Abstract
Kidney stone disease remains a major health and economic burden on the nation. It has been increasingly recognized that nephrolithiasis can be both a chronic or systemic illness. There have been major limitations in the development of new drugs for the prevention and management of this disease, largely due to our lack of understanding of the complex pathophysiologic mechanisms involving the interaction of three major target organs: the kidney, bone, and intestine. We also do not yet understand the molecular genetic basis of this polygenic disorder. These limitations are coupled with the incorrect perception that kidney stone disease is solely an acute illness, and the lack of reliable tests to assess outcome measures. All of these factors combined have diminished the willingness of the pharmaceutical industry to engage in the development of novel drugs.
Index Words: Diabetes mellitus, Nephrolithiasis, Metabolic syndrome, Uric acid, UrolithiasisI, Oxalobacter formigines
Kidney stone disease continues to have a major impact on the health and economy of the United States. The lifetime risk for nephrolithiasis exceeds 6% to 12% in the general population, and it is also projected that the prevalence of kidney stones will escalate.1,2 One approximation in 2005 estimated the combined inpatient and outpatient costs to be over $2.1 billion.3 This figure is clearly an underestimate considering the inflation in medical costs over the past 3 years. It has been increasingly recognized that the spectrum of the presentation of nephrolithiasis is wide, and it may be characterized as an acute, localized, chronic, or systemic illness. It has been shown that nephrolithiasis may be associated with an increased risk of end-stage renal disease,4,6 coronary artery disease, the metabolic syndrome, hypertension, and diabetes mellitus.7 Therefore, targeted pharmacologic treatments are imperative in the management of this disorder. The developments of such treatments require a comprehensive understanding of the pathophysiologic and molecular genetic basis of this disease. This review highlights some of our current approaches to this complex disorder, the obstacles involved in newer treatment modalities, and future targeted interventions. At the present time, conservative measures such as increased fluid intake to ensure sufficient urinary dilution and conventional treatments including alkali therapy, thiazide diuretics, and allopurinol are the mainstay treatments for various types of nephrolithiasis.
Spectrum of Kidney Stone Disease
Acute Illness
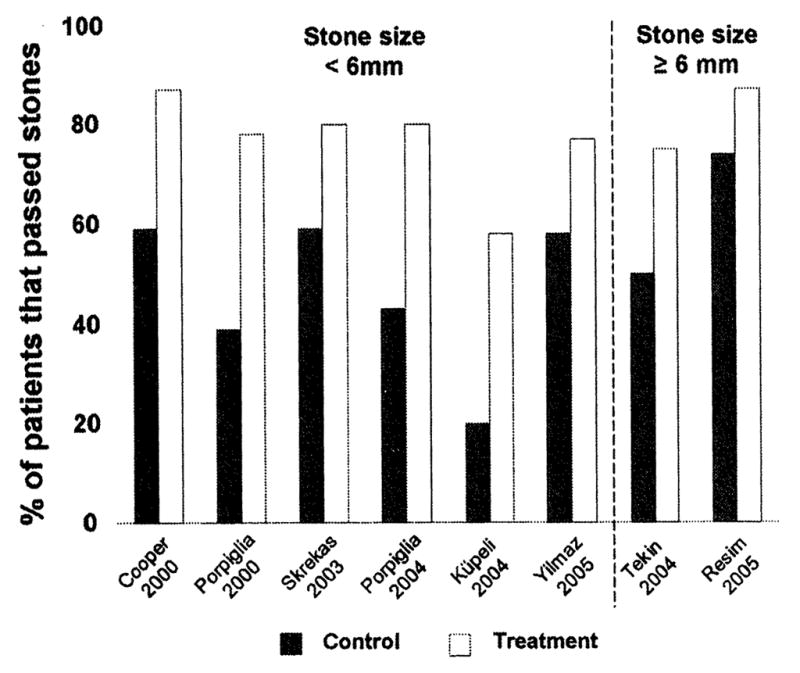
As an acute illness, kidney stone disease is associated with significant pain, disability, and loss of productivity.8 Up to 50% of patients experiencing acute upper urinary tract stones require surgical intervention because stone dissolution is impractical.9,10 It has recently been shown that medical expulsive treatment facilitates urethral stone passage. There is growing interest in the usage of medical treatment such as calcium-channel blockers and α-adrenergic antagonists to enhance stone passage.11,12 A MEDLINE meta-analysis of all scientific data up to 2005, using calcium-channel blockers or α-blockers search criteria, showed that medical treatment with such agents, with or without steroid treatment and regardless of stone size, enhanced the percentage of ureteral stone expulsion11 (Fig 1). Despite such successful results, chemical expulsive treatment has been underused largely because of (1) a lack of widespread physician awareness, (2) the absence of large-scale randomized controlled trials, and (3) limitations in our understanding of the mechanisms of action of these agents. It is crucial to pursue randomized trials because expulsive medical treatment would significantly decrease the cost imposed by shock-wave lithotripsy and ureteroscopy.8,13 Furthermore, these treatments may vastly improve the quality of life of the patients because they have been shown to reduce episodes of pain,14,15 lower analgesic requirements,16 and reduce the time of stone passage.16,17
Figure 1.
