Abstract
Effector CD4+ T cell subsets, whose differentiation is facilitated by distinct cytokine cues, amplify the corresponding type of inflammatory response. Regulatory T (Treg) cells integrate environmental cues to suppress particular types of inflammation. In this regard, STAT3, a transcription factor essential for T helper 17 (Th17) cell differentiation, is necessary for Treg cell-mediated control of Th17 cell responses. Here, we showed that anti-inflammatory interleukin-10 (IL-10), and not pro-inflammatory IL-6 and IL-23 cytokine signaling, endowed Treg cells with the ability to suppress pathogenic Th17 cell responses. Ablation of the IL-10 receptor in Treg cells resulted in selective dysregulation of Th17 cell responses and colitis similar to that observed in mice harboring STAT3-deficient Treg cells. Thus, Treg cells limit Th17 cell inflammation by serving as principal amplifiers of negative regulatory circuits operating in immune effector cells.
Introduction
Protective immunity against different classes of pathogens is dependent upon generation of distinct types of immune responses mediated and coordinated by T helper 1 (Th1), Th2, and Th17 effector CD4+ T cells. The signals promoting differentiation of naïve CD4+ T cells into a particular T helper cell subset are provided by distinct sets of secreted and membrane bound cytokines elaborated upon triggering of innate immune sensors of infection (e.g. Toll-like receptors, inflammasomes, RIG-I and MDA5) displayed by antigen presenting cells (APCs). Activation of members of the STAT transcription factor family downstream of corresponding cytokine receptors is critical for generation of the effector CD4+ T cell subsets. For example, activation of STAT3 downstream of IL-6, IL-23, and IL-21 receptors is required for efficient generation of highly inflammatory Th17 cells essential for protective immunity against yeast, fungi, and extracellular bacteria (Adamson et al., 2009; Littman and Rudensky, 2010; Xu and Cao, 2010). Th17 cells specific for self-antigens and, possibly, for commensal microbiota have been also implicated in autoimmune diseases such as inflammatory bowel disease, arthritis, and psoriasis (Ahern et al., 2008; McKenzie et al., 2006). In both immunity to infection and autoimmunity, effector CD4+ T cells act foremost as critical amplifiers and “recruiters” of the appropriate types of inflammatory immune responses mediated by cells of both the innate and adaptive immune systems (Littman and Rudensky, 2010).
In addition to a response mode tailored to protect against a particular type of pathogen, a successful immune defense strategy requires intricate negative regulation to restrict host tissue damage caused by the inflammation. Besides cell-intrinsic down-modulation of signaling through antigen-specific and innate receptors, these mechanisms include elaboration of inhibitory soluble mediators by immune effector cells acting in both an autocrine and paracrine manner. Among these mediators, IL-10, which can be produced by multiple immune cell types, plays a particularly prominent role in curtailing immune-mediated inflammation in the context of infection, allergy, and autoimmunity (Moore et al., 2001; Saraiva and O'Garra, 2010).
The negative regulation afforded by immune effector cells themselves is complemented by suppression of inflammatory responses by regulatory T (Treg) cells. These cells, characterized by the expression of the X-chromosome-encoded transcription factor Foxp3, are vital for preventing autoimmunity and immune-mediated inflammation elicited by commensal microbiota and by pathogens, especially, during chronic infection. Treg cell deficiency resulting from deletion or loss-of-function mutations of the Foxp3 gene causes a fatal lympho- and myeloproliferative inflammatory syndrome characterized by massive cytokine storm including sharply increased amounts of Th1, Th2, and Th17 cell cytokines (Fontenot et al., 2003). Likewise, ablation of Treg cells in adult healthy mice leads to augmented generation of Th1, Th2, and Th17 cells and death within two weeks from highly aggressive inflammatory lesions in a variety of organs (Kim et al., 2007). These observations suggest a possibility that Treg cells are able to control different types of the immune response by tailoring their suppressive function to a particular inflammatory environment. In support of this notion, in Treg cells, expression of T-bet and IRF-4, transcription factors involved in Th1 and Th2 cell differentiation, respectively, facilitates Treg cell-mediated suppression of the corresponding type of response (Koch et al., 2009; Zheng et al., 2009). Along the same lines, Treg-specific deletion of STAT3, a transcription factor necessary for Th17 cell differentiation, results in a fatal Th17 cell-driven colitis (Chaudhry et al., 2009). In Treg cells, activated STAT3 and Foxp3 co-operatively regulate a subset of genes, which likely endows Treg cells with the ability to suppress Th17 cell-mediated inflammation (Chaudhry et al., 2009).
STAT3 can be activated downstream of receptors for several pro-inflammatory cytokines including IL-6 and IL-23, which serve as critical inducers of Th17 cell responses, as well as IL-10, known to restrain Th17 cell-mediated inflammation (Gu et al., 2008; McGeachy et al., 2007; Yen et al., 2006). These observations raise a question as to whether the ability of Treg cells to suppress Th17 cell-mediated inflammation is imparted upon sensing pro-inflammatory (IL-6 and IL-23) or anti-inflammatory (IL-10) cues. The former scenario would suggest that Treg cell-mediated suppression represents negative feedback regulation induced by an inflammatory environment. In contrast, the latter scenario implies that Treg cells amplify negative regulators elicited by immune effector cells. Here, we demonstrate that IL-10R, but not IL-6R or IL-23R was required for Treg cell-mediated suppression of spontaneous Th17 cell driven colitis. Thus, in analogy with amplification of effector immune responses by distinct types of Th cells, Treg cells act as amplifiers of negative regulatory circuits of immune effector cells to restrain Th17 cell-mediated inflammation.
