Abstract
Early-life experience including maternal care profoundly influences hormonal stress responses during adulthood. Daily handling on postnatal day (P) 2–9, eliciting augmented maternal care upon returning pups to their cage, permanently modifies the expression of the stress neuromodulators corticotropin-releasing factor (CRF) and glucocorticoid receptor (GR). We have previously demonstrated reduced hypothalamic CRF expression already at the end of the handling period, followed by enhanced hippocampal GR mRNA levels (by P45). However, the initial site(s) and time of onset of these enduring changes have remained unclear. Therefore, we used semiquantitative in situ hybridization to delineate the spatiotemporal evolution of CRF and GR expression throughout stress-regulatory brain regions in handled (compared with undisturbed) pups. Enhanced CRF mRNA expression was apparent in the amygdaloid central nucleus (ACe) of handled pups already by P6. By P9, the augmented CRF mRNA levels persisted in ACe, accompanied by increased peptide expression in the bed nucleus of the stria terminalis and reduced expression in the paraventricular nucleus. The earliest change in GR consisted of reduced expression in the ACe of handled pups on P9, a time point when hippocampal GR expression was not yet affected. Thus, altered gene expression in ACe, bed nucleus of the stria terminalis as well as paraventricular nucleus may contribute to the molecular cascade by which handling (and increased maternal care) influences the stress response long term.
Early-life experience influences hormonal and behavioral responses to stress long term, a fact with implications for emotional health and cognitive function (1–3). In animal models, brief daily handling of rat pups for the first 1–3 wk of life evokes neuroplastic processes that lead to long-term reduction of the neuroendocrine stress responses (4–6).
Expression of key effector molecules involved in regulating the hypothalamic-pituitary-adrenal (HPA) axis is permanently altered in adult animals handled early in life (6, 7). The levels of corticotropin-releasing factor (CRF) mRNA in the hypothalamic paraventricular nucleus (PVN), where CRF release elicits ACTH and glucocorticoid secretion, are reduced in early-life handled rats. This reduction of hypothalamic CRF is associated with attenuated hormonal stress responses: after restraint stress, plasma ACTH and corticosterone levels are significantly lower in early-life handled rats compared with undisturbed controls (6, 7). In addition, glucocorticoid receptor (GR) expression in hippocampal CA1 is increased in early-life handled rats, consistent with increased sensitivity to circulating glucocorticoids [corticosterone (CORT)] and more efficient glucocorticoid-mediated negative feedback (Ref. 8, but see Ref. 9). These changes in CRF and GR expression and function are considered to underlie the enduring attenuation of neuroendocrine stress response that follows early-life handling (7). Therefore, determining the origin and evolution of these expression changes should provide an important foundation for understanding this long-term plasticity of the stress response.
We have previously found that modulation of CRF expression in the PVN preceded changes in the hormonal stress response and alterations in hippocampal GR expression (6). The aims of the current studies were: 1) to determine the stress-regulatory regions that are initially involved in handling-induced changes in CRF and GR expression; and 2) to delineate the temporal evolution of these region-specific expression changes by examining CRF and GR expression during, as well as at the completion of the daily handling paradigm.
Materials and Methods
Animals
Timed-pregnancy Sprague Dawley rats (Zivic-Miller Laboratories, Inc., Zelienople, PA) were maintained in animal facilities approved by National Institutes of Health (NIH) and kept on a 12-h light, 12-h dark cycle with access to unlimited lab chow and water. Delivery was verified at 12-h intervals, and the day of birth was considered d 0. If needed, litters were adjusted to 12 pups on postnatal day (P) 1. Experimental conditions were assigned per litter, and cages were not changed during the course of the experiment. All of the experimental procedures were approved by Institutional Animal Care Committee and conformed to NIH guidelines.
Early-life handling
Immature rats were handled at 0830 according to previously described procedures (6). Briefly, cages were brought from the vivarium into the laboratory, and the dam and pups were placed in separate fresh cages. After 15 min, pups were returned to the home cage first, followed by the dam. For mRNA analysis, rats were handled on P2 to P5 (killed on P6, without handling), or from P2 to P8 (killed on P9). For analysis of plasma CORT, rats were handled daily from P2 to P6, or from P2 to P9 and killed on the last day of handling.
