Endothelial and smooth muscle morphogenesis involves actin cytoskeletal elements and cytoskeletal regulatory molecules. Selective actin blockade interferes with several key components of neovascularization, including adhesion and growth factor–induced proliferation, and reduces neovessel formation and fibrosis in vivo.
Abstract
Purpose.
The efficacy of the peptide Ac-EEED on reducing cell adhesion and proliferation in vitro and choroidal neovascularization (CNV) in vivo was examined.
Methods.
The peptide chimera containing the Ac-EEED sequence was chemically linked to the N terminus of the XMTM delivery peptide from the Erns viral surface protein. Ac-EEED or scrambled control peptide (SCRAM) was added to cultures of vascular smooth muscle cells, pericytes, endothelial cells, and fibroblasts, and adhesion, growth, and matrix production was assessed. Ac-EEED or SCRAM was injected into the vitreous of mice undergoing laser rupture of Bruch's membrane to induce CNV and lesion volume, neovascularization and lesion fibrosis were assessed.
Results.
Ac-EEED–induced changes in the morphology of the actin cytoskeleton by inhibiting polymerization of G-actin and disrupting the formation of stress fibers. Pretreatment with Ac-EEED resulted in endothelial cells becoming less responsive to the mitogenic and pro-adhesive effects of VEGF. Ac-EEED treatment in fibroblasts reduced TGF-β–induced fibrosis as assessed by decreased levels of connective tissue growth factor, cysteine-rich 61, collagen I (COL1A2), and collagen III (COL3A1). CNV lesion size and fibrosis were reduced in a concentration-dependent manner by up to 60%.
Conclusions.
In vitro studies showed that Ac-EEED affects a broad range of mechanical properties associated with cytoskeletal actin to reduce growth factor effects. The utilization of Ac-EEED in vivo may offer a novel therapeutic strategy by both suppressed neovessel growth and curtailing fibrosis typically associated with the involutional stage of CNV.
Vision-threatening conditions such as age-related macular degeneration are associated with choroidal neovascularization (CNV) and lead to vision loss in the elderly. Although the cellular and molecular bases for CNV are not entirely understood, a disruption of Bruch's membrane commonly caused by a traumatic break, degeneration of the retinal pigment epithelium, tissue traction, and/or inflammation can lead to development of CNV.1,2 When this occurs, choriocapillary endothelial cells, pericytes, fibroblasts, and inflammatory cells invade the subretinal space. Vascular endothelial growth factor (VEGF), along with platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β, mediate growth of endothelial cells. Endothelial cell and pericyte proliferation, deposition, and remodeling of the extracellular matrix are critical elements of the angiogenic cascade responsible for CNV. Strategies to inhibit the action of these molecules include intravitreal injection of neutralizing anti-VEGF antibodies, antagonistic VEGF mutants, soluble receptors, and upstream inhibitors of VEGF regulators such as PKC.3 However, anti–VEGF-based pharmacotherapies have been associated with retinal detachment, subretinal fibrosis, and chorioretinal atrophy.
The morphogenetic processes through which proliferating vascular cells organize themselves into neovessels remain incompletely understood. Many lines of evidence suggest that endothelial and smooth muscle morphogenesis involves actin cytoskeletal elements and cytoskeletal regulatory molecules such as Rho GTPases.4 The actin cytoskeleton provides a continuous and dynamic link between virtually all cellular structures and thus enables nuclear elements, such as chromatin, to respond directly and immediately to chemical and physical insults. Pharmacological inhibitors of the Rho GTPase–dependent cytoskeletal actin organization suppress VEGF-mediated angiogenesis in ex vivo retinal explants and strongly disrupt vasculogenesis in pluripotent embryonic stem cell cultures.5,6 However, the GTPase Rho is required for a variety of complex and vital biological processes, including focal adhesions, microtubule dynamics, vesicle trafficking, cell polarity, cell cycle progression, and cytokinesis, which if suppressed may compromise the proper function of cells and tissues. Previously we demonstrated that a fusion peptide chimera containing the N-terminal acetylated nanopeptide Ac-EEEDSTALVK (Ac-EEED) reversibly blocks actin and actin-binding protein interactions in isolation from other Rho GTPase–dependent activities.7 The present study was undertaken to test the specific effects of the fusion peptide on steps relevant to angiogenesis in vitro and to the process of in vivo angiogenesis using the rodent model of CNV.