Results
IL-10 elicits potent STAT3 phosphorylation in Treg cells
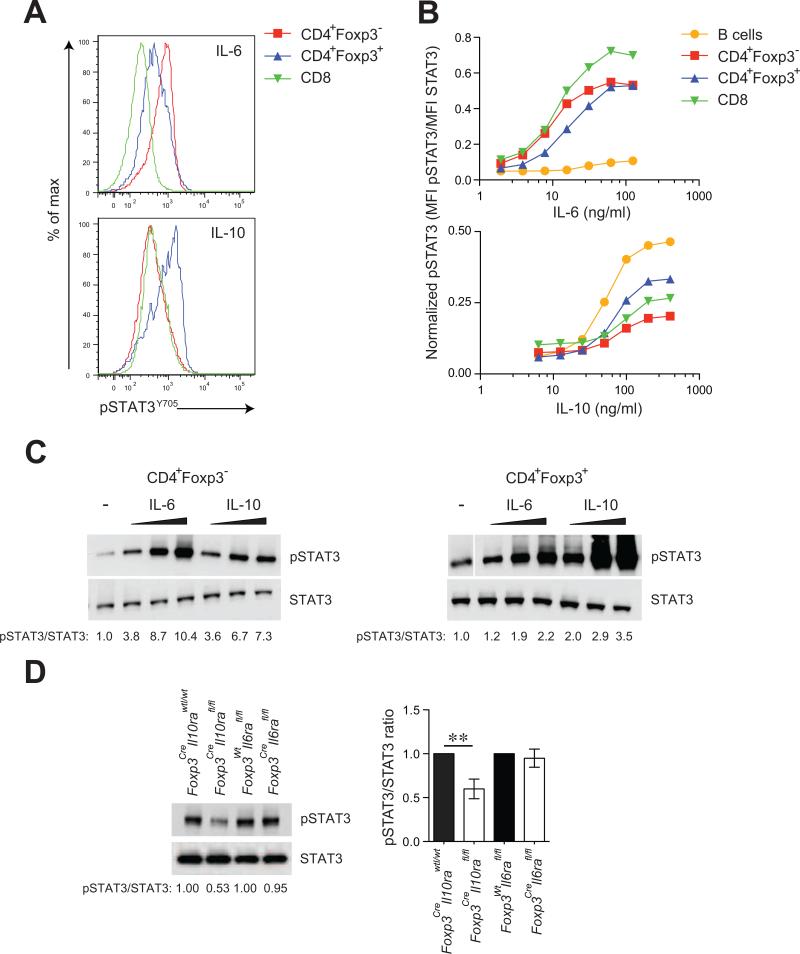
STAT3 activation elicited by IL-6, IL-23, and IL-21 is indispensable for differentiation of activated T cells into Th17 effector cells. In addition to these pro-inflammatory cytokines, STAT3 phosphorylation can be induced by the anti-inflammatory cytokine IL-10, known to oppose Th17 cell responses (O'Shea and Murray, 2008). Our recent studies showed that in suppressive Treg cells, STAT3 plays an essential role for their ability to suppress pathogenic Th17 cell responses (Chaudhry et al., 2009). Thus, we sought to explore whether pro- or anti-inflammatory cytokine signaling confers upon Treg cells the ability to restrain Th17 cell-dependent inflammation. Since thresholds and consequences of STAT3 activation elicited by different cytokines differ between various cell types, we first assessed STAT3 phosphorylation in response to titrated amounts of IL-6 and IL-10 in conventional naïve CD4+ Foxp3- T cells and Treg cells. Amounts of phosphorylated STAT3 (pSTAT3; Y705) were determined using flow cytometric or immunoblot analyses. We found that stimulation of naïve CD4+ and CD8+ T cells with IL-6 induced markedly higher pSTAT3 as compared to IL-10. In contrast, IL-10 was a more potent inducer of STAT3 phosphorylation in Treg cells as compared to naïve T cells (Fig. 1A, B and C). The total STAT3 amounts were not changed upon cytokine treatment. These findings indicate that the pro-inflammatory cytokine IL-6 is more effective in inducing STAT3 activation in naïve T cells, whereas in Treg cells, the anti-inflammatory cytokine IL-10 triggers more efficient STAT3 phosphorylation. Furthermore, basal amounts of pSTAT3 in unstimulated T cells and Treg cells following IL-23 treatment were not different (data not shown). Several factors can account for these differences including varying surface expression of the corresponding cytokine receptors and altered expression of either positive or negative regulators of STAT3 activation. Regardless of the mechanism underlying the contrasting pattern of Treg and naïve T cell responsiveness to IL-6 and IL-10, these results suggest that Treg cells are poised to sense IL-10 and that IL-10R on Treg cells plays a pivotal role in their function.
Figure 1. IL-10 induces robust STAT3 phosphorylation in Treg cells.
(A) Flow cytometric analysis of pSTAT3 (Y705) amounts after treatment of splenic CD4+Foxp3- T cells, CD4+Foxp3+ Treg cells and CD8 T cells with recombinant IL-6 (16 ng/ml) or IL-10 (100 ng/ml) for 15 mins. Analysis of pSTAT3 levels by either flow cytometry (B) or western blotting (C) after stimulation of indicated cells with titrating doses (3, 10 and 15 ng/ml for IL-6; 50, 150 and 300 ng/ml for IL-10) of recombinant IL-6 or IL-10. (D) Immunoblot detection of pSTAT3 and STAT3 in Treg cells isolated from IL-10R or IL-6R deficient and sufficient mice. Data are representative of four independent experiments.
Impaired STAT3 phosphorylation in IL-10R, but not IL-6R-deficient Treg cells
To directly explore this possibility, we induced Treg-specific ablation of IL-10R and IL-6R by breeding mice harboring a conditional allele encoding IL-10Rα (Il10rafl) or IL-6Rα (Il6rafl) chain with Foxp3Cre mice (Pils et al., 2010; Wunderlich et al., 2010; Rubtsov et al., 2008). Foxp3CreIl10rafl/fl and Foxp3CreIl6rafl/fl mice were born at an expected Mendelian ratio and efficiency of deletion was confirmed by quantitative PCR (Fig. S1). We then tested the assumption that IL-10R plays a non-redundant role in STAT3 activation in Treg cells by analyzing pSTAT3 in MACS purified CD4+CD25+ Treg cells isolated from spleens and lymph nodes of healthy 3-4 week-old Foxp3CreIl10rafl/fl or littermate control Foxp3CreIl10rawt/wt mice. IL-10R-deficient Treg cells showed reduced pSTAT3 as compared to littermate controls. In contrast, no differences in pSTAT3 amounts were observed in IL-6R-deficient as compared to IL-6R-sufficient Treg cells (Fig. 1D). These results indicated that IL-10R signaling is an important contributor to STAT3 activation in Treg cells in accordance with the in vitro analysis of STAT3 phosphorylation in Treg cells.