Hormonal assay
Rats were decapitated on P6 or P9 (0800–0930 h). Trunk blood was collected from undisturbed and handled rats. For the latter group, samples were collected at different time points after the pups’ handling and return to the home cage. Thus, 0 min denotes pups who were not returned to the home cage and were killed immediately after handling; 15 min and 30 min denote that rats were killed after a 15- or 30-min sojourn in the home cage after handling. Plasma CORT was analyzed using a commercial RIA kit (ICN, Costa Mesa, CA) as previously described (10); assay sensitivities were 0.5 µg/dl.
In situ hybridization histochemistry
Rats were decapitated on P6 or P9 (0800–0900). Brains were rapidly removed, frozen on powdered dry ice, and stored at −80 C. In situ hybridization histochemistry for CRF and GR mRNA was performed as previously described (6, 11). Briefly, 20-µm coronal sections were collected on gelatin-coated slides and stored at −80 C. Sections were thawed, air-dried, and postfixed in 4% paraformaldehyde in phosphate buffer. Sections were dehydrated/rehydrated through graded ethanols, exposed to 0.25% acetic anhydride in 0.1 m triethanolamine (pH 8), and dehydrated. Sections were incubated with prehybridization buffer (at 42 C for CRF probe and 55 C for GR probe) for 1 h in a humidified chamber then hybridized overnight with CRF deoxyoligonucleotide probe or with GR ribonucleotide probe (originally from Dr. K. Yamamoto, courtesy Dr. J. Masters, Pfizer, Inc., Ann Arbor, MI). Sections hybridized with the riboprobe underwent a 30 min ribonuclease digestion at 37 C (6). All sections were washed in serial standard sodium citrate buffers of increasing stringency, dehydrated, and apposed to film for 7–14 d (6, 11). Selected sections were dipped in emulsion (NTB-2; Eastman Kodak Co., Rochester, NY), and developed after 3 wk.
Semiquantitative analysis and statistical considerations
Four anatomically matched sections per brain region per rat (n = 8–12 rats per group) were used for each analysis. Unbiased methods for section sampling have been previously described (12) and analyses were conducted without knowledge of treatment. CRF and GR mRNA in situ hybridization histochemistry signal was analyzed on digitized films using the ImageTool software program (UTHSC, San Antonio, TX) as described elsewhere (12). The linear range of ODs was determined using 14C standards. The significance of differences between groups were determined using Student’s t test (Prism GraphPad, San Diego, CA) with Welch’s correction for unequal variance, when needed. Significance levels were set at P < 0.05.