Methods
Fusion Peptide Synthesis
The active Ac-EEEDSTALVK (Ac-FP) and scrambled (SCRAM) [Ac-EDDESTALVK] peptides were each fused to the XMTM delivery peptide from the Erns viral surface protein (GRQLRIAGRRLRGRSR) and synthesized to a purity of >98% (Pepscan Systems, Lelystad, The Netherlands). The XMTM molecule was used as a delivery vector for its ability to efficiently translocate any attached cargo molecule into the cells.
Cell Cultures and Treatments
Mouse aortic smooth muscle cells and NIH3T3 mouse fibroblast cell line were obtained from American Type Tissue Culture (Manassas, VA). Rat retinal pericytes were isolated from a pool of Sprague–Dawley rat retinas by selective sieving at QBM Cell Science Laboratories (Ottawa, Canada). Rat retinal endothelial cells (RECs) were obtained from Cellpro (San Pedro, CA). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics in a humidified atmosphere containing 5% CO2 in air at 37°C°. For most experiments cells were grown to subconfluence either in 25 cm2 culture flasks or in 35-mm 6-well plates. Cells were then washed with serum-free medium to remove traces of serum and pretreated with either Ac-EEED or SCRAM peptide (10 μg/mL) for 30 minutes followed by the addition of either 10 ng/mL of TGF-β1 (BD Bioscience, San Jose, CA) or 1 ng/mL of VEGF-A (Biovision, Mountain View, CA) for the indicated period.
For visualization of actin stress fibers, tissue cross sections or cells plated on glass cover slips were fixed in 0.4% formaldehyde-PBS for 30 minutes, permeabilized in 0.1% Triton X-100 at room temperature for 5 minutes, and stained with rhodamine-phalloidin (Cytoskeleton, Denver, CO).
Proliferation Assay
Cell proliferation was determined using a proliferation assay (CyQUANT Direct Cell Proliferation Assay; Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The fluorescence intensity was measured with a fluorescence microplate reader using the FITC filter set.
Adhesion Assay
Cell adhesion was examined in 96-well plates. RECs were seeded at 5 × 104 cells/well in 100 μL. As indicated, cells were pretreated with Ac-EEED or SCRAM peptide (10 μg/mL) for 30 minutes at 37°C. Adhesion was carried out for 4 hours at 37°C, 5% CO2 in the presence of each of the fusion peptides. After removal of nonadherent cells by two washing steps with basal medium containing 0.1% BSA at room temperature, attached cells were fixed with 4% paraformaldehyde at room temperature for 10 minutes and stained for 10 minutes with crystal violet (5 mg/mL in 2% ethanol). After several washes with water, the wells were dried and the dye was extracted with 2% SDS for 30 minutes at room temperature. Adhesion was quantified by measurement of absorbance at 550 nm. Nonspecific cell attachment (attachment to wells coated with BSA) was always <5%.
RNA Isolation and Quantitative Analysis of mRNA
Total RNA was extracted from cells using RNAEasy column purification protocol (Qiagen, Valencia, CA) as previously described.8 Quantitative real-time reverse transcriptase–polymerase chain reaction (Q-RT-PCR) assay was performed to quantify the mRNA levels of numerous genes using hydrolysis probe technology (TaqMan; Applied Biosystems, Carlsbad, CA) on an a sequence detection system (ABI Prism 7000; Applied Biosystems). Highly specific primers for cysteine-rich protein 61 (Cyr61), connective tissue growth factor (CTGF), type I collagen α2 chain (COL1A2), and type III collagen α1 chains (COL3A1) were designed using the Web-based primer design program Primer3 (http://frodo.wi.mit.edu/primer3/). These primers were designed to span exon-exon junctions so that genomic DNA would not be detected. These primers were designed to span exon-exon junctions so that genomic DNA would not be detected. The forward and reverse primers were the following: 5′GCACCTCGAGAGAAGGACAC3′ and 5′CAAACCCACTCTTCACAGCA3′ for Cyr61; 5′GTAACCGGGGAGGGAAATTA3′ and 5′GCTTTATCACCTGCACAGCA3′ for CTGF; 5′GGGACCATCAACACCAACTC3′ and 5′CCCTTTGCCATTTTTGTCTT3′ for COL1A2 and 5′TGGAGAATGTTGTGCAGTTTGC3′ and 5′GCCTTGAGGTCCTTGACCATTA3′ for COL3A1. The cycling parameters for PCR amplification reactions were DNA polymerase (AmpliTaq; Applied Biosystems) activation 95°C for 10 minutes, denaturation 95°C for 15 seconds, and annealing/extension 60°C for 1 minute (40 cycles). Triplicate CT values were analyzed with commercial software (Excel; Microsoft, Redmond, WA) using the comparative CT (hhCT) method as described by the manufacturer (Applied Biosystems). Transcript levels (2−hhCT) were obtained by normalizing to an endogenous reference (18S rRNA).
