Abstract
Two proteins involved in nitrogen fixation contain ferredoxin-type [4Fe4S] clusters that exist in paramagnetic ground state upon oxidation, a property never observed since the discovery of ferredoxins 50 years ago. This unique characteristic suggests a specific coupling in these clusters necessary for nitrogen fixation and implies an evolutionary connection between the clusters in the two proteins.
FeS clusters have been extensively studied since their first discovery in the early 60’s in biological oxidoreductases1,2. The most common and best studied form among them is the [4Fe4S] cluster, which can serve either as an electron transfer unit, or as a structural or catalytic unit. As an electron transfer unit, the cluster typically exists as either a ferredoxin ([4Fe4S]1+/2+)- or a HiPIP ([4Fe4S]2+/3+)-type redox couple. A property of these clusters that never varies is that the 2+ state always has a diamagnetic (S = 0) ground state. This diamagnetism is associated with two delocalized Fe2.5+-Fe2.5+ pairs (S = 9/2 each) that are antiferromagnetically coupled, resulting in a net zero spin ground state1. Similarly, theoretical analyses also predict S = 0 as the lowest spin state of these clusters3. Here, we present the first report of paramagnetic ground states of [4Fe4S]2+ clusters in two oxidized Nif proteins of the nitrogen fixation system.
ΔnifB NifEN (Fig. 1) is an α2β2-tetrameric protein that serves as a scaffold for the biosynthesis of the active FeMo-cofactor (Fe-Moco) center of Mo-nitrogenase4. Metal, EPR, Fe-XAS, Magnetic Circular Dichroism (MCD) and Resonance Raman (RR) analyses show that this protein contains two identical, yet well-separated, [4Fe4S]-like clusters—one at each α/β-subunit interface—which may mediate the electron transfer for the reductive insertion of Mo and homocitrate into the Fe-only FeMoco precursor5,6,10,11. This hypothesis has been verified with the recent determination of the x-ray diffraction structure of NifEN12. As expected, upon oxidative titration, the S = ½ EPR spectrum of the reduced [4Fe4S]+ cluster in ΔnifB NifEN disappears at Em7 ~ −350 mV and n = 1 (Fig. 2A), resulting in an EPR silent oxidized state. The reversibility of this redox change, as well as the RR spectrum10 of the oxidized cluster, clearly indicates [4Fe4S]2+ as the cluster species in the oxidized state. However, MCD spectra recorded before and immediately after such an oxidation reveal that both states exhibit dominant MCD features (Fig. 2B).
Figure 1.
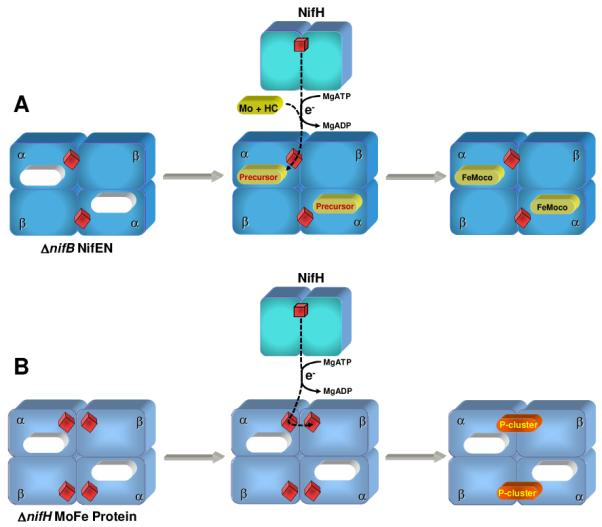
Schematic presentation of the composition and function of ΔnifB NifEN (A) and ΔnifH MoFe protein (B). The two proteins are α2β2-tetrameric proteins that are highly homologous in primary sequence and cluster composition4. Both proteins are deficient of FeMoco-related cluster species (indicated by empty ovals) as a result of the deletion of nifB (A) or nifH (B), two genes encoding for proteins that are essential for the biosynthesis of FeMoco (4). Furthermore, they either contain a single [4Fe4S] cluster (A) or a pair of [4Fe4S]-like clusters (B) at each α/β-subunit interface that likely mediates the electron transfer from Fe protein for the reductive insertion of Mo and homocitrate (abbreviated as HC) into the FeMoco precursor (A)5,6 or the reductive coupling of paired [4Fe4S] clusters into a mature [8Fe7S] P-cluster (B)7-9.