Results
CRF mRNA, but not GR mRNA, levels are altered in amygdaloid central nucleus (ACe) already by P6
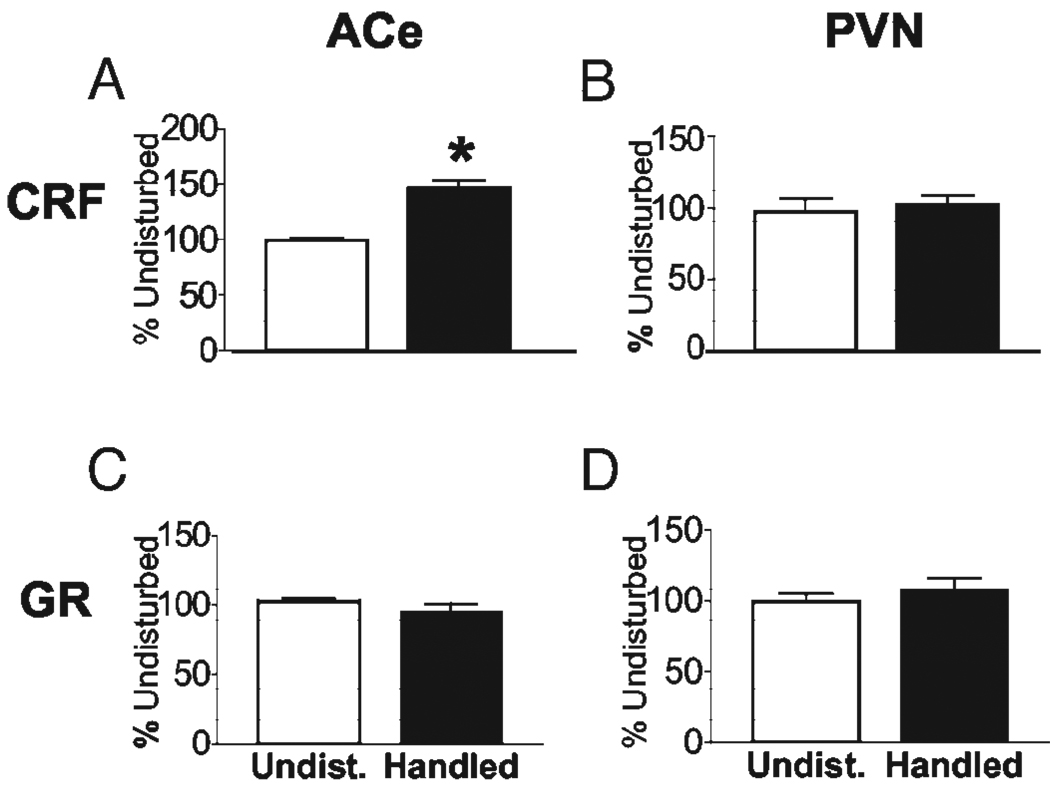
The temporal onset and progression of CRF and GR mRNA expression changes were determined in rats that were handled from P2 to P5 and killed on P6. Enhanced CRF mRNA expression in ACe was evident already by P6 in handled rats compared with age-matched undisturbed controls (146.0 ± 7.76%; P < 0.001; Fig. 1A). CRF mRNA levels in PVN were not yet reduced in this group (compare Fig. 1B with Fig. 2C), and the peptide’s expression in bed nucleus of the stria terminalis (BnST) was unchanged (108 ± 4.6% of control levels, P = 0.87; data not shown). Handling-induced differences in GR expression in ACe (Fig. 1C) or PVN (Fig. 1D) were not observed in handled rats killed on P6.
FIG. 1.
CRF mRNA expression is altered already in rats handled from P2 to P5 and killed on P6, whereas GR expression is not affected. Semiquantitative analyses of CRF (A and B) and GR (C and D) in situ hybridization signals in the ACe (A and C) and the PVN (B and D) were conducted. At this age, CRF mRNA expression was increased in ACe of handled rats (A); CRF mRNA levels in PVN and GR mRNA levels in both regions did not differ between handled and undisturbed rats. Values are expressed as percent of undisturbed values (means ± sem); *, P < 0.05. n = 8–12 animals per group.
FIG. 2.
CRF mRNA is differentially regulated in stress-responsive brain regions in 9-d-old (P9) rats that have experienced handling from P2 to P8. Photomicrographs of coronal brain sections—from undisturbed (Undist.) and handled rats—after in situ hybridization show typical CRF mRNA signals. Quantification of each region is located on the right. A, The ACe; B, anteriodorsal BnST (AD-BnST), at the level shown in the diagram. In both of these regions, handling led to increased CRF mRNA expression. C, The PVN, where handling led to reduced CRF expression. BLA, Basolateral nucleus of the amygdala; Piri, piriform cortex. ODs were averaged from four sections per brain-region per animal (n = 8–12 animals per group) and are expressed as percent of undisturbed values (means ± sem); *, P < 0.05.