Pharmacologic treatment, facilitating ureteral stone passage: α-blockers versus calcium-channel blockers (±steroids). (Adapted with permission from Hollingsworth et al.11)
Chronic Disease
Nephrolithiasis has been shown to be a chronic disease because its cumulative recurrence rate progressively increases from the onset of acute stone passage and approaches 50% over 10 years. Given the chronicity of this illness, long-term pharmacological treatment is necessary to reduce stone recurrence in this population. Most drugs applied in the treatment of kidney stones have been in use for many years. Since the advent of potassium citrate treatment,19–20 no new drug has been developed. Studies using potassium-magnesium citrate have shown its efficacy against recurrent calcium oxalate nephrolithiasis 21–23 However, this drug has not yet been marketed.
Alkali Treatment
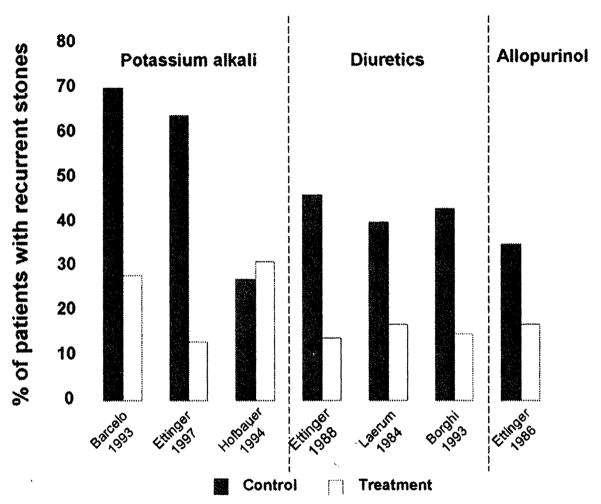
Three separate randomized trials conducted in the 1990s examining the effect of potassium alkali in recurrent calcium oxalate nephrolithiasis showed a significant decrease in stone recurrence 20,21,24 These studies encompassed a total of 231 subjects with recurrent hypocitraturia calcium nephrolithiasis who were treated with potassium alkali, 30 mEq to 60 mEq/d for a duration ranging between 12 and 36 months. However, a similar fourth study did not show superiority of potassium alkali over the placebo treatment.25 This study was conducted in a total of 50 hypocitraturia calcium nephrolithiasis patients treated with 90 mEq potassium alkali per day over the course of 3 years. The reported lack of efficacy of potassium alkali treatment in this investigation was, in part, attributed to the small size of the study population (Fig 2).
Figure 2.
Randomized treatment trials for the prevention of recurrent calcium oxalate nephrolithiasis. (Adapted with permission from Coe et al.31)
Thiazide Diuretics
Calcium oxalate is the most prevalent type of kidney stone disease in the United States and has been shown to occur in 70% to 80% of the kidney stone population.26 The effects of thiazide diuretics on recurrent calcium nephrolithiasis have been studied in 6 randomized trials. Four of these studies, conducted in a total of 408 patients over a duration of 26 to 36 months, showed a significant decrease in recurrent kidney stones with thiazides and their analog indapamide27–31 (Fig 2). Conversely, no difference was noted between thiazide treatment and the placebo in 2 of these trials.32,33 Three of these studies alluded to high dietary fluid intake to ensure sufficient urinary volume.27,28,30 However, 2 studies did not indicate whether patients were instructed to maintain a high urinary volume through sufficient fluid intake.29,32 In 2 studies, an index of obesity was calculated by dividing weight in pounds by the square of height.27,30 The remaining studies made no indication of the influence of body weight or obesity.
