Abstract
Objective
Converging evidence from both experimental and epidemiological studies indicates that there is a bidirectional association between depression and cardiovascular disease, however the precise neurobiological mechanisms underlying this relationship are not well understood. Disruptions in the social environment may influence this relationship. The primary objective of the present study was to investigate the hypothesis that long-term social isolation in an animal model would produce depression-relevant behaviors and disruptions in 24-hour autonomic and activity parameters, and to further demonstrate the utility and validity of an animal model for the study of the social environment, behavior, and autonomic function.
Methods
Depression-relevant behaviors and ambulatory electrocardiographic and activity data were measured in 12 adult, socially monogamous prairie voles (rodents), during a period of chronic social isolation or social pairing (control conditions).
Results
Prairie voles exposed to 4 weeks of social isolation, versus control conditions (social pairing), exhibited anhedonia, increased 24-hour heart rate, reduced 24-hour heart rate variability, and predictable correlations between the behavioral measure (anhedonia) and the autonomic measures.
Conclusions
Social isolation is associated with depressive behaviors, 24-hour autonomic dysfunction, and predictable interrelationships between these variables in prairie voles, but does not appear to be associated with rhythmicity changes in activity level or autonomic function. These findings have implications for understanding the role of the social environment in mediating the association of mood and cardiovascular disorders in humans.
Keywords: Heart rate variability, Isolation, Parasympathetic nervous system, Prairie vole, Social behavior, Sympathetic nervous system
INTRODUCTION
Converging evidence from both experimental and epidemiological studies indicates that there is a bidirectional association between depression and cardiovascular disease, such that the presence of one of these conditions increases one’s likelihood of developing the other condition (1–5). Further, a recent meta-analysis suggests that major depression is an independent risk factor for several cardiovascular diseases including myocardial infarction, coronary artery disease, and cerebrovascular diseases (6). However, understanding the precise neurobiological mechanisms that underlie this association requires further experimental research. The association of mood and cardiovascular disorders may be modulated by reactions to environmental and social stressors, as well as the absence of positive social interactions. For instance, studies in humans indicate that the disruption of social bonds (e.g., through isolation) and perceived loneliness are associated with maladaptive grief, mood disorders, and autonomic dysfunction, as well as altered interactions among these variables (7–11). In particular, individuals with smaller social networks have shown increased depressive symptomatology (elevated scores on the Beck Depression Inventory) (12). Further, several studies have demonstrated that social isolation and lack of social connections (for instance being single or unmarried, having fewer social interactions with friends/family, and/or being less involved in social organizations/clubs) may be associated with an increased risk of cardiovascular morbidity and mortality both in men and women (12–16). Social isolation and the presence of smaller social networks in humans also are associated with several cardiovascular risk factors including coronary artery calcification, increased blood glucose levels, hypertension, and diabetes (14, 17).
Similar to the results found in human samples, experimental investigations with rodents and non-human primates demonstrate that alterations in the social environment (such as acute social stressors, long-term social isolation, and social subordination stress) produce several negative behavioral and physiological changes including behaviors related to depression and anxiety, autonomic dysregulation, atherosclerosis, and exaggerated reactivity to stressors (18–21). For instance, in studies with cynomolgus monkeys, subordinate females have been shown to exhibit behavioral signs of depression, increased heart rate (HR), and atherosclerotic plaques (see for review 20, 21). In studies with rodents, long-term social isolation has been associated with depressive and anxiety behaviors, increased resting HR, and autonomic imbalance (19).
Studies that focus on interactions of the social context with autonomic regulation of the heart can provide insight into the mechanisms underlying depression and cardiovascular disease. Depression has been characterized by activation of the sympathetic nervous system and withdrawal of parasympathetic (vagal) tone to the heart, which are associated with elevations both in resting and 24-hour HR and reductions in HR variability (22–25). Similar autonomic changes are observed in acute and chronic cardiovascular conditions, and are hypothesized to be predictive of cardiovascular morbidity and mortality (26–30). In studies with humans, the analysis of 24-hour electrocardiographic (ECG) parameters, such as HR and HR variability, has been a useful strategy for investigating autonomic consequences of depression and predictors of mortality both in patient and community samples (26, 31–33).
In combination with research involving human subjects, experimental approaches that focus on reliable and valid animal models provide novel methods for investigating mechanisms underlying the link between mood and cardiovascular regulation. The prairie vole (Microtus ochrogaster) is one of very few socially monogamous rodent species, displaying social behaviors similar to humans including an active engagement in the surrounding social context, living in pairs or family groups, exhibiting bi-parental care, and forming long-term familial and opposite-sex bonds (34, 35). Furthermore, autonomic regulation of the heart in this species includes a high level of parasympathetic cardiac tone (relative to sympathetic tone), similar to humans (36). The unique social behaviors coupled with high parasympathetic regulation of the heart suggest that the prairie vole is a useful translational model for studying the integration of behavior and autonomic function.