Mice harboring IL-10R deficient Treg cells developed severe immune-mediated colitis
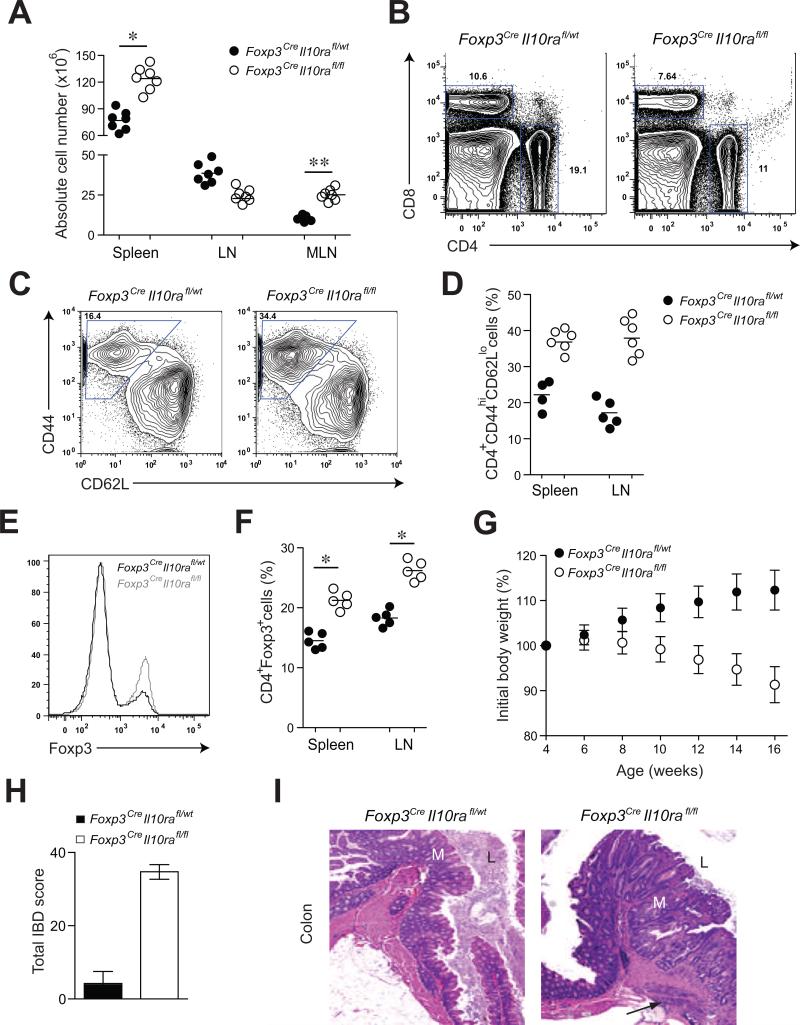
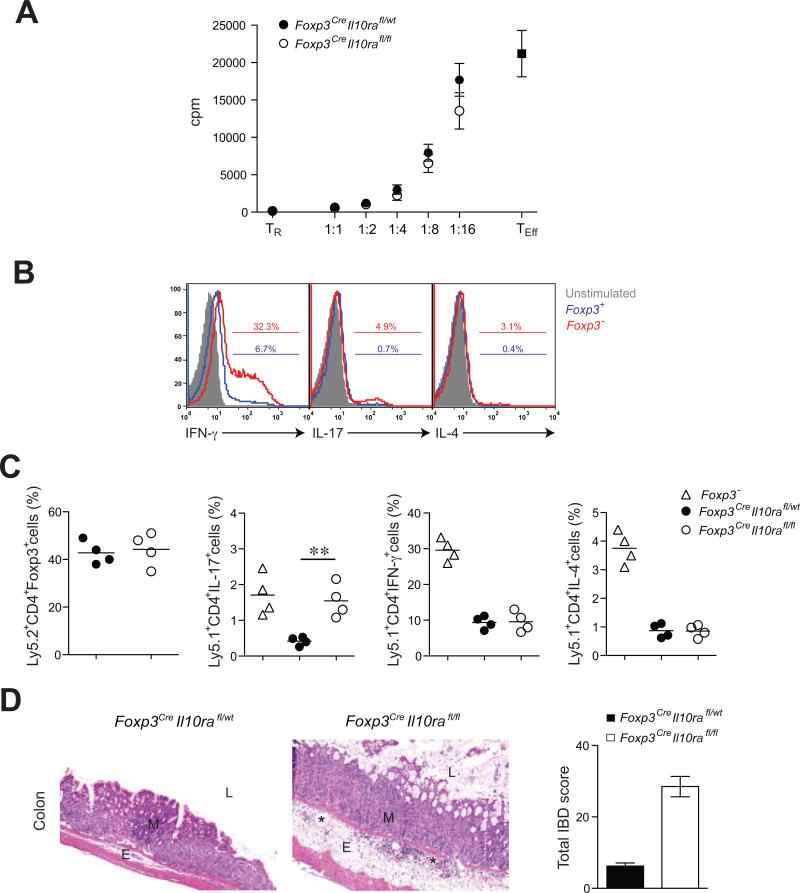
Although Foxp3CreIl10rafl/fl mice appeared healthy up to 8-10 weeks of age, these mice developed clinical symptoms of immune mediated colitis later in life. At ~13-14 weeks of age they exhibited failure to thrive, splenomegaly and pronounced enlargement of mesenteric lymph nodes (Fig. 2A). Despite unchanged or even somewhat reduced numbers of T cells in the lymph nodes and spleen, we observed a marked increase in activated CD44hiCD62LloCD4+ T cells in these mice as compared to littermate controls (Fig. 2B-D). Moreover, these mice developed weight loss, rectal prolapse, and colon thickening and succumbed to the disease by 18-20 weeks of age (Fig. 2G). These clinical manifestations are hallmarks of inflammatory bowel disease (IBD), which was confirmed by histological examination of the intestinal tissues (Fig. 2H). In particular, the colon of the affected mice exhibited marked thickening of the mucosa with dense lymphoid and lesser neutrophilic infiltration, crypt loss and abscessation, regions of glandular hyperplasia with irregular gland formation and increased mitotic figures. In the most severely affected mice, the inflammation was transmural extending to the attached mesentery (Fig. 2I). Unlike colitic Foxp3CreIl10rafl/fl mice, where essentially all Treg cells lacked IL-10R, heterozygote female Foxp3Cre/WtIl10rafl/fl mice, harboring both IL-10R-sufficient and -deficient Treg cells, remained disease-free (Fig. S2). The heightened activation of CD4+ T cells in the presence of IL-10R-deficient Treg cells was not due to diminished numbers, rather, the frequency and absolute numbers of Treg cells were increased in Foxp3CreIl10rafl/fl mice (Fig. 2E and F). Furthermore, the IL-10R-sufficient and -deficient Tregs expressed similar amounts of Foxp3 (Fig. 2E) excluding diminished Foxp3 expression as an explanation for the pathology in Foxp3CreIl10rafl/fl mice.
Figure 2. Treg cell-specific IL-10R deletion results in severe intestinal inflammation.
(A, B) Spleen and lymph node cellularity in Foxp3CreIl10rafl/wt and Foxp3CreIl10rafl/fl mice (** p<0.001; * p<0.01). (C, D) Frequencies of activated T cells (E, F) Absolute numbers of CD4+Foxp3+ T cells and (G) body weights of Foxp3CreIl10rafl/fl mice (n=7). (H) IBD scores derived from evaluation of colon and cecum from 14-16 week-old Foxp3CreIl10rafl/wt and Foxp3CreIl10rafl/fl mice (n=4/group). (I) Representative H&E stained sections of large bowel at the level of the ileocecal-colic junction from Foxp3CreIl10rafl/wt and Foxp3CreIl10rafl/fl mice. Note: Mucosa (M) with expanded irregular, hyperplastic crypts, glandular loss and fibrosis with multifocal transmural inflammation (arrow). Lumen (L). Original magnification, 5x (n=7).