In addition, a widely quoted study involving thiazide diuretics in patients with recurrent calcium nephrolithiasis determined chlorthalidone to be significantly superior to both magnesium treatment and placebo.27 However, only 28.5% of this total study population had hypercalciuria. It appears that the successful response to this treatment also occurred in calcium stone formers without hypercalciuria. Therefore, because this study was performed over 36 months, it is logical to conclude that the reported effectiveness against stone recurrence could not be attributed to regression to the mean or stone clinic effect. In general clinical practice, however, thiazide diuretics are typically only prescribed for those with hypercalciuric calcium nephrolithiasis.
In most of the thiazide trials, both symptomatic and clinical stone passage rates were used to determine kidney stone recurrence. Because most of these studies were performed in the 1980s and 1990s, clinical stone passage rate was determined by routine x-ray procedures including kidney-ureter-bladder, intravenous pyelography, and tomography. The appearance of new calculus or the enlargements of preexisting calculi were considered as new stones. In symptomatic patients, new stone passage was verified, and any surgical procedure for stone removal was also considered as a new stone. To exclude stone clinic effect, a grace period of several months was used to mark the washout period.
Allopurinol
Hyperuricosuria is detected in 15% to 20% of patients with calcium nephrolithiasis.34 The first double-blind study showing the efficacy of allopurinol was conducted over 20 years ago. In this study, 60 patients with hyperuricosuria and normocalciuria received either the placebo or allopurinol, 100 mg, 3 times a day for 39 months. Compared with the placebo, allopurinol treatment significantly reduced new calcium oxalate stone incidence and also prolonged the time between new stone recurrences. The findings of this study have not been challenged by new controlled studies. To date, this treatment has largely been safe with the only severe side effect being the possible increased risk of allergic reactions when used in combination with thiazide diuretics36 (Fig 2).
The Risk-Benefit Ratio of Pharmacologic Treatment
The risk-benefit ratio of pharmacologic interventions with thiazide diuretics and alkali treatment in the kidney stone-forming population has not been fully investigated. However, alkali treatment has been shown to be relatively free of side effects with only minor gastrointestinal complaints. The main concern with this treatment is its overuse, which may increase the propensity for calcium phosphate stone formation. Because thiazide diuretics may have harmful effects extending beyond their efficacy against kidney stone formation, it remains unclear whether its risks may outweigh its benefits.
In a recent retrospective meta-analysis of all clinical trials conducted between 1966 and 2004 in thiazide-treated populations, an inverse relationship was shown between changes in serum glucose and potassium concentrations.37 The result of this study was also supported by The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial in which a 4-year incidence of new-onset diabetes mellitus was shown to be significantly higher with chlorthalidone compared with amlodipine or lisinopril treatments.38 Although the association between thiazide use and glucose intolerance has been known for many years,39,40 the exact pathophysiologic mechanism is not well known. One possibility may be that low serum potassium concentrations may cause a decrease in insulin secretion.41. As of yet, no prospective studies have shown a causal relationship between serum potassium concentrations and the development of diabetes mellitus in the general thiazide-treated population and, more specifically, in thiazide-treated kidney stone patients. This is important because a relationship between type 2 diabetes mellitus and the risk of kidney stone disease has been shown in 3 separate longitudinal studies.43
Systemic Disorder
It has become more apparent that nephrolithiasis may be a systemic illness. Several prospective epidemiologic studies have shown that obesity and weight gain increase the risk of kidney stone disease 43,44 In addition, 2 studies conducted in a kidney stone population and in normal subjects showed an inverse relationship between urinary pH and body weight and also a progressive decrease in urinary pH with additional features of the metabolic syndrome.45,46 However, the pathophysiologic link between obesity, insulin resistance, and calcium stone formation has not yet been established. A direct physiological relationship between insulin sensitivity and urinary pH has been shown in a carefully conducted metabolic study using the hyperinsulinemic euglycemic clamp procedure.47 The result of this study has a major clinical impact on the future development of novel drugs targeting insulin resistance.