Previous research, from our laboratory and others, has indicated that prairie voles (like humans) are highly sensitive to disruptions of the social environment. For instance, short- or long-term social isolation (from siblings or opposite-sex partners) induces depression-relevant behaviors in validated, operational tasks, including both anhedonia (that is, the reduced responsiveness to pleasurable stimuli) and learned helplessness (i.e., behavioral “despair”) (18, 37, 38). Similarly, long-term isolation sensitizes prairie voles to several autonomic disruptions indicative of cardiovascular pathophysiology, including increased resting HR, reduced resting HR variability, altered cardiac responsiveness to acute stressors, and autonomic imbalance (characterized by both increased sympathetic tone and a reduction in parasympathetic tone to the heart) (19, 39).
While several recent studies using the prairie vole model have investigated primarily resting cardiac and autonomic function and reactivity to acute environmental stressors (19, 36, 39), the effects of negative social experiences on long-term autonomic parameters in this species are not known. The study of 24-hour variations in autonomic function may provide insight into the influence of the social environment on circadian and ultradian rhythms, and will provide increased translational potential for understanding the interactions among the social environment, mood, and autonomic function in humans. Therefore, the present study included several key goals: (a) to investigate the reliability of social bond disruptions in prairie voles by replicating previous findings (19) illustrating that social isolation produces depression-relevant behaviors; (b) to investigate the hypothesis that social isolation produces disruptions in 24-hour autonomic regulation, including increased HR, reduced HR variability, and altered rhythmicity of autonomic control of the heart; (c) to investigate the hypothesis that depression-relevant behaviors are predictably correlated with 24-hour autonomic dysfunction in socially isolated prairie voles, and more specifically that the degree of anhedonia would be positively related to the degree of autonomic dysregulation; and (d) to further characterize the prairie vole as a useful animal model for informing our understanding of the influence of long-term social stressors on mood and autonomic function in humans.
METHODS
Animals
Twelve adult (75 ± 5 days of age) female prairie voles (42 ± 2 g body weight), descendants of a wild stock caught near Champaign, Illinois, were maintained on a 14/10 h light/dark cycle (lights on at 0630 h), with a temperature of 25 ± 1°C and relative humidity of 31 ± 2 %. Animals were allowed food (Purina rabbit chow) and water ad libitum, unless otherwise specified. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs. For all procedures described herein, only one animal from each sibling pair was studied. All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the local university Institutional Animal Care and Use Committees.
Females were chosen for these experiments because female rodents are an understudied group both in behavioral and physiological investigations relating to mood and cardiovascular disorders (see 40). Also, female prairie voles may be especially sensitive to the effects of social stressors (37, 41). Finally, they do not show a spontaneous puberty or estrous cycle; the ovaries remain inactive until the female has physical contact with a male, allowing for the use of reproductively intact animals without the need for controlling the estrous cycle (42).
Telemetric Transmitter Implantation
Wireless radiofrequency transmitters [Data Sciences International (DSI), St. Paul, MN; model TA10ETA-F20] were implanted intraperitoneally for continuous ECG and activity recordings, under aseptic conditions, during the light period, using procedures described previously (39). Care was taken to ensure that animals did not experience unnecessary pain and distress during the procedures. Briefly, animals were anesthetized (ketamine 67mg/kg, sc; xylazine 13.33mg/kg, sc; NLS Animal Health, Owings Mills, MD), and the transmitter was inserted into the abdominal cavity. The leads were directed rostrally and anchored in place on either side of the heart with permanent sutures. All skin incisions were sutured closed, and subcutaneous sterile saline was administered as necessary. Following the surgical procedures, all animals were housed for 5 days in custom-designed divided cages (Scientific Instrument Shop, University of Illinois at Chicago, Chicago, IL) to permit adequate healing of suture wounds (see 36), and then were returned to the home cages (with respective sibling) to recover for an additional 5–7 days.
Radiotelemetric Recordings
ECG signals were recorded with a radiotelemetry receiver (DSI, St. Paul, MN; sampling rate 5 kHz, 12-bit precision digitizing). Activity level was monitored via the receiver (sampling rate 256 Hz). The radiotelemetry receiver was controlled by Dataquest ART, Version 4.1 Acquisition software (DSI). ECG and activity data were recorded continuously throughout all periods of the study, including recovery from surgery, collection of baseline data, and during social isolation (described below).