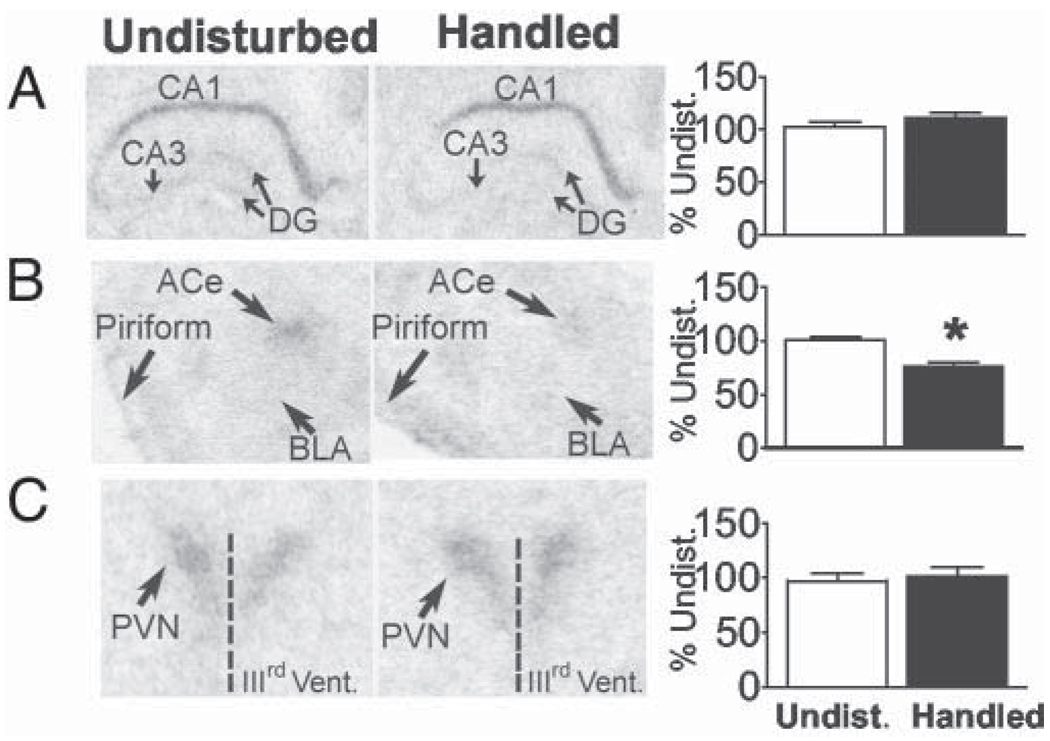
CRF mRNA expression is enhanced in ACe and BnST and reduced in PVN at the end of the handling paradigm (P9)
Changes in CRF expression in several stress-responsive brain regions of handled pups were found by P9. At this age, handling increased CRF mRNA expression in ACe (151.4 ± 9.2%; Fig. 2A) and in BnST (163.6 ± 9.12%; Fig. 2B) compared with undisturbed controls (P < 0.001). In contrast, CRF mRNA levels in PVN were significantly reduced in handled rats (55.04 ± 4.47%) compared with undisturbed controls (P < 0.001; Fig. 2C), consistent with our previous findings (6). Representative bright-field and dark-field photomicrographs of coronal brain sections from undisturbed and handled rat pups depict the region-specific alteration of CRF mRNA signal intensity in ACe, BnST, and PVN.
GR mRNA levels are reduced in ACe but remain unchanged in the hippocampus on P9
Handling-induced changes in GR mRNA expression in ACe, PVN, and hippocampal CA1 were examined in P9 rats. Consistent with earlier findings, handling did not alter GR mRNA levels in hippocampal CA1 (P = 0.261; Fig. 3A). However, ACe GR expression levels were lower in handled pups (75.82 ± 4.55%; P < 0.001; Fig. 3B), whereas expression levels in PVN (P = 0.676; Fig. 3C) were unaffected.
FIG. 3.
GR mRNA expression is modified in rats that have been handled from P2 to P8 and killed on P9 in a region-specific manner. Comparative bright-field photomicrographs from undisturbed and handled rats indicate unaltered GR expression levels in hippocampal CA1 region (A) and PVN (C) and significant down-regulation in ACe (B) of handled rats. BLA, Basolateral nucleus of the amygdala; DG, dentate gyrus. Quantitative analysis is shown on the right, and values are expressed as percent of undisturbed values (means ± sem); *, P < 0.05. n = 8–12 animals per group.