Figure 2.
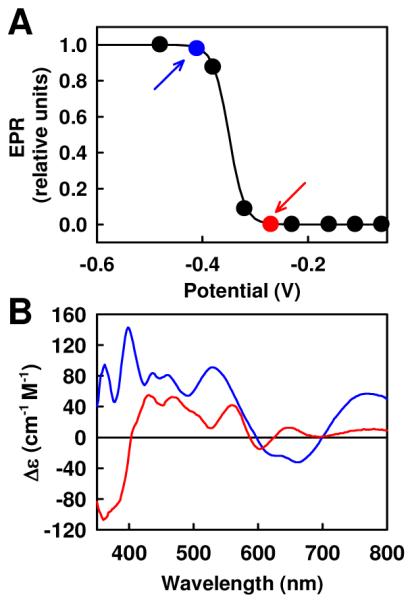
(A) Reversible redox titration of ΔnifB NifEN, where a Nernst curve with n = 1 and Em7 = −350 ± 10 mV is represented by the solid line. Points labeled blue and red represent samples used to collect the MCD spectra in B. (B) MCD spectra of ΔnifB NifEN recorded at −420 mV (reduced; blue) and −278 mV (oxidized; red).
A variable-temperature study of the oxidized cluster of ΔnifB NifEN clearly shows an inverse relationship between the MCD spectral intensity and temperature (Fig. 3A). This inverse relationship establishes a paramagnetic ground state of the oxidized [4Fe4S] cluster, contrary to conventional wisdom. The n = 1 oxidation generating this state (see Fig. 2A) implies that it is an integer spin system, while the lack of an EPR signal (perpendicular or parallel modes) suggests S = 1 or 2 as the most likely spin states for this species. The spin state of this species is further explored through simulations of the magnetization curves (i.e., plots of MCD spectral intensity versus magnetic field at various temperatures). Although magnetization curves are helpful in determining the spin states of half-integer systems, they are not as exact in assessing the spin states of integer spin systems, where little or no EPR spectral parameters exist and non-zero rhombic interactions (E/D > 0) produce further splitting of ±ms spin sublevels. Magnetization curves of oxidized ΔnifB NifEN are simulated for the series of spin states S = 1, 2 and 3. The closest fit occurs using the parameters S = 2, D = 1 cm−1 and E/D = 0.05, with polarizations Mxy = 1.0, Mxz = 1.0 and Myz = 1.0 (Fig. 3B).
Figure 3.
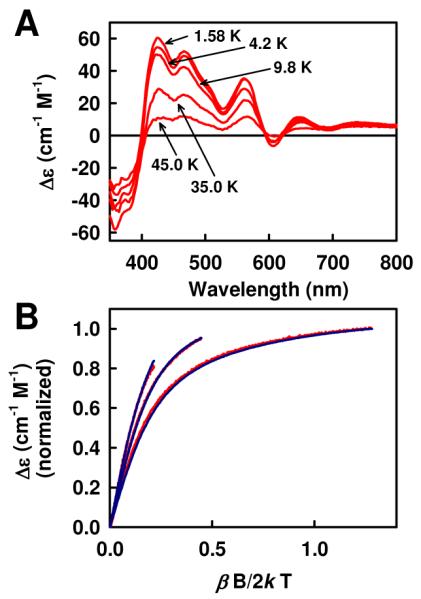
(A) Variable-temperature MCD spectra of oxidized ΔnifB NifEN (0.67 mM) showing a decrease in spectral intensity with an increase in temperature, which arises from a paramagnetic ground state of the [4Fe4S] cluster in this protein. (B) Magnetization curves of oxidized ΔnifB NifEN (red) and the simulation of data (blue) assuming S = 2, D = 1 cm−1 and E/D = 0.05, with polarizations Mxy = 1.0, Mxz = 1.0 and Myz = 1.0. Curves were recorded at 1.58 K (far right), 4.2 K (middle) and 9.8 K (far left).