Social Isolation
Following recovery from the surgical procedures, prairie voles were randomly divided into two independent groups of either: (a) paired (control; n = 6); or (b) isolated (n = 6) conditions. Isolated animals were separated from their respective siblings and housed individually (in a separate room, without visual or olfactory cues) for 4 weeks, while paired animals were continually housed with the siblings during this period. This time period was chosen to be consistent with previous studies showing that 4 weeks of social isolation in female prairie voles results in a disruption of affective behaviors (e.g., depression- and anxiety-relevant behaviors) (38, 43) and resting cardiac function (19). Handling, cage changing, and measuring of body weight were matched between the two groups. Body weight was recorded prior to isolation, and following 2 and 4 weeks of this period.
Fluid Intake
Ad libitum 2% sucrose was available for 1 week before beginning the experimental procedures to allow for adaptation to its taste. Intake of water and 2% sucrose were measured as an operational index of anhedonia, defined as reduced sucrose intake and sucrose preference relative to control animals and baseline values (44, 45). Food and water were removed from the cage for 16 hours prior to the test; cardiac and activity parameters were monitored to ensure that the deprivation produced no adverse effects. One hour prior to the test, all animals (paired and isolated) were moved into clean, individual cages to ensure accurate fluid intake measurements of paired animals. Water and 2% sucrose were placed on the cage in premeasured bottles, and fluid intake was monitored for 1 hour. Animals were returned to the home cages immediately following the test. Fluid intake tests were conducted prior to isolation (baseline) and following 2 and 4 weeks of this period.
Data Quantification
Quantification of Telemetric Variables
Quantification of telemetric variables was conducted according to procedures described elsewhere (19, 36), and is therefore described briefly here. Multiple segments of stable, continuous data were used to evaluate HR, HR variability, and activity throughout the period of isolation. Data segments of 3–5 minutes in duration were collected every hour, on the hour, for a 24-hour period prior to isolation (baseline) and following 2 and 4 weeks of this period. All data segments were matched between groups, such that the analyses were conducted on data from all subjects (paired and isolated) at the same time during each hour of the day.
Quantification of 24-Hour Autonomic and Activity Variables
24-Hour ECG parameters were evaluated using Dataquest ART, Version 4.1 Analysis software (DSI) and custom-designed software (Department of Psychiatry, University of Illinois at Chicago). Each raw ECG signal was manually inspected to ensure that all R-waves were correctly detected by the recording software.
The analysis of 24-hour HR was conducted by plotting the number of R waves per unit time (reported in beats per minute; bpm). The analysis of 24-hour R-R intervals (i.e., HR variability) included two measures: (a) the standard deviation of all R-R (normal-to-normal; N-N) intervals (SDNN index); and (b) amplitude of respiratory sinus arrhythmia (RSA). The SDNN index was evaluated according to procedures described previously (46), and is hypothesized to represent the convergence of both sympathetic and parasympathetic innervation on the sino-atrial node. RSA was assessed with a modification of procedures described elsewhere (47); and has been hypothesized to be influenced by several central nervous system processes (48), including myelinated vagal efferent pathways originating in the nucleus ambiguus (see 49). Preliminary pharmacological analyses in prairie voles have demonstrated that peripheral cholinergic receptor blockade with atropine methyl nitrate reduces RSA to near zero, providing evidence that there is a prevalent vagal influence mediating this variable (36). Additional spectral analyses identified spectral peaks within the approximate frequency band in which breathing is observed in mammals of similar size (36), confirming that prairie voles express a spectral peak in the range of 1.0–4.0 Hz (50, 51). The R-R intervals were resampled at 20 Hz and, to comply with the assumption of stationarity, detrended with a 21-point cubic moving polynomial to remove low frequency (trend) components below 0.5 Hz. The residuals of this procedure were free of aperiodic and slow periodic processes that may have violated the assumption of stationarity. A bandpass filter was applied to define RSA by extracting only the variance in the HR spectrum between the frequencies of 1.0 and 4.0 Hz.
24-hour activity was quantified using Dataquest ART Version 4.1 Acquisition software (DSI), with a sampling rate of 256 Hz. Activity was recorded at the same time as the ECG parameters, once per hour, on the hour, for a 24-hour period prior to isolation (baseline) and following 2 and 4 weeks of this period. The mean activity measure during each recording was used in the analyses.
Data Analysis
Analyses of Variance and Multiple Comparisons
Data are presented as means ± standard error of the mean (SEM) for all analyses, tables and figures. A value of P < 0.05 was considered to be statistically significant. Repeated variables were analyzed with repeated measures or mixed-design analyses of variance (ANOVA), with group (paired/isolated) as the independent groups factor and the behavioral or physiological variable as the repeated measures factor. Multiple comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) or a priori Student’s t-tests. For all cardiac analyses, care was taken to not include segments of data that included artifact from animal movement.
Periodicity Analyses
Autocorrelation function analyses were performed on each time-series (activity, HR, SDNN index, and RSA amplitude), over the course of the 24-hour period, at each experimental time point (baseline, week 2, and week 4).