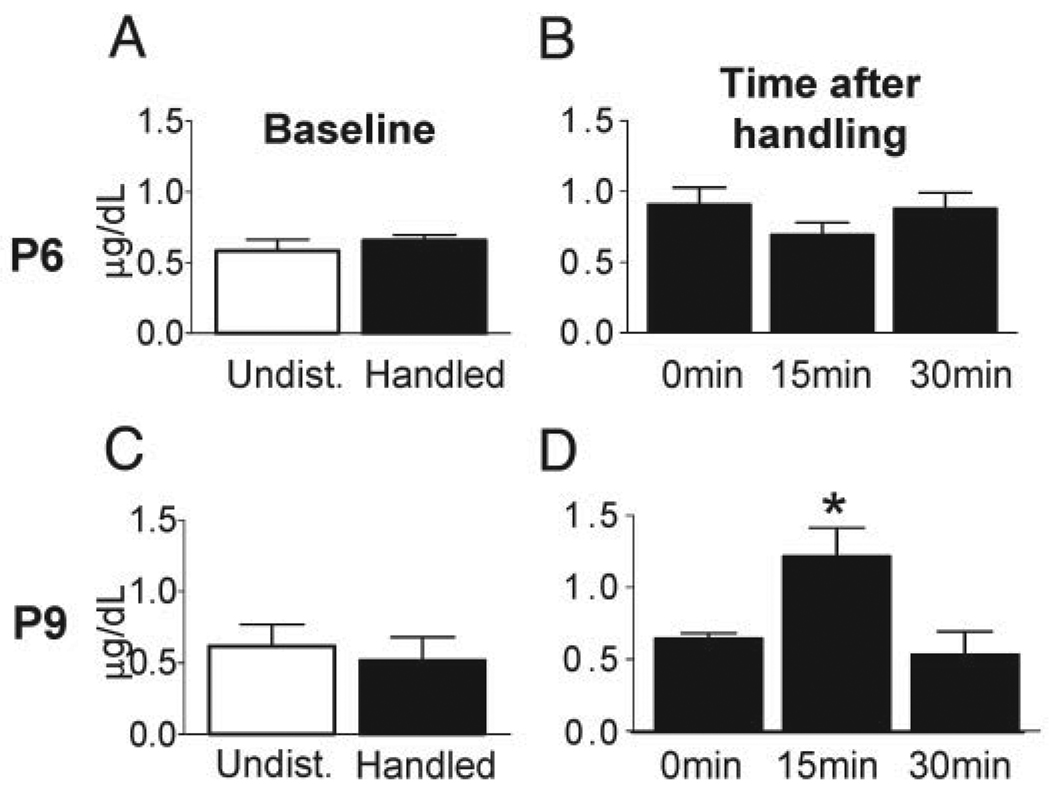
The return of the pups to their mothers during the last days of the handling paradigm, induces a transient elevation of plasma corticosterone levels
To investigate the potential role of recurrent activation of the HPA axis in the handling-induced programming of the stress response, we measured plasma CORT levels at baseline, immediately after handling, and 15 min and 30 min after the pups’ return to their home cage on P6 and P9. As shown in Fig. 4, baseline plasma CORT levels of handled pups, and hormone levels obtained after handling (time 0 of pups’ return to the cage), did not differ from those of undisturbed pups on either day. However, on P9, plasma CORT levels were higher in handled pups 15 min after their return to the dam (P < 0.05). This transient enhancement of CORT returned to basal levels 30 min after the pups’ return to the cage.
FIG. 4.
Plasma CORT levels in 9-d-old handled rats are elevated after pups are returned to their cage. Baseline plasma CORT levels in the handled and undisturbed groups were not significantly different on P6 (A) or on P9 (C). B and D, Plasma CORT levels were measured at the end of the handling paradigm (0 min) and in rats killed 15 min or 30 min after the pups’ return to the dam. B, Transient elevation of plasma CORT was not found in rats handled from P2 to P6 and evaluated on P6. D, Plasma CORT levels in P9 pups killed 15 min after their return to the dam were increased and this elevation was transient. Values are means ± sem; *, P < 0.05. n = 6 animals per group.