Limitations of Drug Development
There are several obstacles to overcome in the development of novel agents for the treatment of nephrolithiasis. These limitations can be divided into 2 different categories: (1) the incorrect perception that kidney stone disease is a self-limiting local disorder without consideration for chronic, recurrent, and systemic illnesses and (2) an incomplete understanding of the pathophysiologic and molecular genetic basis of nephrolithiasis. The misconception that kidney stone disease is a self-limiting disorder hinders nephrologists and urologists in the prevention of recurrent kidney stone disease, indirectly impeding the development of new drugs. Additionally, the field is deficient in new agent testing because of the lack of availability of concisely measured outcomes. Although the stone events appear to be ideal for an outcome measure, it is cumbersome because it requires a long-term follow up because the median time for recurrence after the first event is approximately 5 years. This misconception, coupled with poor patient compliance, results in a lack of interest from the pharmaceutical industry in the development of a novel drug because of its perceived low profitability. Moreover, testing new agents requires a surrogate marker other than a stone event. These markers may include the use of spiral computed tomography scans for the detection of small and large stones. To date, computer-based approaches have been used in kidney stone risk assessment but are becoming increasingly recognized as having major flaws.
Second, the pathophysiologic mechanisms and molecular genetic basis of nephrolithiasis are diverse and complex. They involve a multi-faceted physiologic interaction of 3 major organs participating in calcium homeostasis: the bone, intestine, and kidney. The molecular genetic pathways of nephrolithiasis have not been fully elucidated largely because this illness appears to be a polygenic trait. Moreover, there has been limited success49–53 in the establishment of accepted animal models that recapitulate the human disease to further explore kidney stone pathophysiology and response to treatment.
Future Innovative Therapies
In the past 10 to 15 years, there have been significant advances in our understanding of the epithelial cell transport of calcium and oxalate,54,55 the role of insulin resistance,45–47,56,57 and the link between renal lipotoxicity and the development of uric acid nephrolithiasis. We have also witnessed a major progression in the field by defining the role of interstitial plaque formation as a precursor to calcium oxalate nephrolithiasis.58,59
Epithelial Cell Regulation of Calcium Transport
Renal and intestinal transcellular calcium absorption plays a key role in calcium homeostasis. The rate-limiting step in transcellular calcium transport across the apical membrane of the epithelial cell is tightly controlled by 2 members of the transient receptor potential (TRP) superfamily, TRPV5 and TRPV6.55 TRPV5 primarily controls renal epithelial calcium transport, and TPRV6 is the main gatekeeper in the small intestine.61 Future drug development can be targeted at the intestinal tract gatekeeper, TRPV6, if concurrent intervention is available to offset the development of negative calcium balances. Conversely, novel drugs may be developed to upregulate TRPV5 in the renal epithelial cell to enhance renal tubular calcium reabsorption. The development of such targeted treatments specific to the kidney may be a difficult task to achieve.
Epithelial Cell Regulation of Oxalate
Hyperoxaluria is a condition commonly encountered in kidney stone formers.62–64 The metabolic cascade leading to hyperoxaluria may be classified into increased oxalate production, increased dietary oxalate intake, or enhanced intestinal oxalate absorption. Our knowledge of intestinal oxalate absorption has recently advanced with a study defining the role of Oxalobacter formingenes (OF)64,65 and another study outlining the significant role of putative anion transporter Slc25a6.66
OF is a gram-negative obligate anaerobe microorganism that principally uses oxalate as a main source of energy for cellular biosynthesis 67 It has been shown that OF colonization is significantly lower in patients with recurrent calcium oxalate stone formation.68 Despite this finding, as of yet, OF colonization has not been shown to be associated with changes in urinary oxalate levels.68 The existence of this bacterium can be used as a potential tool in the treatment of hyperoxaluria. One study in human subjects with type 1 primary hyperoxaluria has shown a reduction in urinary oxalate after the oral administration of OF.65 One conclusion that can be drawn from this study is that OF, by degrading intestinal oxalate content, will stimulate luminal oxalate secretion, thereby lowering urinary oxalate excretion. One limitation of this approach is the short lifespan of OF on the complete utilization of its principal nutrient source, oxalate. In the future, this may be overcome by the development of recombinant enzymes or by the introduction of engineered bacteria which are not solely dependant on oxalate (Table 1).