Correlational Analyses
Correlations between behavioral and electrocardiographic variables were computed using Pearson’s r correlation coefficients.
RESULTS
Recovery from Surgery and Body Weight
All animals in the study showed a predictable recovery pattern following implantation of the radiotelemetry transmitters, including visible signs of eating, drinking, and activity level within 24 hours after surgery, appropriate healing of suture wounds within 5 days after surgery, and evidence of a stable ECG signal within 12 days after surgery (data not shown). Baseline body weight was 42 ± 3 g in the paired group and 43 ± 2 g in the isolated group; these values were not significantly different (P > 0.05). Body weight following 4 weeks of social isolation was 43 ± 4 g in the paired group and 45 ± 3 in the isolated group; these values also were not significantly different. The ANOVA yielded a main effect of time [F(2,20) = 6.90, P < 0.05], indicating that both groups gained weight during the 4-week paradigm (versus baseline body weight).
Fluid Intake and Preference
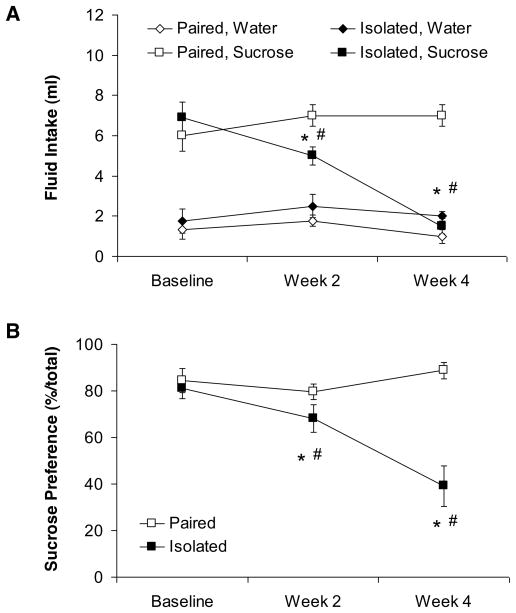
Isolation significantly reduced sucrose intake and sucrose preference relative to control (paired) conditions, but did not affect water intake, indicative of anhedonia (Figure 1). The ANOVA for water intake yielded no significant effects (P > 0.05 for both main effects and interact effect); no follow-up tests were conducted. The ANOVA for sucrose intake yielded main effects of group [F(1,10 = 6.37, P < 0.05] and time [F(2,20) = 17.89, P < 0.05], and a group by time interaction [F(2,20) = 17.88, P < 0.05]. The paired and isolated groups did not differ in baseline sucrose intake (P > 0.05). However, following both 2 and 4 weeks of isolation or pairing, sucrose consumption in the isolated group was significantly lower than its respective baseline consumption and that of the paired group (P < 0.05 for all comparisons). In contrast to the isolated group, the sucrose intake of the paired group did not differ from its respective baseline intake following either 2 or 4 weeks of pairing (P > 0.05 for both comparisons).
Figure 1.
Mean (± SEM) absolute fluid intake (Panel A) and sucrose preference (Panel B) at baseline and following 2 and 4 weeks of social isolation or pairing. *P < 0.05 vs. respective paired value; #P < 0.05 vs. respective baseline value. Statistical significance symbols are displayed below the respective data point in Panel B.
The ANOVA for sucrose preference yielded main effects of group [F(1,10) = 19.94, P < 0.05] and time [F(2,20) = 4.30, P < 0.05], and a group by time interaction [F(2,20) = 7.41, P < 0.05]. Similar to absolute sucrose intake, the two groups did not differ in baseline sucrose preference (P > 0.05). Following 2 and 4 weeks of isolation or pairing, sucrose preference in the isolated group was lower than its respective baseline preference and that of the paired group (P < 0.05 for all comparisons). The paired group did not differ from its respective baseline sucrose preference following either 2 or 4 weeks of pairing (P > 0.05 for both comparisons).
24-Hour Electrocardiographic and Activity Parameters
All 24-hour ECG and activity parameters were plotted across the light and dark periods prior to isolation (baseline) and following 2 and 4 weeks of this period, and the data were analyzed for variations in ECG and activity parameters in both groups. Neither paired nor isolated animals differed in light vs. dark HR, SDNN index, RSA amplitude, or activity measures, consistent with studies showing that variations in these parameters follow an ultradian pattern in prairie voles (36). Light and dark period ECG and activity data were collapsed across the 24-hour period for the purpose of the following analyses.