IL-10R deficiency does not impair stability of Foxp3 expression in Treg cells
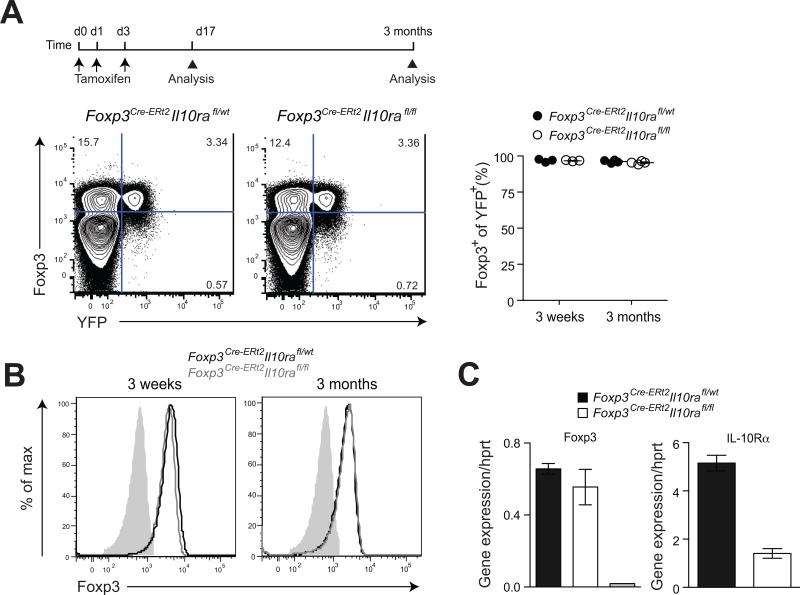
A recent study suggested that upon adoptive transfer of naive CD4+ T cells and Treg cells into lymphopenic hosts, innate immune cell production of IL-10 in the recipients is required to maintain Foxp3 expression (Murai et al., 2009). In this study, Treg cells lacking IL-10R lost Foxp3 expression and thus their suppressor function under inflammatory conditions and, hence, severe colitis commenced in lymphopenic hosts. Since Foxp3CreIl10rafl/fl mice develop similar pathology it was plausible that the phenotype observed in our studies was due to a loss of Foxp3 expression in the absence of IL-10R. Although flow cytometric analysis did not reveal any difference in Foxp3 amounts on per cell basis between Foxp3CreIl10rafl/fl mice and control littermates (Fig. 2E), it was still possible that loss of Foxp3 expression by some IL-10R-deficient Treg cells was masked by proliferation of newly generated Treg cells. To address this important caveat, we employed inducible ablation of the Il10rafl allele in Treg cells using Cre-ERt2 recombinase and assessed Foxp3 expression (Rubtsov et al., 2010). For these experiments, we crossed the Il10rafl mice to knock-in Foxp3Cre-ERt2 mice that express a triple fusion protein of the enhanced green fluorescent protein (eGFP) with Cre recombinase and mutated human estrogen receptor ligand-binding domain (ERt2) under control of the Foxp3 gene. These mice were equipped with a Cre-mediated recombination reporter allele containing a loxP site-flanked STOP cassette followed by a DNA sequence encoding yellow fluorescent protein (YFP) knocked into the ubiquitously expressed ROSA26 locus (R26Y). Treatment of these mice with tamoxifen induced Cre-ERt2-mediated IL-10R ablation and YFP tagging upon excision of the STOP cassette. Genetic labeling of Foxp3-expressing cells with YFP using inducible recombination avoids the continuous incorporation into the labeled population of cells that transiently up-regulate Foxp3 and affords assessment of Foxp3 stability in differentiated Treg cells. Thus, Foxp3Cre-ERt2Il10rafl/flR26Y and littermate control mice were administered tamoxifen and analyzed 3 and 12 weeks later for Foxp3 expression in YFP+CD4+ T cells. We found that IL-10R ablation did not affect Foxp3 expression within splenic and lymph node cells and that essentially all YFP+ tagged cells expressed unaltered amounts of Foxp3 at both time points (Fig. 3A). These results were also confirmed by intracellular staining for Foxp3 and flow cytometric analysis of FACS-purified YFP+ cells at both time points (Fig. 3B). Moreover, analysis of Foxp3 mRNA expression in the sorted YFP+ cells by quantitative PCR (qPCR) also did not detect a measurable loss of Foxp3 in IL-10R ablated Treg cells. As expected, expression of IL-10R mRNA were substantially lower in YFP+ Treg cells isolated from Foxp3Cre-ERt2Il10rafl/flR26Y mice as compared to the control animals (Fig. 3C). Further characterization of the YFP-tagged IL-10R deficient Treg cells did not reveal changes in expression of Th1, Th2 and Th17 specific transcription factors and corresponding cytokines (Fig. S3). These findings effectively exclude a loss of Foxp3 expression as an explanation for the observed inability of IL-10R-deficient Treg cells to suppress intestinal inflammation.
Figure 3. IL-10R ablation does not affect Foxp3 expression and Treg cell lineage stability.
(A) Flow cytometric analysis of Foxp3 and YFP expression on splenic CD4+ T cells in Foxp3CreIl10rafl/flR26Y and control littermate mice. (B) Expression of Foxp3 in flow cytometry-sorted CD4+YFP+ T cells from indicated mice. (C) Quantitative PCR (qPCR) analysis of Foxp3 and IL-10Rα expression in flow cytometry-sorted CD4+YFP+ T cells. The grey bar represents CD4+YFP- T cells. The results represent the mean+/-SD of relative expression values for the indicated genes relative to hypoxanthine-guanine phosphoribosyl transferase. Data are representative of three independent experiments.
Unimpaired function of IL-6R and IL-23R-deficient Treg cells in vivo
In contrast to immuno-suppressive effects of IL-10, IL-6 signaling on T cells has been suggested to be critical for mediating as well as sustaining enteric inflammation (Fasnacht et al., 2009). To identify if IL-6 signaling plays a similar role in Treg cells, we examined Foxp3CreIl6rafl/fl mice. IL-6R ablation in Treg cells did not result in noticeable changes in cytokine production, or activation status and numbers of T cells and other immune cell types including granulocytes, macrophages, B cells, NK cells and dendritic cells (Fig. S4; data not shown). Accordingly, phenotype of Treg cells in Foxp3CreIl6rafl/fl mice was indistinguishable from that in littermate controls and both groups of mice remained healthy.
To assess potential role for IL-23R in Treg cell function we generated mixed bone marrow (BM) chimeras by transferring T cell-depleted BM cells from IL-23R-deficient (Il23r-/-) or -sufficient Ly5.2 B6 mice mixed with BM cells from Ly5.1+Foxp3- mice into lethally irradiated (900 rads) Rag2-/- recipients. In these experimental chimeric mice all Treg cells lacked IL-23R expression. Irradiated Rag2-/- recipients reconstituted with Foxp3- BM cells alone, which served as a positive control for immune-mediated lesions, died within 5-6 weeks after BM transfer (data not shown). In contrast, IL-23R-deficient Treg cells appeared fully functional and (Il23r-/- + Foxp3-) → Rag2-/- BM chimeras were healthy and devoid of measurable signs of T cell activation, lymphoproliferation, and immune-mediated pathology (Fig. S5). Thus, in contrast to IL-10R ablation, IL-6R or IL-23R deficiency in Treg cells did not have noticeable effect on their function.