Table 1.
Future Directions Regulating Intestinal Oxalate Transport
Intestinal Luminal Oxalate Degradation and Secretion
|
It has recently been shown that the putative ion exchange transporter coded by gene Slc26a6 plays a role in intestinal oxalate transport.69 This transporter is expressed in the luminal portion of various segments of the small intestine and large bowel, which facilitates oxalate secretion into the bowel lumen, thereby regulating the net intestinal oxalate absorption. 70 A study using Slc26a6-null mice on controlled oxalate diets reported high urinary oxalate excretion associated with increased plasma oxalate concentration and diminished fecal oxalate excretion.54 Because the differences in urinary oxalate excretion was shown to be abrogated after restricted dietary oxalate in Slc26a6-null mice, this study suggests the potential role of diminished intestinal oxalate secretion in the development of elevated urinary oxalate excretion. For the first time, this study highlights the significance of this transporter in urinary oxalate excretion. From this novel study, one may surmise that future drugs up-regulating intestinal luminal oxalate secretion may be developed. Such drugs will target the Slc26a6 putative anion transporter activity.
Additional tools controlling intestinal oxalate absorption may also involve the development of agents that enhance intestinal luminal oxalate complexation. One such drug, a polymeric positively charged drug, chitosan, has been tested. However, it has not been shown to be effective in lowering urinary oxalate excretion.71
The Role of Insulin Resistance and Renal Lipotoxicity in Uric Acid Nephrolithiasis
The association between obesity and insulin resistance and its link to the pathogenesis of uric acid stone formation have been studied in patients with uric acid nephrolithiasis.46,47,56,57,72 It has been suggested that the disturbance in caloric intake and caloric utilization may lead to the deposition of fat in tissues other than adipocytes. This process has been termed tissue lipotoxicity. There is a potential role of renal lipotoxicity in the development of acidic urine, the main culprit in the formation of uric acid nephrolithiasis.56
Magnetic resonance imaging has shown evidence of fat accumulation in skeletal muscle, liver tissue, and cardiac muscle and its reversal with insulin-sensitizing drugs.73–75 Currently, a double-blind study in Dallas is underway in patients with uric acid nephrolithiasis to explore the presence of fat in the renal cortex and its response to the insulin-sensitizing drug pioglitazone. As of yet, there is only 1 animal model found to recapitulate the main pathophysiologic defects found in human uric acid nephrolithiasis, the Zucker diabetic fatty rat.76 Similar to humans with uric acid stone disease, this animal model presents low urinary pH, impaired urinary ammonium excretion, and increased urinary titratable acid excretion.76 These abnormalities have been shown to reverse after the administration of insulin-sensitizing drugs such as thiazolidinediones, which are Food and Drug Administration approved for the treatment of type 2 diabetes. However, unlike patients with uric acid stone disease, this animal model lacks the formation of uric acid crystals and consequent uric acid stones. This is because of the presence of uricase, which poorly converts soluble uric acid into soluble allantoin. At the present time, oral alkali treatment remains the principal agent for the treatment of uric acid nephrolithiasis.
Interstitial Papillary Plaque
Dr Alexander Randall first suggested that interstitial calcium phosphate deposits, referred to as plaques, are the initial sites that harbor urinary crystals beneath the uroepithelial cells of the renal papilla.77 This important finding was performed in cadaveric kidney specimens and has been widely disputed because it was not tested in a targeted kidney stone-forming population. Recent developments of modern techniques visualizing Randall’s plaque in vivo have shown that Randall’s plaque occurs more frequently in patients with kidney stones compared with nonstone formers undergoing endoscopic evaluation.78
Conclusion
Our knowledge of the pathogenic cascades and molecular genetic basis leading to nephrolithiasis has changed significantly over the past decade. In the future, we should use this knowledge to formulate a novel method for the diagnosis and management of nephrolithiasis. There are many questions to be answered and barriers to overcome before such an approach will become reality. The gap in current trials on the management of kidney stone disease is wide. This is largely because of the availability of only a few longitudinal trials in a small number of patients with a short duration of treatment that was conducted over 2 to 3 decades ago. It is anticipated that this gap will gradually fill with the advances in our understanding of the pathophysiology and the genetic basis of kidney stone disease. This knowledge will be helpful in the design of long-term outcome trials of conventional treatments and, hopefully, new novel drugs that target the rectification of specific physiologic abnormalities.