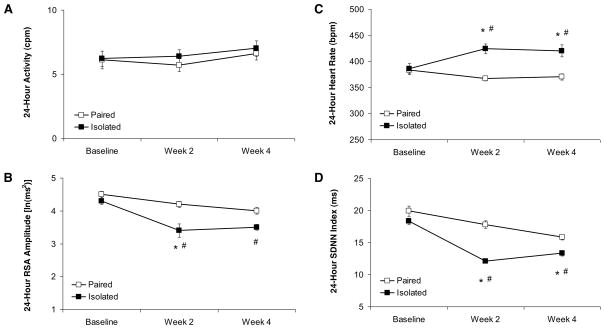
Isolation significantly increased 24-hour HR and reduced 24-hour HR variability, but did not produce an alteration in general activity level, relative to control (paired) conditions (Figure 2). The ANOVA for 24-hour HR yielded a main effect of group [F(1,10) = 9.93, P < 0.05] and group by time interaction [F(2,20) = 9.46, P < 0.05]. The two groups did not differ in baseline 24-hour HR (P > 0.05); following 2 and 4 weeks of isolation or pairing, HR in the isolated group was significantly higher than its respective baseline HR and that of the paired group (P < 0.05 for all comparisons). The paired group did not differ from its respective baseline 24-hour HR across time (P > 0.05 for both comparisons).
Figure 2.
Mean (± SEM) 24-hour activity level (Panel A), amplitude of respiratory sinus arrhythmia (Panel B), heart rate (Panel C), and SDNN index (Panel D) at baseline and following 2 and 4 weeks of social isolation or pairing. Note the scale differences among the 4 panels. *P < 0.05 vs. respective paired value; #P < 0.05 vs. respective baseline value. RSA, respiratory sinus arrhythmia; SDNN, standard deviation of normal-to-normal intervals.
The ANOVA for 24-hour SDNN index yielded main effects of group [F(1,10) = 5.07, P < 0.05] and time [F(2,20) = 9.18, P < 0.05]. The two groups did not differ in baseline 24-hour SDNN index (P > 0.05). The isolated group exhibited a significantly lower 24-hour SDNN index versus its respective baseline value and that of the paired group following 2 and 4 weeks of isolation or pairing (P < 0.05 for all comparisons). Although the paired group showed a slight, progressive decline in 24-hour SDNN index across the 4-week period, this decline was not statistically significant at either 2 or 4 weeks of pairing (P > 0.05 for both comparisons).
The ANOVA for 24-hour RSA amplitude yielded a main effect of time [F(2,20) = 9.83, P < 0.05] and a trend toward a significant main effect of group [F(1,10) = 3.93, P < 0.07]. The two groups did not differ in baseline 24-hour RSA amplitude (P > 0.05). However, after 2 weeks of isolation the isolated group exhibited a significantly lower 24-hour RSA amplitude versus the paired group following 2 weeks of isolation or pairing (P < 0.05). The isolated group also exhibited a significantly lower 24-hour RSA amplitude following both 2 and 4 weeks of isolation versus its respective baseline RSA amplitude (P < 0.05 for both comparisons). Although the paired group showed a slight, progressive decline in 24-hour RSA amplitude across the 4-week period, this decline was not statistically significant at either 2 or 4 weeks of pairing (P > 0.05 for both comparisons).
The ANOVA for 24-hour activity level did not yield any significant effects (P > 0.05 for all comparisons). No follow-up analyses were conducted.
Periodicity Measures
Autocorrelations were calculated for each subject during each of the three time points (baseline, week 2 isolation/pairing, week 4 isolation pairing), using the hour-by-hour measures of activity, HR, RSA amplitude, and SDNN index. During baseline conditions, the activity exhibited a significant autocorrelation with a periodicity of three hours, confirming that prairie voles show increased levels of activity approximately every three hours but do not differ in their activity levels during the light and dark periods (see 36). The measures of HR, RSA amplitude, and SDNN index were not significantly periodic; and the patterns of activity and ECG parameters following 2 and 4 weeks of isolation were similar to the baseline patterns (data not shown).
Behavior-Cardiac Function Correlations
Absolute sucrose intake was predictably correlated with 24-hour electrocardiographic parameters following 4 weeks of isolation, but not following 2 weeks of isolation (see Table 1). In the isolated group, sucrose intake was negatively correlated with 24-hour HR, and was positively correlated with 24-hour SDNN index and RSA amplitude. In the paired group, sucrose intake was negatively correlated with 24-hour HR, positively correlated with 24-hour SDNN index, but was unrelated to RSA amplitude. The Pearson’s r values in Table 1 are shown for illustrative purposes only; statistical comparisons of these values are not shown given the small sample sizes.
Table 1.
Pearson’s r correlations between anhedonic behavior (sucrose intake) and autonomic variables following 4 weeks of isolation or pairing.
| RSA Amplitude | Heart Rate | SDNN Index | |
|---|---|---|---|
| Paired | 0.00 | −0.53 | 0.22 |
| Isolated | 0.34 | −0.30 | 0.23 |
Note: Correlations were calculated using data following 4 weeks of isolation or pairing. RSA, respiratory sinus arrhythmia; SDNN, standard deviation of normal-to-normal intervals.