An identical phenomenon of redox change (Fig. 1) is observed in the case of ΔnifH MoFe protein, which is another Nif protein related to the biosynthesis of Mo-nitrogenase4. This α2β2-tetrameric protein is highly homologous to ΔnifB NifEN in primary sequence and cluster type, except that it contains four (instead of two) ferredoxin-type, [4Fe4S]-like clusters—one pair at each α/β-subunit interface—that could be reductively coupled into two mature, [8Fe7S] P-clusters7-8. Like ΔnifB NifEN, the oxidized ΔnifH MoFe protein is paramagnetic9. Interestingly, its MCD spectrum (Fig. 4A) has transitions (Fig. 4B) virtually identical to those of the oxidized ΔnifB NifEN, but with twice the spectral intensity of the latter. This spectral increase corresponds to the ratio of cluster content between the two proteins (i.e., 4:2), which is also observed in the spectra of the reduced clusters (Fig. 4C)11. Thus, all of the oxidized [4Fe4S] clusters in both proteins exhibit the same paramagnetism. The similarity between the spectra of the two proteins is unexpected and suggests that these transitions may be a general fingerprint of the ground state paramagnetic [4Fe4S]2+ clusters.
Figure 4.
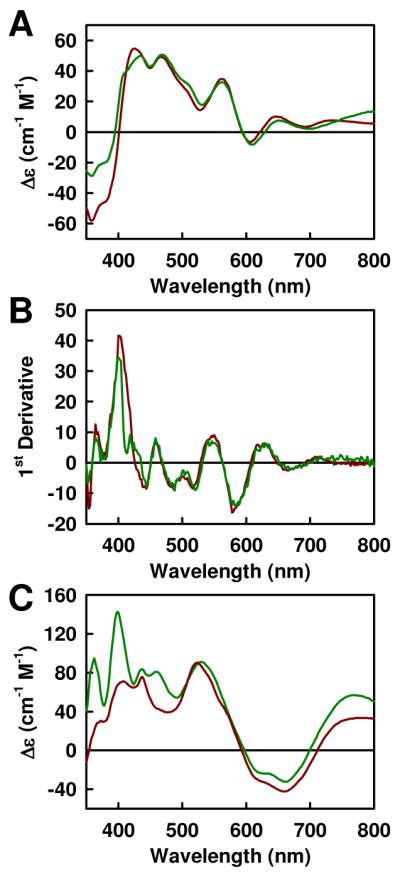
(A) MCD spectra of IDS-oxidized ΔnifB NifEN (dark red) and ΔnifH MoFe protein (green; scaled down by a factor of 0.5). (B) First derivative MCD spectra of oxidized ΔnifB NifEN (dark red) and ΔnifH MoFe protein (green; scaled down by a factor of 0.5), showing nearly identical transitions in both cases. All spectra were recorded at a temperature of 1.6 K and a magnetic field of 6.0 T and standardized per αβ subunit of protein. (C) MCD spectra of reduced ΔnifB NifEN (dark red) and ΔnifH MoFe protein (green; scaled down by a factor of 0.5). All spectra were recorded at a temperature of 1.6 K and a magnetic field of 6.0 T and standardized per αβ subunit of protein. Broad inflections in the 450 – 800 nm region are characteristic of classic [4Fe4S]+ clusters; and intensities at 540 nm quantify to 2 and 4 [4Fe4S]+-like clusters per ΔnifB NifEN and ΔnifH MoFe protein, respectively.