Acknowledgments
Supported in part by the National Institutes of Health grants P01-DK20543 and M01-RR00633.
The author would also like to acknowledge the editorial support of Ms Hadley Armstrong.
References
- 1.Johnson CM, Wilson DM, O’Fallon WM, et al. Renal stone epidemiology: A 25-year study in Rochester, Minnesota. Kidney Int. 1979;16:624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: Urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 4.Worcester E, Parks JH, Josephson MA, et al. Causes and consequences of kidney loss in patients with nephrolithiasis. Kidney Int. 2003;64:2204–2213. doi: 10.1046/j.1523-1755.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 5.II. Incidence and prevalence of ESRD. Am J Kidney Dis. 1999;34:S40–S50. doi: 10.1053/AJKD034s00040. [DOI] [PubMed] [Google Scholar]
- 6.Jungers P, Joly D, Barbey F, et al. ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44:799–805. [PubMed] [Google Scholar]
- 7.Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens. 2008;17:304–309. doi: 10.1097/MNH.0b013e3282f8b34d. [DOI] [PubMed] [Google Scholar]
- 8.Lotan Y, Gettman MT, Roehrborn CG, et al. Management of ureteral calculi: A cost comparison and decision making analysis. J Urol. 2002;167:1621–1629. [PubMed] [Google Scholar]
- 9.Krepinsky J, Ingram AJ, Churchill DN. Metabolic investigation of recurrent nephrolithiasis: Compliance with recommendations. Urology. 2000;56:915–920. doi: 10.1016/s0090-4295(00)00822-0. [DOI] [PubMed] [Google Scholar]
- 10.Glowacki LS, Beecroft ML, Cook RJ, et al. The natural history of asymptomatic urolithiasis. J Urol. 1992;147:319–321. doi: 10.1016/s0022-5347(17)37225-7. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth JM, Rogers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171–1179. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- 12.Parsons JK, Hergan LA, Sakamoto K, et al. Efficacy of alpha-blockers for the treatment of ureteral stones. J Urol. 2007;177:983–987. doi: 10.1016/j.juro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Bierkens AF, Hendrikx AJ, De La Rosette JJ, et al. Treatment of mid- and lower ureteric calculi: extracorporeal shock-wave lithotripsy vs laser uretero- scopy. A comparison of costs, morbidity and effectiveness. Br J Urol. 1998;81:31–35. doi: 10.1046/j.1464-410x.1998.00510.x. [DOI] [PubMed] [Google Scholar]
- 14.Resim S, Ekerbicer H, Ciftci A. Effect of tamsulosin on the number and intensity of ureteral colic in patients with lower ureteral calculus. Int J Urol. 2005;12:615–620. doi: 10.1111/j.1442-2042.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz E, Batislam E, Basar MM, et al. The comparison and efficacy of 3 different alphal-adrenergic blockers for distal ureteral stones. J Urol. 2005;173:2010–2012. doi: 10.1097/01.ju.0000158453.60029.0a. [DOI] [PubMed] [Google Scholar]
- 16.Porpiglia F, Destefanis P, Fiori C, et al. Effectiveness of nifedipine and deflazacort in the management of distal ureter stones. Urology. 2000;56:579–582. doi: 10.1016/s0090-4295(00)00732-9. [DOI] [PubMed] [Google Scholar]
- 17.Porpiglia F, Ghignone G, Fiori C, et al. Nifedipine versus tamsulosin for the management of lower ureteral stones. J Urol. 2004;172:568–571. doi: 10.1097/01.ju.0000132390.61756.ff. [DOI] [PubMed] [Google Scholar]
- 18.Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989;111:1006–1009. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 19.Pak CY, Peterson R, Sakhaee K, et al. Correction of hypocitraturia and prevention of stone formation by combined thiazide and potassium citrate therapy in thiazide-unresponsive hypercalciuric nephrolithiasis. Am J Med. 1985;79:284–288. doi: 10.1016/0002-9343(85)90305-5. [DOI] [PubMed] [Google Scholar]
- 20.Barcelo P, Wuhl O, Servitge E, et al. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger B, Pak CY, Citron JT, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 22.Zerwekh JE, Odvina CV, Wuermser LA, et al. Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J Urol. 2007;177:2179–2184. doi: 10.1016/j.juro.2007.01.156. [DOI] [PubMed] [Google Scholar]