Increased IL-17 production in the presence of IL-10R-deficient Treg cells
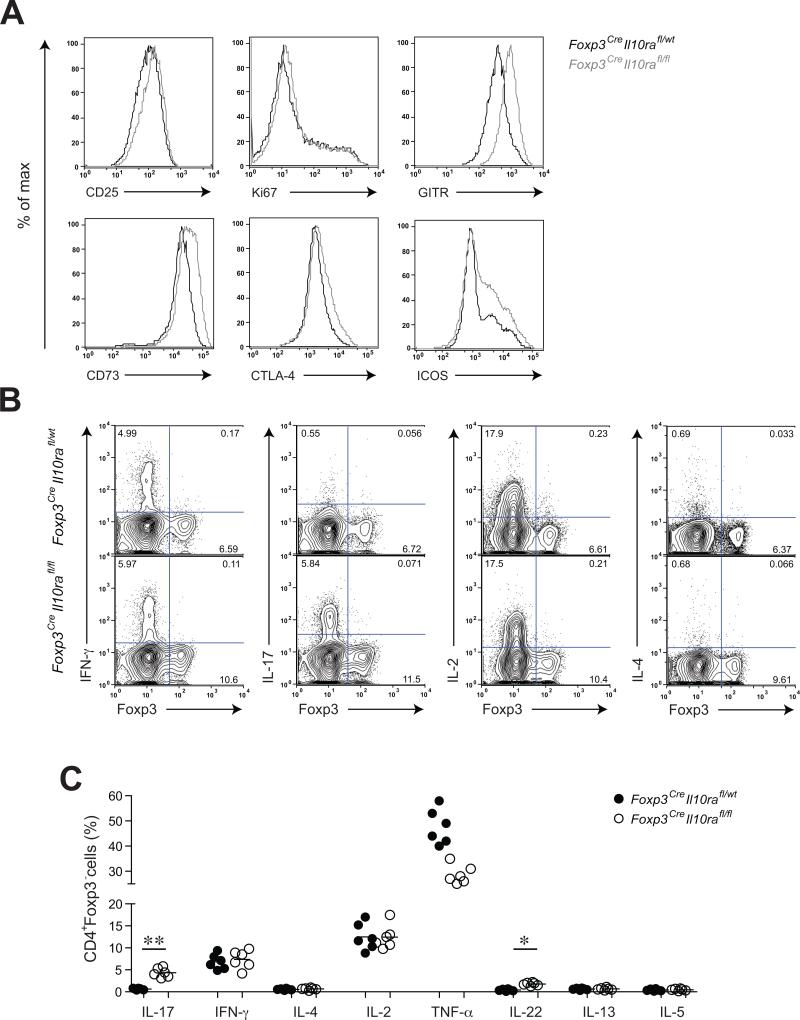
Examination of the surface phenotype of Treg cells from Foxp3CreIl10rafl/fl mice indicated a heightened activation of Treg cells in these mice (Fig. 4A). Although the frequency of proliferating Ki67+ cells within IL-10R-deficient Treg cell population was unchanged, expression of various characteristic Treg cell surface markers including those associated with their suppressor function (eg. CTLA-4) were markedly augmented in Foxp3CreIl10rafl/fl mice in comparison to littermate control mice. The observed Treg cell activation was due to severe colonic inflammation since IL-10R-deficient Treg cells did not exhibit signs of activation in the presence of IL-10R-sufficient counterparts in healthy heterozygote female Foxp3Cre/WtIl10rafl/fl mice (Fig. S2). Thus, IL-10R-deficient Treg cells, like STAT3-deficient Treg cells, fail to restrain colitis likely due to impaired suppressor function.
Figure 4. Increased Treg cell activation and selective dysregulation of Th17 cell responses in Foxp3CreIl10rafl/fl mice.
(A) Flow cytometric analysis of Treg cell activation markers and putative effector molecules on splenic CD4+Foxp3+ T cells in Foxp3CreIl10rafl/fl and control littermate mice. (B, C) Flow cytometric analysis of cytokine production by splenic CD4+Foxp3- T cells in Foxp3CreIl10rafl/wt and Foxp3CreIl10rafl/fl mice after PMA and Ionomycin treatment for 4-6 hours (** p<0.001; * p<0.01).
Recent work has demonstrated that inflammatory Th1 and Th17 responses can both facilitate colitis development and progression (Brand, 2009). Therefore, we assessed T cell production of different effector cytokines in Foxp3CreIl10rafl/fl mice. We observed elevated production of Th17 cell-associated cytokines, IL-17 and IL-22, by CD4+Foxp3- T cells in the presence of IL-10R-deficient Treg cells (Fig. 4B and C). In contrast, production of Th1- and Th2-associated cytokines IFN-γ, IL-4, and IL-5 was comparable in Foxp3CreIl10rafl/fl and littermate control mice. Production of other cytokines such as IL-2 was also unaffected (Fig. 4B and C). Notably, IL-10R-deficient Foxp3+ Treg cells did not produce any of these cytokines (Fig. 4B). Thus, IL-10R-deficient Treg cells were capable of keeping Th1 and Th2 responses in check.
IL-10R-deficient Treg cells fail to suppress Th17 cell responses in vivo
These results were consistent with the idea that only a distinct aspect of the suppressor program was impaired in IL-10R-deficient Treg cells, whereas their general suppressive capacity was intact. This notion was supported by unimpeded ability of IL-10R-deficient Treg cells to suppress proliferative responses of naïve T cells in vitro (Fig. 5A). To examine suppressor function in vivo, we co-transferred flow cytometry-purified IL-10R-deficient or -sufficient Ly5.2+ Treg cells and effector Ly5.1+ CD4 T cells from Foxp3- mice into Rag2-/- recipients. Prior to transfer, the effector CD4+ T cell population was heavily skewed towards Th1 cells, but also contained Th2 and Th17 polarized cells (Fig. 5B). Effector Foxp3- T cells transferred alone into Rag2-/- recipients induced fatal systemic multi-organ autoimmune syndrome associated with lymphoproliferation and increased Th1, Th2 and Th17 cell cytokine production, similar to that observed in unmanipulated Foxp3- mice. Co-transfer of IL-10R deficient Treg cells and Foxp3-effector T cells resulted only in dysregulated Th17 cell responses as evidenced by increased frequencies and absolute numbers of IL-17-producing Ly5.1+ Foxp3- CD4+ T cells in recipient mice (Fig. 5C) and a colitis similar in severity to that seen in Foxp3CreIl10rafl/fl mice (Fig. 5D). Despite failure to suppress Th17 cell-mediated inflammation, IL-10R-deficient Treg cells were able to restrain lymphoproliferation as well as Th1 and Th2 responses (Fig. 5C). In contrast, all autoimmune and clinical IBD manifestations were abrogated upon co-transfer of IL-10R-sufficient Treg cells with only mild subclinical and non-fatal colitis noted histologically (Figure 5D). Thus, these results suggest that IL-10R-deficient Treg cells are selectively impaired in their ability to control inflammatory Th17 cell responses and that this impairment leads to fatal colitis.