DISCUSSION
Prairie voles are socially monogamous mammals that live either in pairs or family groups in nature (35). Studies in this species have provided new insights into both the causes and consequences of sociality (52, 53). The current study was designed to investigate relations among the social environment, anhedonic behaviors, and 24-hour autonomic parameters using the prairie vole model. To investigate these interactions, adult, female prairie voles were exposed to 4 weeks of social isolation from a same-sex sibling, during which time behavioral measures and autonomic parameters were assessed. The findings indicate that social isolation produces anhedonia, as well as disruptions in autonomic regulation of the heart indicated by altered 24-hour HR and HR variability. The present results demonstrate the utility of studying the integration of depressive behaviors and 24-hour ECG parameters in rodent models involving social stressors. Importantly, in humans there are several dimensions of sociality that may have relevance for understanding the influence of the social environment on the link between mood and cardiovascular function (54–56). The present study has translational value for our understanding of how social isolation or the disruption of established social bonds may influence emotion and autonomic function. In combination with studies using human samples, these findings provide a foundation for conducting further experimental investigations of the social environment, behavior, and autonomic function in the context of depression and cardiovascular disease.
Long-term social isolation was associated with anhedonia in prairie voles, using an operational behavioral index (sucrose consumption). In contrast to paired control animals, both absolute sucrose intake and sucrose preference were reduced in isolated prairie voles following 2 and 4 weeks of social isolation. As observed in Figure 1, the isolated group showed no preference for sucrose versus water following the 4-week isolation period. The specific change in sucrose intake (without a corresponding change in water intake), coupled with a lack of change in body weight during the course of isolation, provides evidence that the reduction in sucrose consumption is a specific hedonic deficit, rather than a generalized deficit in ingestive behavior. The anhedonia shown here is similar to previous studies involving social isolation in prairie voles and exposure to chronic behavioral stressors in rats (43, 57), contributing to the predictive validity and reliability of the prairie vole model of social isolation. Importantly, anhedonia is a diagnostic feature and a common characteristic in human depression (58, 59); hence, the ability to mimic this feature in prairie voles also enhances the face and construct validity of this model.
Anhedonic prairie voles in the present study exhibited significant changes in autonomic function during the social isolation period. Isolation was associated with an increase in 24-hour HR and a decrease in 24-hour HR variability (both SDNN index and amplitude of RSA). These autonomic changes in isolated prairie voles were not secondary to locomotor activity or body weight alterations. These changes also were not a result of altered circadian rhythms, as there was no evidence of differences in HR or HR variability during the light (day) vs. dark (night) periods. This finding is consistent with the ultradian rhythm of locomotor activity observed in the current study, as well as previous research showing that prairie voles exhibit periods of increased activity and HR approximately every 3–4 hours (36).
The 24-hour autonomic dysfunction observed here extends previous findings showing that chronic social isolation is associated with increased HR and decreased HR variability during periods of low activity (e.g., resting values) in prairie voles (19). Combined with these previous results, the present observation that social isolation in prairie voles produces 24-hour ECG dysfunction – including changes in cardiac rate and rhythms – enhances the translational potential of the findings to humans. Similar changes in ambulatory autonomic function have been observed in humans with mood disorders and cardiovascular diseases (26, 31, 32). For instance, Carney et al. (32) showed that depression was associated with increased 24-hour HR following acute myocardial infarction, and that elevated nighttime HR was an independent predictor of survival in these patients.
Although the isolated group displayed disruptions in autonomic function and anhedonia following 2 weeks of social isolation, none of the autonomic parameters was predictably related to the behavioral state of anhedonia until the 4th week of isolation. At this time point, sucrose intake in the isolated group was negatively correlated with 24-hour HR, and positively correlated with both 24-hour SDNN index and RSA amplitude. Sucrose intake in the paired group was negatively correlated with 24-hour HR and positively correlated with 24-hour SDNN index (but was unrelated to 24-hour RSA amplitude). While the sample sizes in the present study did not allow for statistical comparisons of these correlational values, the predictable relations indicate that greater levels of anhedonia (i.e., lower sucrose consumption) may be related to greater dysfunction of the autonomic nervous system (that is, higher 24-hour HR or lower 24-hour HR variability values). This hypothesis highlights the need for investigating individual differences in the vulnerability to behavioral and autonomic responses to the social context using animal models. Further investigation of relationships between behavioral and physiological variables in rodent models also may allow for studies of common mechanisms underlying the integration of these changes.