- 23.Jaipakdee S, Prasongwatana V, Premgamone A, et al. The effects of potassium and magnesium supplementations on urinary risk factors of renal stone patients. J Med Assoc Thai. 2004;87:255–263. [PubMed] [Google Scholar]
- 24.Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after Shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. 2002;16:149–152. doi: 10.1089/089277902753716098. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer J, Hobarth K, Szabo N, et al. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis—A prospective randomized study. Br J Urol. 1994;73:362–365. doi: 10.1111/j.1464-410x.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 26.Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:927–949. doi: 10.1016/s0889-8529(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger B, Citron JT, Livermore B, et al. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 28.Laerum E, Larsen S. Thiazide prophylaxis of urolithiasis. A double-blind study in general practice. Acta Med Scand. 1984;215:383–389. [PubMed] [Google Scholar]
- 29.Ohkawa M, Tokunaga S, Nakashima T, et al. Thiazide treatment for calcium urolithiasis in patients with idiopathic hypercalciuria. Br J Urol. 1992;69:571–576. doi: 10.1111/j.1464-410x.1992.tb15624.x. [DOI] [PubMed] [Google Scholar]
- 30.Borghi L, Meschi T, Guerra A, et al. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol. 1993;22(Suppl 6):S78–S86. [PubMed] [Google Scholar]
- 31.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brocks P, Dahl C, Wolf H, et al. Do thiazides prevent recurrent idiopathic renal calcium stones? Lancet. 1981;2:124–125. doi: 10.1016/s0140-6736(81)90302-0. [DOI] [PubMed] [Google Scholar]
- 33.Scholz D, Schwille PO, Sigel A. Double-blind study with thiazide in recurrent calcium lithiasis. J Urol. 1982;128:903–907. doi: 10.1016/s0022-5347(17)53269-3. [DOI] [PubMed] [Google Scholar]
- 34.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977;87:404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 35.Ettinger B, Tang A, Citron JT, et al. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315:1386–1389. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 36.Young JL, Jr, Boswell RB, Nies AS. Severe allopurinol hypersensitivity. Association with thiazides and prior renal compromise. Arch Intern Med. 1974;134:553–558. doi: 10.1001/archinte.134.3.553. [DOI] [PubMed] [Google Scholar]
- 37.Zillich AJ, Garg J, Basu S, et al. Thiazide diuretics, potassium, and the development of diabetes: A quantitative review. Hypertension. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 38.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 39.Goldner MG, Zarowitz H, Akgun S. Hyperglycemia and glycosuria due to thiazide derivatives administered in diabetes mellitus. N Engl J Med. 1960;262:403–405. doi: 10.1056/NEJM196002252620807. [DOI] [PubMed] [Google Scholar]
- 40.Sagild U, Andersen V, Andreasen PB. Glucose tolerance and insulin responsiveness in experimental potassium depletion. Acta Med Scand. 1961;169:243–251. doi: 10.1111/j.0954-6820.1961.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 41.Houston MC. The effects of antihypertensive drugs on glucose intolerance in hypertensive nondiabetics and diabetics. Am Heart J. 1988;115:640–656. doi: 10.1016/0002-8703(88)90816-2. [DOI] [PubMed] [Google Scholar]
- 42.Carter BL, Einhorn PT, Brands M, et al. Thiazide-in- duced dysglycemia: Call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52:30–36. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 43.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 44.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 45.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 46.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 47.Abate N, Chandalia M, Cabo-Chan AV, Jr, et al. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 48.Grampsas SA, Moore M, Chandhoke PS. 10-year experience with extracorporeal Shockwave lithotripsy in the state of Colorado. J Endourol. 2000;14:711–714. doi: 10.1089/end.2000.14.711. [DOI] [PubMed] [Google Scholar]