Figure 5. IL-10R-deficient Treg cells efficiently control in vitro T cell proliferation but fail to suppress Th17 cell differentiation and colitis.
(A) IL-10R-deficient and -sufficient Treg cell-mediated suppression of effector T cell (TEff) proliferative responses in vitro. (B) Frequencies of Th1, Th2 and Th17 skewed cells among the transferred T cell population isolated from the Foxp3- mice. (C) Frequencies of splenic Treg cells, CD4+IL-17+Foxp3- T cells, CD4+IFN-γ+ Foxp3- T cells, and CD4+IL-4+Foxp3- T cells within the indicated donor-derived population 5-6 weeks after co-transfer of either Foxp3CreIl10rafl/wt or Foxp3CreIl10rafl/fl Treg cells with Foxp3- CD4+ T cells into Rag2-/- recipients. (D) Representative H&E stained sections of colon collected from recipient mice 5-6 week after adoptive T cell transfer (original magnification, 10x). Note: Mucosa (M), submucosal edema (E), dilated lymphatics (*) and lumen (L).
IL-10R signaling facilitates IL-10 production by Treg cells
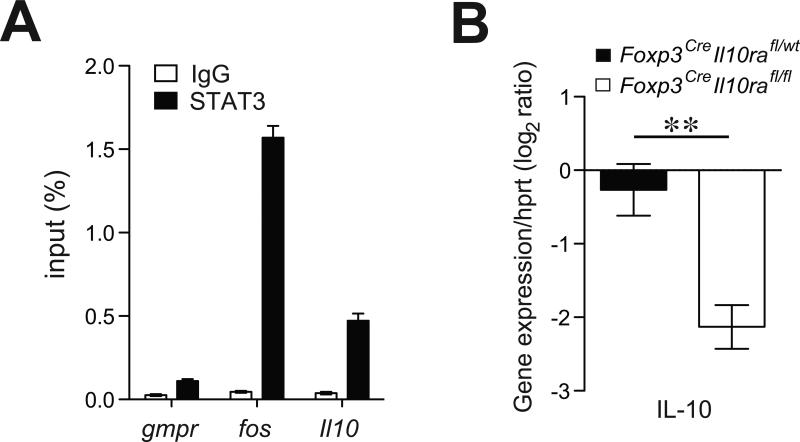
Although Treg cells employ multiple mechanisms of suppression, IL-10 produced by Treg cells plays an important role in preventing colonic inflammation. Mice with Treg-specific ablation of IL-10 develop spontaneous colitis (Rubtsov et al., 2008). Thus, it was plausible that IL-10 produced by immune effector cells elicited STAT3 phosphorylation in Treg cells, which in turn induced IL-10 production in these cells as well as other mediators of suppression. STAT3 has recently been shown to directly bind to a diverse array of targets (Durant et al., 2010). Thus, we examined STAT3 binding to regulatory elements of the Il10 gene in Treg cells. Indeed, we found that STAT3 binds at the Il10 locus in Treg cells (Fig. 6A). To test whether IL-10R deficiency in Treg cells impairs their ability to produce IL-10, we examined IL-10 expression at the mRNA level in IL-10R-deficient and -sufficient Treg cells using qPCR. These experiments showed significantly diminished production of IL-10 by IL-10R-deficient Treg cells (Fig. 6B). Thus, IL-10R signaling in Treg cells leads to STAT3-dependent expression of IL-10.
Figure 6. IL-10R signaling is required for IL-10 expression by Treg cells.
(A) qPCR analysis of STAT3-bound chromatin isolated from wild type Treg cells using primers corresponding to the indicated genes. House-keeping gene Gmpr was used as a specificity control while IgG-immunoprecipitated chromatin was used as a negative control. (B) qPCR analysis of relative expression of indicated genes in Treg cells isolated from either Foxp3CreIl10rafl/wt or Foxp3CreIl10rafl/fl mice. The results represent the mean+/-SD of relative expression values for the indicated genes relative to hypoxanthineguanine phosphoribosyl transferase.
In aggregate, our studies implicate sensing of anti-inflammatory cytokine IL-10, but not pro-inflammatory IL-6 and IL-23, by Treg cells as an essential facilitator of their suppressor function. IL-10 produced by diverse immune effector cells induces activation of STAT3 in Treg cells and, thereby, facilitates their ability to suppress Th17 cell responses, which includes IL-10 production by Treg cells. These results suggest that Treg cells suppress Th17 cell-mediated inflammation by acting as amplifiers of negative regulatory mechanisms elaborated by immune effector cells (Fig. 6C).
Discussion
IL-17- and IL-22-producing Th17 cells mediate protection against mycobacterial, fungal, and bacterial infections. Under physiologic conditions, Th17 cells are found predominantly in the small and large intestine and associated lymphoid tissues where they facilitate production of antimicrobial peptides, enforce integrity of the epithelial barrier, and recruit and activate granulocytes and macrophages to restrain pathogenic bacteria (Weaver et al., 2007). Recent studies demonstrated a critical role of certain components of commensal flora in induction of Th17 cell responses. In germ-free (GF) mice devoid of commensal flora, Th17 cells are essentially undetectable, but are prominently present upon colonization of GF mice with a single commensal microorganism, segmented filamentous bacterium (Ivanov et al., 2009). Th17 cells play a prominent role not only in resistance against intestinal pathogens such as Shigella or Citrobacter (Ouyang et al., 2008), but also in autoimmune and inflammatory disorders in the gastrointestinal tract including Crohn's disease, and ulcerative colitis (Ahern et al., 2008). The preferential generation of Th17 cells in the small and large intestine associated lymphoid tissues and draining lymph nodes is due to high expression of factors supporting Th17 cell differentiation. These factors include IL-1, TGF-β, and STAT3-signaling inflammatory cytokines IL-6, IL-21 and IL-23 (Korn et al., 2009). A complete block of Th17 cell differentiation in the absence of STAT3 illustrates a critical role for the latter three cytokines (Mathur et al., 2007; Yang et al., 2007).
Intestinal homeostasis requires restraint of highly inflammatory Th17 cells by a variety of cell-intrinsic and extrinsic negative regulatory mechanisms. Among mechanisms limiting Th17 cell responses, another STAT3-signaling cytokine IL-10 plays a prominent role (Gu et al., 2008; Li and Flavell, 2008; McGeachy et al., 2007). IL-10 is a powerful negative regulator of the immune-mediated inflammation with a broad range of target cell types, primarily of hematopoietic origin (Jankovic et al., 2010). IL-10 has been implicated in limiting inflammation at environmental interfaces, in particular, in the gut and lung mucosa (Groux and Cottrez, 2003; Sun et al., 2009). Germ-line IL-10 deficiency in mice or inability of T cells to produce IL-10 leads to immune-mediated colitis (Roers et al., 2004) that is completely dependent on enteric microflora (Stehr et al., 2009) defining a major tissue-protective role for IL-10. Not surprisingly, impaired IL-10 signaling has also been implicated in the pathogenesis of IBD and recently mutations in the IL-10R genes have been described in two un-related families with a heritable and poorly treatable form of Crohn's disease (Glocker et al., 2009).