Discussion
These studies demonstrate that: 1) CRF expression in ACe is enhanced by daily handling already by P6. 2) By P9, enduring up-regulation of CRF mRNA in ACe is associated with similar changes in the BnST and by reduced CRF mRNA in PVN (3). The earliest changes in GR expression are found in ACe by P9 (4). In the latter days of the handling paradigm (after P6), daily return of pups to the mother is accompanied by a significant and transient activation of glucocorticoid release. These findings provide a spatiotemporal fingerprint of the stress-related regions involved in the early phases of HPA programming by handling (and subsequent enhancement of maternal care). In addition, this sequence of events, together with the hormonal response evoked by maternal care, suggest plausible mechanisms for the profound and long-lasting modulation of the neuroendocrine stress responses by early-life experience.
The initial molecular change evoked by recurrent daily handling consisted of increased CRF mRNA levels in ACe. ACe is involved in complex integrative pathways stimulated by maternal-derived sensory input, particularly licking and grooming (2, 10, 13). The primary role of augmented maternal care (initiated by the return of handled pups to their cages) in handling-evoked programming of the HPA axis has been well documented (13), and was evident also in our hands (data not shown). Maternal stimulation of anogenital areas may be conveyed via brain stem nuclei including CRF-expressing neurons in Barrington’s nucleus to the paraventricular nucleus of the thalamus (14). Paraventricular nucleus of the thalamus neurons project heavily to ACe (15), the central integrator of maternal-derived input and of stress signals (10). Our data suggest that a key consequence of daily activation of this circuitry is augmentation of CRF gene expression in ACe already by P6.
By P9, the handling-evoked changes in ACe were accompanied by reduced CRF mRNA in the hypothalamic PVN, a reduction that persisted and probably contributed to the lifelong diminished stress response of handled rats. The relationship of increased CRF expression in ACe and the subsequent reduced expression of the gene in the PVN is not entirely clear: ACe may inhibit PVN directly (16) or multi-synaptically (17), but extensive research indicates that the ACe generally facilitates PVN CRF expression (18–20) and function (21–23). Therefore, handling may exert independent and opposing effects on CRF expression in these regions. Clues to such mechanisms arise from analyzing plasma CORT levels evoked by the procedure: we found modest but significant elevations of plasma CORT in handled pups after their return to the dam. Levels were not increased immediately after handling, (i.e. ~20 min from the initial disturbance of the pups), and CORT levels peaked approximately 15 min after the return of pups to the dam, suggesting that this glucocorticoid output was initiated by maternal stimulation of the pups. Glucocorticoids exert a differential effect on CRF mRNA expression in ACe and PVN: CORT increases CRF expression in ACe and reduces CRF mRNA levels in PVN in mature animals (21, 24, 25), as well as in developing rats (26–29). Thus, the recurrent elevations of plasma CORT (presumably starting on P7) may underlie the differential effects of handling on CRF expression in ACe and PVN.
Thus, the cascade of molecular changes elicited by handling may commence with CORT-independent increase of CRF synthesis in ACe by P6. This may facilitate CORT release upon subsequent daily return of the pups to the dam (28, 30). Given that GR expression remains unaltered in the PVN, these elevations of plasma CORT act to down-regulate CRF expression in PVN by P9, when sensitivity of the transcript to glucocorticoids is established (26). The enduring reduction of PVN CRF then decreases cumulative CORT release long term, leading to the long-lasting increase in hippocampal GR observed after P45. In ACe, whereas the transient elevation of plasma CORT on P7–P9 might contribute to the observed augmentation of CRF expression on P9, reduced CORT at older ages prevents long-lasting enhancement of the peptide’s expression. Indeed, we did not find long-term increase of ACe CRF mRNA (6, 7), a finding consistent with the reduced behavioral stress responses and anxiety of adult animals handled early in life. In this context, the reduction of GR expression in ACe on P9, although transient (Avishai-Eliner, S., unpublished observation), may function to reduce the sensitivity of CRF-ACe to the handling-induced plasma CORT elevation, mitigating enduring up-regulation of the peptide’s expression in the amygdala.