The ground state paramagnetism of the oxidized clusters in ΔnifB NifEN and ΔnifH MoFe protein implies an unusual coupling among the Fe sites within the cluster, which could originate from a subtle conformational rearrangement of these [4Fe4S] clusters upon oxidation. Such a coupled structural/redox change may allow the cluster to effectively mediate the electron transfer for the maturation of FeMoco precursor (in the case of ΔnifB NifEN), or prime the clusters in the correct conformation/oxidative state for their subsequent coupling into a mature P-cluster (in the case of ΔnifH MoFe protein) (Fig. 1). In the latter case, it is interesting to note that the mature P-cluster, much like its precursor, can exist both in an all-ferrous diamagnetic state and in (at least) three more oxidized states—all of which are paramagnetic and capable of undergoing structural/redox changes upon interconversion.
The high sequence4,10 and structural12 homologies of NifEN and the MoFe protein have been used in the past to suggest an evolutionary link between these proteins. The current finding of similar, unusual ferredoxin-type clusters in both proteins further supports these suggestions. It will be interesting to see whether these unusual cluster properties are confined to only Nif proteins and, as such, are related to specific functions in nitrogenase assembly and/or function.
Supplementary Material
Acknowledgment
This work was supported by NIH grant GM-67626 (M.W.R.) and NSF grant (2010)-Pfund-177 (B.J.H.).
Footnotes
Supporting Information Available: Materials and methods are included. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Beinert H, Holm RH, Münck E. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- (2).Beinert H. J. Biol. Inorg. Chem. 2000;5:2–15. doi: 10.1007/s007750050002. [DOI] [PubMed] [Google Scholar]
- (3).Noodleman L, Peng CY, Case DA, Mouesca JM. Coord. Chem. Rev. 1995;144:199–244. [Google Scholar]
- (4).Hu Y, Fay AW, Lee CC, Yoshizawa J, Ribbe MW. Biochemistry. 2008;47:3973–3981. doi: 10.1021/bi7025003. [DOI] [PubMed] [Google Scholar]
- (5).Corbett MC, Hu Y, Fay AW, Ribbe MW, Hedman B, Hodgson KO. Proc. Natl. Acad. Sci. USA. 2006;103:1238–1243. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hu Y, Corbett MC, Fay AW, Webber JA, Hodgson KO, Hedman B, Ribbe MW. Proc. Natl. Acad. Sci. USA. 2006;103:17119–17124. doi: 10.1073/pnas.0602647103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Corbett MC, Hu Y, Naderi F, Ribbe MW, Hedman B, Hodgson KO. J. Biol. Chem. 2004;279:28276–28282. doi: 10.1074/jbc.M403156200. [DOI] [PubMed] [Google Scholar]
- (8).Lee CC, Blank M, Fay AW, Yoshizawa JM, Hu Y, Hodgson KO, Hedman B, Ribbe MW. Proc. Natl. Acad. Sci. USA. 2009;106:18474–18478. doi: 10.1073/pnas.0909149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Broach RB, Rupnik K, Hu Y, Fay AW, Cotton M, Ribbe MW, Hales BJ. Biochemistry. 2006;45:15039–15048. doi: 10.1021/bi061697p. [DOI] [PubMed] [Google Scholar]
- (10).Goodwin PJ, Agar JN, Roll JT, Roberts GP, Johnson MK, Dean DR. Biochemistry. 1998;37:10420–10428. doi: 10.1021/bi980435n. [DOI] [PubMed] [Google Scholar]
- (11).Rupnk K, Hu Y, Fay AW, Ribbe MW, Hales BJ. J. Biol. Inorg. Chem. 2011;16:325–332. doi: 10.1007/s00775-010-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kaiser JT, Hu Y, Wiig JA, Rees DC, Ribbe MW. Science. 2011;331:91–94. doi: 10.1126/science.1196954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