Multiple immune effector cells, including T cells, B cells, macrophages, dendritic cells, NK cells, neutrophils, eosinophils, and mast cells, express IL-10 (Saraiva and O'Garra, 2010). In a naïve mouse, B cells have been reported to constitute a dominant source of IL-10 in several lymphoid tissues and B cell-derived IL-10 plays a non-redundant role in restraining T cell responses (Madan et al., 2009). Unlike several other cell types where IL-10 plays an immunosuppressive function, IL-10 on B cells acts to prevent apoptosis, enhances proliferation, differentiation, and MHC II expression in addition to playing a role in regulation of Ig class switching (Sabat et al., 2010). The differential activation of STAT3 observed in B cells upon IL-10 as opposed to IL-6 stimulation probably reflects the crucial role of IL-10 in B cell function.
Within the T cell subset, activated CD8+ T cells and all three main effector CD4+ T cell types, Th1, Th17, and Th2 cells are capable of secreting IL-10 (Li and Flavell, 2008). Thus, IL-10 production represents an essential negative feedback mechanism elaborated by immune effector cells to limit their activation.
In addition to immune effector T cells, suppressive Foxp3- Tr1 and Foxp3+ Treg cells produce IL-10 in the small and large intestine. Recently, we demonstrated that expression of STAT3 in Treg cells is a prerequisite for their ability to suppress Th17 responses and that ablation of STAT3 in Treg cells results in fatal Th17 cell-mediated colitis (Chaudhry et al., 2009). Since STAT3 is required for both elaboration of inflammatory Th17 cell responses and for their suppression by Treg cells, two models of Treg cell-mediated control of Th17 inflammatory responses can be considered. First, Treg cells directly sense the inflammatory environment favoring Th17 cell differentiation, i.e. high amounts of IL-6 and IL-23, which activate STAT3 and, thereby, enable Treg-mediated suppression of Th17 cell-dependent inflammation. Alternatively, Treg cells respond to the anti-inflammatory mediator IL-10 produced by the immune effector cells. According to this scenario, Treg cells act as amplifiers of the negative regulatory mechanisms elaborated by immune effector cells in analogy to the aforementioned amplification of immune responses by effector T cell subsets. Our finding that IL-10R signaling in Treg cells was necessary for their suppression of Th17 cell responses, whereas IL-6R or IL-23R was dispensable strongly argues in favor of the latter possibility. In addition, this finding suggests that the essential role for STAT3 in Treg cells for suppression of Th17 cell inflammation is dependent upon its phosphorylation downstream of cytokine receptors and subsequent changes in gene expression.
Colitis observed in Foxp3CreIl10rafl/fl mice was similar to that observed in mice harboring STAT3-deficient Treg cells suggesting that IL-10 is the predominant activator of STAT3 signaling in Treg cells. Like STAT3-deficient Treg cells, IL-10R deficient Treg cells also fail to suppress Th17 cell responses, whereas no increases in Th1 or Th2 cell cytokines were noted. Nevertheless, clinical colitis progression in the presence of IL-10R-deficient Treg cells was slower than that in the presence of STAT3-deficient Treg cells implying that an additional STAT3 signaling cytokine(s) can contribute to some extent to STAT3 activation in Treg cells.
Recent work has suggested that Treg cells may lose Foxp3 expression in inflammatory settings and these so called “ex-Treg” cells can contribute to pathology (Murai et al., 2010; Zhou et al., 2009). In particular, IL-10 signaling was shown to be necessary for Treg cell stability in an experimental colitis model. A prominent loss of Foxp3 was observed upon transfer of purified Il10rb-/- Treg cells together with naive CD4+CD45RBhi T cells into lymphopenic recipients (Murai et al., 2009). However, we found similar Foxp3 protein expression in IL-10R-deficient and -sufficient Treg cells and increased numbers of Foxp3+ cells were present in Foxp3CreIl10rafl/fl mice. Furthermore, we failed to detect loss of Foxp3 expression upon co-transfer of effector Foxp3- cells and IL-10R-deficient Treg cells into lymphopenic recipients. Finally, we did not observe detectable loss of Foxp3 expression upon inducible deletion of IL-10R in Treg cells combined with the cell fate mapping approach using Foxp3Cre-ERt2 and R26Y recombination reporter allele (Rubtsov et al., 2010). These results indicate that despite loss of IL-10 signaling, Treg cells remain stable and potential down-regulation of Foxp3 cannot explain the inflammatory colitis observed with Treg-specific deletion of IL-10R. One possible explanation for discrepant results is that few contaminating Foxp3- cells can rapidly expand in lymphopenic settings (Komatsu et al., 2009) and this expansion in numbers can be further exacerbated by a lack of IL-10 signaling. Furthermore, these incongruent results can also be potentially dependent on whether Il10ra or Il10rb was utilized to inactivate IL-10 signaling. Whereas IL-10Rα, required for ligand binding, is unique to the IL-10R complex, IL-10Rβ, essential to mediate downstream signaling, is shared between several members of the class II cytokine receptors such as IL-22R and IFN-λR family members (Pestka et al., 2004). Although the expression and function of these receptors on Treg cells have not been characterized, it remains possible that functions of IL-10Rβ unrelated to IL-10 signaling can account for this discordance.
Although Treg cell-mediated suppression is not fully understood in mechanistic terms, various studies indicate that Treg cells utilize multiple means to accomplish suppression of different inflammatory responses (Shevach, 2009; Vignali et al., 2008). Our observation that phosphorylated STAT3 binds to regulatory elements of the Il10 gene in Treg cells and that IL-10R deficiency in these cells is associated with drastically reduced IL-10 expression is indicative of a feed-forward IL-10 loop operating in Treg cells, i.e. IL-10 produced by variety of cellular sources augments its own production by Treg cells. Importantly, ablation of IL-10 in Treg cells leads to spontaneous colitis albeit less severe than that in Foxp3CreStat3fl/fl or Foxp3CreIl10rafl/fl mice (Rubtsov et al., 2008). Furthermore, impaired IL-10 signaling in effector T cells results in augmented Th17 cell, but not Th1 cell responses and Treg cells can restrain these Th17 cell responses in an IL-10 dependent manner (Huber et al., 2011). These observations corroborate our findings that reduced IL-10 production by IL-10R deficient Treg cells is likely to affect Th17 but not other T cell responses.