Understanding the precise neural mechanisms underlying the autonomic and behavioral consequences of social isolation will require further investigation. Several lines of evidence suggest that a likely site of dysfunction in the central nervous system is the hypothalamus, with possible involvement from serotonin, oxytocin, vasopressin, and stress hormones such as corticotropin-releasing factor. First, the hypothalamic paraventricular nucleus, which produces oxytocin, vasopressin, and corticotropin-releasing hormone, receives serotonergic innervation and projects to the intermediolateral cell column of the spinal cord, rostral ventrolateral medulla and dorsal vagal complex to influence both sympathetic and parasympathetic outflow (60, 61). Additionally, chronic stressors have been shown to produce profound changes in the synthesis and levels of serotonin and the function of serotonin receptors in several brain regions, including the hypothalamus (62, 63). Further, chronic social isolation in prairie voles has been shown to alter the function of both oxytocin and corticotropin-releasing hormone neurons in the paraventricular nucleus (38). Similarly, circulating levels of oxytocin, vasopressin, and corticosterone are elevated in isolated prairie voles (versus paired animals) following exposure to an acute social stressor (37). Finally, administration of exogenous oxytocin to isolated prairie voles was protective against both depression-relevant behaviors and autonomic dysfunction (39). To gain a more comprehensive understanding of the role of the hypothalamus in integrating emotion, endocrine function, and autonomic regulation of the heart, future studies may benefit from investigating the specific interactions among the various neuropeptides and neurotransmitters in this region in animal and human models of social isolation.
A limitation of the present study is that the results were obtained in a small sample of rodents. However, the current findings are strengthened by observations of statistically significant results in a validated operational behavioral index of depression coupled with valid and reliable indices of continuous HR and HR variability. Another possible limitation, which presents a challenge in rodent models of social interactions, is that the experimental design may have included uncontrolled variance associated with short-term isolation experienced by the paired animals. For instance, paired animals were required to be temporarily separated from their siblings during the sucrose intake test to ensure accurate fluid intake measurements; this manipulation may therefore have led to differential stress levels in the paired versus isolated groups. However, if this temporary separation of paired animals was interpreted as a significant stressor, the difference between paired and isolated groups may have been less pronounced or absent. In contrast, the present results suggest a robust difference between paired and isolated animals during this test.
While the majority of studies exploring the association of mood and cardiovascular function have focused on human samples, the present study illustrates the utility of experimental investigations of this link using animal models. The findings described here are consistent with human studies showing that the lack of positive social interactions (for instance, fewer social connections) and social isolation can contribute to depressive disorders (64) and cardiovascular dysfunction (9, 10, 16, 64), as well as interactions of mood and autonomic dysfunction (65). Further validation of the prairie vole as a useful model for the study of depressive behaviors and autonomic dysfunction will provide a foundation for investigating novel behavioral and pharmacological treatments for mood and cardiovascular disorders. For instance, studies in humans suggest that treatment of depression with pharmacotherapy or psychotherapy may not lead to a full normalization of HR or HR variability (see 25, 32), while other studies indicate that antidepressant drugs (including tricyclic antidepressants and serotonin reuptake inhibitors) may have cardiotoxic side effects such as promotion of arrhythmias, vasoconstriction, QT prolongation, and decreased HR variability (66–68). The lack of consistent findings regarding the use of antidepressant drugs in patients with mood disorders and cardiovascular conditions highlights the importance of conducting experimental investigations of causal and common mechanisms underlying mood and cardiovascular regulation. Additional studies might include a combination of animal models and human samples, to provide a more comprehensive understanding of the mechanisms of depression and cardiovascular disease.
Acknowledgments
The investigators thank Parag Davé, Narmda Kumar, Damon Lamb, Gregory Lewis, Brett Pinkepank, and Diane Trahanas for technical assistance. This research was supported by National Institutes of Health Grants MH72935 (Carter), MH73233 (Grippo), MH77581 (Grippo), and MH67446 (Porges).