In summary, daily handling of neonatal rats provokes profound and lasting alterations of the neuroendocrine (and behavioral) stress response. The current studies provide information about the early phases of this programming effect; identify key alterations in the expression of the stress neuromodulators CRF and GR; and point to the likely underlying mechanisms.
Acknowledgments
We thank Farah Akhtar and Karen LeBlanc for excellent technical support.
This work was supported by National Institutes of Health Grants NS 39307, NS 28912, and NS 07444.
Abbreviations
- ACe
Amygdaloid central nucleus
- BnST
bed nucleus of the stria terminalis
- CA
cornu ammonis
- CORT
corticosterone
- CRF
corticotropin-releasing factor
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- P
postnatal day
- PVN
paraventricular nucleus
References
- 1.Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:146–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 3.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess JL, Denenberg VH, Zarrow M, Peiffer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiol Behav. 1969;4:102–109. [Google Scholar]
- 5.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 6.Avishai-Eliner S, Eghbal-Ahmadi M, Hatalski CG, Schultz L, Baram TZ. Downregulation of hypothalamic corticotropin-releasing factor-mRNA precedes early-life experience-induced changes in hippocampal glucocorticoid receptor-mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 8.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:3072–3082. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 9.Tuvnes FA, Steffenach H-A, Murison R, Moser M-B, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci. 2003;23:4345–4354. doi: 10.1523/JNEUROSCI.23-10-04345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ. Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology. 1997;138:5048–5051. doi: 10.1210/endo.138.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Diorio j, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal response to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 14.Otake K, Nakamura Y. Sites of origin of corticotropin-releasing factor-like immunoreactive projection fibers to the paraventricular thalamic nucleus in the rat. Neurosci Lett. 1995;201:84–86. doi: 10.1016/0304-3940(95)12148-w. [DOI] [PubMed] [Google Scholar]
- 15.Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- 16.Marcilhac A, Siaud P. Identification of projections from the central nucleus of the amygdala to the paraventricular nucleus of the hypothalamus which are immunoreactive for corticotropin-releasing hormone in the rat. Exp Physiol. 1997;82:273–281. doi: 10.1113/expphysiol.1997.sp004022. [DOI] [PubMed] [Google Scholar]
- 17.Larsen PJ, Hay-Schmidt A, Mikkelsen JD. Efferent connections from the lateral hypothalamic region and the lateral preoptic area to the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 1994;342:299–319. doi: 10.1002/cne.903420211. [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu S, Pelletier G, Vaudry H, Barden N. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 1989;49:255–261. doi: 10.1159/000125125. [DOI] [PubMed] [Google Scholar]
- 19.Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 20.Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 21.Palkovits M, Young WS, 3rd, Kovacs K, Toth Z, Makara GB. Alterations in corticotropin-releasing hormone gene expression of central amygdaloid neurons following long-term paraventricular lesions and adrenalectomy. Neuroscience. 1998;85:135–147. doi: 10.1016/s0306-4522(97)00621-0. [DOI] [PubMed] [Google Scholar]
- 22.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 23.Walker CD, Tankosic P, Tilders FJ, Burlet A. Immunotargeted lesions of paraventricular CRF and AVP neurons in developing rats reveal the pattern of maturation of these systems and their functional importance. J Neuroendocrinol. 1997;9:25–41. doi: 10.1046/j.1365-2826.1997.00544.x. [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 25.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 26.Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 27.Grino M, Burgunder JM, Eskay RL, Eiden LE. Onset of glucocorticoid responsiveness of anterior pituitary corticotrophs during development is scheduled by corticotropin releasing factor. Endocrinology. 1989;124:2686–2692. doi: 10.1210/endo-124-6-2686. [DOI] [PubMed] [Google Scholar]
- 28.Hatalski CG, Guirguis C, Baram TZ. Corticotropin-releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 30.Walker CD, Dallman MF. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinology. 1993;132:1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]