Our studies indicate that Treg cell-mediated suppression of Th17-driven pathology is facilitated by activation of STAT3 downstream of IL-10R engagement by IL-10. Subsequent induction of a STAT3-dependent Th17-suppression program including IL-10 production by Treg cells can then result in dampening of this Th17 response. However, it is also possible that Treg cells can act on effector T cells to induce IL-10 expression (Kearley et al., 2005). In either scenario, we suggest that Treg cells can act as amplifiers of negative regulatory mechanisms, such as IL-10, that are initially elaborated by immune effector cells upon their activation. This is in analogy with amplification of a particular type of innate and adaptive immune responses by a given effector CD4+ T cell type.
Methods
Animals
Foxp3-, Foxp3Cre, Foxp3Cre-ERt2, Rosa26YFP, Il6rafl and Il10rafl mice have been previously described (Fontenot et al., 2003; Pils et al., 2010; Rubtsov et al., 2010; Rubtsov et al., 2008; Wunderlich et al., 2010). IL-23R-deficient mice were kindly provided by Vijay Kuchroo (Harvard Medical School). C57BL/6J were purchased from the Jackson Laboratories (Bar Harbor, ME). All the mice were bred and housed in the specific pathogen-free animal facility at the Memorial Sloan-Kettering Cancer Center and used in accordance with institutional guidelines. Tamoxifen (Sigma) was dissolved in olive oil (Fluka) to a final concentration of 40mg/ml. Mice received three doses of tamoxifen (8 mg each) at days 0, 1, and 3 by oral gavage.
Reagents, flow cytometry and cell isolation
Fluorophore-conjugated antibodies were purchased from BD-Biosciences and eBioscience. Stained cells were analyzed using a FACSCanto or LSRII flow cytometer (BD Biosciences) and data were analyzed using FlowJo software (Treestar). Intracellular Foxp3 staining was performed using Foxp3 mouse Treg cell staining kit (eBioscience). Flow cytometric analysis of pSTAT3 staining was performed according to manufacturer's instructions by immediate fixation using BD Phosflow Fix Buffer and Perm Buffer III (BD Biosciences). Cytokine staining was performed after stimulation of splenocytes with PMA (50ng/ml) and Ionomycin (500ng/ml) for 4-6 h in the presence of Golgi-Plug (BD Biosciences). All antibodies used for flow cytometry staining were purchased from eBioscience or BD Biosciences.
Single-cell suspensions from lymph nodes and spleen were generated by mechanical disruption. CD4+CD25- naïve T cells and CD4+CD25+ regulatory T cells were purified using MACS isolation kits (Miltenyi Biotec, Germany) according to the manufacturer's protocol. Foxp3-YFP+ and Foxp3-YFP- cells were isolated by sorting on a FACS Aria cell sorter (BD Biosciences).
In vitro suppression assays
For in vitro suppression assays, Treg cells and effector T cells were purified by positive selection using CD4-specific beads (Invitrogen) followed by FACS sorting. Antigen-presenting cells (APC) were prepared from wild-type C57BL/6J splenocytes by T cell depletion using Thy1-specific MACS beads. Effector T cells (2×104 cells/well) were co-cultured with Treg cells at indicated ratios in the presence of irradiated (2000 rad) APCs (1×105 cells/well) in 96-well plates in the presence of CD3 antibody (1 μg/ml) for 60 h and pulsed with 1 μCi 3H-thymidine for additional 8-12 hr. Cell proliferation was measured using a scintillation counter. The data are presented as mean cpm 3H-thymidine incorporation in triplicate cultures and standard deviation.
Western blot analysis
Nuclear extracts from MACS (Miltenyi)-purified CD4+CD25- and CD4+CD25+ T cells or FACS-sorted CD4+Foxp3- and CD4+Foxp3+ T cells were prepared using nuclear lysis buffer (Active Motif) according to the manufacturer's protocol and subjected to western blot analysis with antibodies specific for STAT3 and pSTAT3 (Y705) (Cell Signaling Technologies).
Quantitative PCR
Total RNA was extracted with TriZOL reagent (Invitrogen) from FACS purified IL-10R-sufficient and -deficient CD4+YFP+ Treg cells. cDNA was synthesized using SuperscriptIII Reverse Transcriptase (Invitrogen), followed by real-time PCR analysis (SYBR green; Applied Biosystems).
Histopathological evaluation of colitis
Samples of cecum and colon from 3-5 animals per group were fixed in 10% neutral buffered formalin and routinely processed for hematoxylin and eosin staining. Histological evaluation of colitis was performed as described previously (Burich et al., 2001). In brief, the cecum, proximal, middle and distal colon were scored on a 0-4 scale each for mucosal changes (erosion, ulceration, and/or hyperplasia), inflammation, and extent of section involvement by a pathologist. The total inflammation score (IBD score) was derived by summing the scores from the individual segments and subsequently, the mean was derived for each experimental group.
Chromatin immunoprecipitation
ChIP was performed as described previously (Zheng et al., 2007). CD4+CD25+ Treg cells from 6-8 month old mice were isolated using Treg isolation kit (Miltenyi Biotec). Antibody to STAT3 was used from Cell Signaling Technology. Relative abundance of regions of interest in precipitated DNA was measured by qPCR using Power SYBR-Green PCR master mix (Applied Biosystems).
Statistical Methods
Unless otherwise noted statistical analysis was performed using the Student's t test on individual biological replicates in Prism (GraphPad).
Supplementary Material
Acknowledgements
We would like to thank Antonio Bravo, Jennifer Herlihy, Julia Gerard and Payam Zarin for help with the mouse colony management, Hana Lee for superb technical assistance, Jean-Christophe Renauld for anti-IL-22, Vijay Kuchroo for Il23r-/- mice, Dominik Schenten, Simone Nish and Ruslan Medzhitov for help in facilitating key experiments. This work was supported by grants from the National Institutes of Health (A.Y.R). A.C. is supported by the Irvington Institute Fellowship Program of the Cancer Research Institute. R.M.S. was supported by NIH MSTP grant GM07739. A.Y.R. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson AS, Collins K, Laurence A, O'Shea JJ. The Current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21:161–166. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G764–778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasnacht N, Greweling MC, Bollati-Fogolin M, Schippers A, Muller W. T-cell-specific deletion of gp130 renders the highly susceptible IL-10-deficient mouse resistant to intestinal nematode infection. Eur J Immunol. 2009;39:2173–2183. doi: 10.1002/eji.200838710. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20:281–285. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Conner W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudenksy AY, Battaglia M, Flavell RA. Immunity. 2011 doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J. Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443–449. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, Rozell B, Jack RS, Muller W. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol. 2010;40:443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;5:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Stehr M, Greweling MC, Tischer S, Singh M, Blocker H, Monner DA, Muller W. Charles River altered Schaedler flora (CRASF) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab Anim. 2009;43:362–370. doi: 10.1258/la.2009.0080075. [DOI] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, Juntti-Berggren L, Li LS, van Rooijen N, Libert C, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12:237–249. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.