Acronyms
- ANOVA
analysis of variance
- DSI
Data Sciences International
- ECG
electrocardiographic
- HR
heart rate
- RSA
respiratory sinus arrhythmia
- SDNN
standard deviation of normal-to-normal intervals
- SEM
standard error of the mean
Reference List
- 1.Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 2.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–80. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 4.Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–7. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–7. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 6.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 7.Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–17. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. Am J Epidemiol. 2004;159:167–74. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- 9.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 10.Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosom Med. 2009;71:836–42. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspec Biol Med. 2003;46 (Suppl 3):S39–S52. [PubMed] [Google Scholar]
- 12.Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, York K, McClure C, Kelsey SF, Reis SE, Cornell CE, Vaccarino V, Sheps DS, Shaw LJ, Krantz DS, Parashar S, Merz CN. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom Med. 2008;70:282–7. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. Am J Epidemiol. 1988;128:370–80. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- 14.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Bairey Merz CN, Sopko G, Cornell CE, Sharaf B, Matthews KA. Social networks are associated with lower mortality rates among women with suspected coronary disease: the National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2004;66:882–8. doi: 10.1097/01.psy.0000145819.94041.52. [DOI] [PubMed] [Google Scholar]
- 15.Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155:700–9. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, Wannamethee SG. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population based study of older men. Annals of Epidemiology. 2008;18:476–83. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, Shin M, Bruce M, Krantz DS, Rozanski A. Social network and coronary artery calcification in asymptomatic individuals. Psychosom Med. 2005;37:343–52. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- 18.Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–15. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62:1162–70. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–44. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- 22.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–4. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 23.Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, Mastropasqua F, Russo GD, Rizzon P. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J. 2001;141:765–71. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- 24.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 25.Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Int Med. 2005;165:1486–91. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 26.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif J-C, Tavazzi L, Tendera M for the Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 27.Palatini P, Julius S. Association of tachycardia with morbidity and mortality: pathophysiological considerations. J Hum Hypertens. 1997;11 (Suppl 1):S19–S27. [PubMed] [Google Scholar]
- 28.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 29.Tapanainen JM, Thomsem PEB, Køber L, Torp-Pedersen C, Mäkikallio TH, Still A-M, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–52. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- 30.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35 (Suppl 4):S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 31.Guzzetti S, La Rovere MT, Pinna GD, Maestri R, Borroni E, Porta A, Mortara A, Malliani A. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26:357–62. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- 32.Carney RM, Steinmeyer B, Freedland KE, Blumenthal JA, Stein PK, Steinhoff WA, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Domitrovich PP, Burg MM, Hayano J, Jaffe AS. Nighttime heart rate and survival in depressed patients post acute myocardial infarction. Psychosom Med. 2008;70:757–63. doi: 10.1097/PSY.0b013e3181835ca3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronow WS, Ahn C, Mercando AD, Epstein S. Association of average heart rate on 24-hour ambulatory electrocardiograms with incidence of new coronary events at 48-month follow-up in 1311 patients (mean age 81 years) with heart disease and sinus rhythm. Am J Cardiol. 1996;78:1175–6. doi: 10.1016/s0002-9149(96)90077-6. [DOI] [PubMed] [Google Scholar]
- 34.Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- 35.Getz LL, Carter CS. Prairie-vole partnerships. Am Scientist. 1996;84:56–62. [Google Scholar]
- 36.Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90:386–93. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konkle AT, Baker SL, Kentner AC, Barbagallo LSM, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–38. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 41.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 42.Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 43.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–17. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- 45.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 46.Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 47.Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19:426–32. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 48.Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biol Psychol. 2007;74:295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- 51.Ishii K, Kuwahara M, Tsubone H, Sugano S. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab Animals. 1996;30:359–64. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- 52.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–14. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 53.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 54.Cohen S, Janicki-Deverts D. Can we improve our physical health by altering our social networks? Perspectives on Psychological Science. 2009;4:375–8. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berkman LF. The role of social relations in health promotion. Psychosom Med. 1995;57:245–54. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Cohen S. Social relationships and health. Am Psychologist. 2004;59:676–84. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 57.Grippo AJ, Na ES, Johnson RF, Beltz TG, Johnson AK. Sucrose ingestion elicits reduced Fos expression in the nucleus accumbens of anhedonic rats. Brain Res. 2004;1019:259–64. doi: 10.1016/j.brainres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, Keitner GI, Marin DB. Results of the DSM-IV mood disorders field trial. Am J Psychiatry. 1995;152:843–9. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- 59.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 60.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–9. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 61.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–7. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 62.Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21 (Suppl 1):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 63.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005;179:769–80. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- 64.Prigerson HG, Reynolds CF, III, Frank E, Kupfer DJ, George CJ, Houck PR. Stressful life events, social rhythms, and depressive symptoms among the elderly: an examination of hypothesized causal linkages. Psychiatry Res. 1994;51:33–49. doi: 10.1016/0165-1781(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 65.Schwerdtfeger A, Friedrich-Mai P. Social interaction moderates the relationship between depressive mood and heart rate variability: evidence from an ambulatory monitoring study. Health Psychol. 2009;28:501–9. doi: 10.1037/a0014664. [DOI] [PubMed] [Google Scholar]
- 66.Glassman AH. Cardiovascular effects of antidepressant drugs: updated. Int Clin Psychopharmacol. 1998;13 (Suppl 5):S25–S30. doi: 10.1097/00004850-199809005-00006. [DOI] [PubMed] [Google Scholar]
- 67.Mikuni M, Kusumi I, Kagaya A, Kuroda Y, Mori H, Takahashi K. Increased 5-HT-2 receptor function as measured by serotonin-stimulated phosphoinositide hydrolysis in platelets of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:49–61. doi: 10.1016/0278-5846(91)90040-8. [DOI] [PubMed] [Google Scholar]
- 68.Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, Van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–67. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]