cells and may provide a correlate of protection against parasitic,29 bacterial30 and viral pathogens.31 This finding provides supporting evidence to the suggestion that an impaired repertoire of multifunctionality, as well as lower absolute numbers of respiratory antigen-specific CD4+ T cell immunity, may contribute to the high burden of respiratory diseases in HIV-infected individuals.
In conclusion, the authors have demonstrated that HIV infection is associated with impaired pulmonary antigen-specific CD4+ T cell immunity against viral and bacterial respiratory pathogens. These defects in pulmonary immunity may help to explain the observed increased risk of acute lower respiratory tract infections in HIV-infected adults at any point during the fall in their systemic CD4 count. A greater understanding of antigen-specific T cell responses that occur in the lungs of HIV-infected individuals will potentially enable better planning of clinical care for these individuals – in terms of prophylactic treatments against common infectious pathogens and the timing of anti-retroviral medication. In addition, it will also provide appropriate markers of efficacy in future vaccine trials for common respiratory pathogens such as S pneumoniae, M tuberculosis and influenza virus.
Acknowledgments
The authors would like to thank the volunteers and the staff of the Queen Elizabeth Central Hospital and MLW diagnostic laboratory, Blantyre, Malawi, for their willing cooperation with this study. We thank Professor Malcolm Molyneux for proof reading the manuscript. We thank Anstead Kankwatira and Rose Malamba for their clinical support, and Visopo Harawa for the laboratory support. We thank James Paton for kindly providing the pneumococcal strains. We acknowledge the support of the Malawi-Liverpool-Wellcome Trust Clinical Research Programme, NIHR Biomedical Research Centre in Microbial Diseases, Royal Liverpool University Hospital and the NWDA for infrastructural support.
Footnotes
Funding: This work was supported by a grant to K.C.J from the Commonwealth Scholarship Commission (UK), and grants to S.B.G and to R.S.H. from the Wellcome Trust (UK).
Competing interests: None.
Ethics approval: This study was conducted with the approval of the College of Medicine Research and Ethics Committee (Malawi) and the Liverpool School of Tropical Medicine Research Ethics Committee (United Kingdom).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mathers C, Boerma T, Ma Fat D. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organisation, 2008 [Google Scholar]
- 2.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS 2001;15:143–52 [DOI] [PubMed] [Google Scholar]
- 3.Turner D. Improving Global Health by Preventing Pneumococcal Disease. In: APPG , ed. London, United Kingdom: UK Government, 2008:1–35 [Google Scholar]
- 4.UNAIDS AIDS Epidemic Update: November 2009. Geneva, Switzerland: UNAIDS WH0, 2009 [Google Scholar]
- 5.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009;374:1543–56 [DOI] [PubMed] [Google Scholar]
- 6.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009;27:393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med 2010;88:135–42 [DOI] [PubMed] [Google Scholar]
- 8.Thomas PG, Keating R, Hulse-Post DJ, et al. Cell-mediated protection in influenza infection. Emerg Infect Dis 2006;12:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton DK, Pitts-Meek S, Keshav S, et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 1993;259:1739–42 [DOI] [PubMed] [Google Scholar]
- 10.Laan M, Cui ZH, Hoshino H, et al. Neutrophil recruitment by human IL-17 Via C-X-C chemokine release in the airways. J Immunol 1999;162:2347–52 [PubMed] [Google Scholar]
- 11.Scriba TJ, Kalsdorf B, Abrahams DA, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 2008;180:1962–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol 2008;181:7445–8 [DOI] [PubMed] [Google Scholar]
- 13.de Bree GJ, Daniels H, Schilfgaarde M, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis 2007;195:1718–25 [DOI] [PubMed] [Google Scholar]
- 14.Barry SM, Lipman MC, Bannister B, et al. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J Infect Dis 2003;187:243–50 [DOI] [PubMed] [Google Scholar]
- 15.Brenchley JM, Knox KS, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol 2008;1:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordano Q, Falcón V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004;38:1623–8 [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg P, Glynn JR, Fielding K, et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005;191:150–8 [DOI] [PubMed] [Google Scholar]
- 18.Gordon SB, Malamba R, Mthunthama N, et al. Inhaled delivery of 23-valent pneumococcal polysaccharide vaccine does not result in enhanced pulmonary mucosal immunoglobulin responses. Vaccine 2008;26:5400–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Főrster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12 [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6 [DOI] [PubMed] [Google Scholar]
- 21.Kalsdorf B, Scriba TJ, Wood K, et al. HIV-1 infection impairs the bronchoalveolarT-cell response to mycobacteria. Am J Respir Crit Care Med 2009;180:1262–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakocević N, Worbs T, Davalos-Misslitz A, et al. T cell-dendritic cell interaction dynamics during the induction of respiratory tolerance and immunity. J Immunol 2010;184:1317–27 [DOI] [PubMed] [Google Scholar]
- 23.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003;17:1881–8 [DOI] [PubMed] [Google Scholar]
- 24.Kolte L, Gaardbo JC, Skogstrand K, et al. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol 2009;155:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geldmacher C, Ngwenyama N, Schuetz A, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med 2010;207:2869–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med 2001;161:441–6 [DOI] [PubMed] [Google Scholar]
- 27.Sutherland JS, Young JM, Peterson KL, et al. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J Immunol 2010;184:6537–44 [DOI] [PubMed] [Google Scholar]
- 28.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008;8:247–58 [DOI] [PubMed] [Google Scholar]
- 29.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007;13:843–50 [DOI] [PubMed] [Google Scholar]
- 30.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nebbia G, Mattes FM, Smith C, et al. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant 2008;8:2590–9 [DOI] [PubMed] [Google Scholar]