- 49.Bushinsky DA, Asplin JR, Grynpas MD, et al. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2002;61:975–987. doi: 10.1046/j.1523-1755.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- 50.Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest. 1988;82:1585–1591. doi: 10.1172/JCI113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int. 1997;51:1540–1547. doi: 10.1038/ki.1997.212. [DOI] [PubMed] [Google Scholar]
- 52.Bushinsky DA, Grynpas MD, Nilsson EL, et al. Stone formation in genetic hypercalciuric rats. Kidney Int. 1995;48:1705–1713. doi: 10.1038/ki.1995.468. [DOI] [PubMed] [Google Scholar]
- 53.Krieger NS, Stathopoulos VM, Bushinsky DA. Increased sensitivity to l,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol. 1996;271:C130–C135. doi: 10.1152/ajpcell.1996.271.1.C130. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Z, Asplin JR, Evan AP, et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 55.van de Graaf SF, Hoenderop JG, Bindels RJ. Regulation of TRPV5 and TRPV6 by associated proteins. Am J Physiol Renal Physiol. 2006;290:F1295–F1302. doi: 10.1152/ajprenal.00443.2005. [DOI] [PubMed] [Google Scholar]
- 56.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 57.Cameron MA, Maalouf NM, Adams-Huet B, et al. Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 58.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evan AP, Coe FL, Lingeman JE, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 60.Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol. 2002;64:529–549. doi: 10.1146/annurev.physiol.64.081501.155921. [DOI] [PubMed] [Google Scholar]
- 61.Nijenhuis T, Hoenderop JG, van der Kemp AW, et al. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol. 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- 62.Baggio B, Gambaro G, Favaro S, et al. Prevalence of hyperoxaluria in idiopathic calcium oxalate kidney stone disease. Nephron. 1983;35:11–14. doi: 10.1159/000183037. [DOI] [PubMed] [Google Scholar]
- 63.Laminski NA, Meyers AM, Kruger M, et al. Hyperoxaluria in patients with recurrent calcium oxalate calculi: dietary and other risk factors. Br J Urol. 1991;68:454–458. doi: 10.1111/j.1464-410x.1991.tb15383.x. [DOI] [PubMed] [Google Scholar]
- 64.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: An update of a 1980 protocol. Am J Med. 1995;98:50–59. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 65.Milliner D. Treatment of the primary hyperoxalurias: A new chapter. Kidney Int. 2006;70:1198–1200. doi: 10.1038/sj.ki.5001821. [DOI] [PubMed] [Google Scholar]
- 66.Freel RW, Hatch M, Green M, et al. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastro- intest Liver Physiol. 2006;290:G719–G728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 67.Jonsson S, Ricagno S, Lindqvist Y, et al. Kinetic and mechanistic characterization of the formyl-CoA transferase from Oxalobacter formigenes. J Biol Chem. 2004;279:36003–36012. doi: 10.1074/jbc.M404873200. [DOI] [PubMed] [Google Scholar]
- 68.Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–1203. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danpur C. Primary hyperoxaluria. In: Scriver C, Beaudet A, Sly W, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. New York, NY: 2001. pp. 3323–3367. [Google Scholar]
- 70.Wang Z, Wang T, Petrovic S, Tuo B, et al. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol. 2005;288:C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 71.Wolf J, Asplin JR, Goldfarb DS. Chitosan does not reduce post-prandial urinary oxalate excretion. Urol Res. 2006;34:227–230. doi: 10.1007/s00240-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 72.Maalouf NM, Cameron MA, Moe OW, et al. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Tiikkainen M, Hakkinen AM, Korsheninrtikova E, et al. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 74.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 75.Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 76.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Randall A. Papillary pathology as a precursor of primary renal calculus. J Urol. 1940;44:580–589. [Google Scholar]
- 78.Low RK, Stoller ML, Schreiber CK. Metabolic and urinary risk factors associated with Randall’s papillary plaques. J Endourol. 2000;14:507–510. doi: 10.1089/end.2000.14.507. [DOI] [PubMed] [Google Scholar]