Experimental Induction of Choroidal Neovascularization and Treatment with Fusion Peptides
Twelve-week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal studies were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Florida, and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Mice (n = 10 per treatment group) were subjected to Bruch's membrane rupture by laser injury (532 nm, 100 ms, 50 mm spot size) as previously described.9 Immediately after injury they were injected into the vitreous with 1 μL vehicle (sterile saline), 200 ng scrambled peptide, or increasing doses of Ac-EEED (20, 200, and 500 ng/mL). All animals received a second injection 7 days after initial injury and were euthanatized 14 days post-injury.
Qualitative and Quantitative Analyses of CNV and Fibrosis
Eyes were allocated evenly into two groups. The first set of eyes was preserved by immersion in 4% buffered paraformaldehyde, taken to PBS through two washes, dissected, and stained with rhodamine-conjugated agglutinin to detect vasculature, and image captures were made through the entire depth of each lesion (z-depth 3 μm, average 25 images per stack) with a spinning disc confocal microscope, as previously described.9 Volumetric morphometry to determine lesion volume was done using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) as described previously.10
The second set of eyes was preserved in Trump's fixative and then embedded in paraffin, sectioned (6-μm sections, collecting every 10th section) and finally stained using Masson's trichrome to examine collagen deposition in CNV lesion loci. Software-assisted morphometry (ImageJ) was used to determine fibrosis by color thresholding. Area measurements were taken from at least four serial sections of each lesion, and the average area for all lesions in a given eye considered an n = 1 for that treatment group. Those values were then averaged to determine collagen deposition and expressed as the percent of lesion area ± SE from the mean.
Statistical Analyses
Data were expressed as mean ± SE. A paired Student's t-test was used to analyze differences between two groups, and P values of 0.05 or 0.01 were considered significant.
Results
Ac-EEED Peptide Reduces Actin Polymerization and Stress Fiber Formation in Cultured Aortic Smooth Muscle Cells and Retinal Pericytes
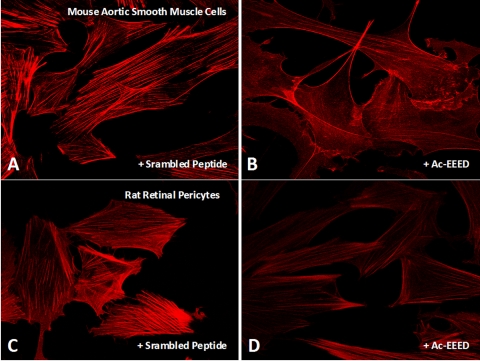
The hallmark of differentiated vascular smooth muscle cells (SMCs) and pericytes is an organized contractile apparatus consisting of an extensive actomyosin-based cytoskeletal network of stress fibers that is dynamically regulated under physiological conditions. SMCs are an ideal cellular model to test the effectiveness of the fusion peptide. Exposure to the Ac-EEED fusion peptide resulted in a marked qualitative reduction in stress fiber formation in both macrovascular (mouse aortic SMCs) and microvascular (rat pericytes) cells in culture (Fig. 1). Stress fibers remained unaltered in cells treated with the scrambled peptide (Figs. 1A, 1C). SMCs, in particular, displayed a nearly stress fiber-free pattern and little or no change in cell morphology. Stress fibers reform spontaneously on incubation of the cells in a peptide-free medium (data not shown), indicating the reversibility of the interactions of the Ac-EEED peptide with cytoskeletal proteins.
Figure 1.
Effect of the Ac-EEED peptide on stress fiber formation in aortic SMC and retinal pericytes. SMCs (A, B) and retinal pericytes (C, D) were cultured in 2% serum and incubated with either scrambled peptide (A, C) or Ac-EEED fusion peptide (B, D) for 30 minutes, Cells were then fixed, permeabilized, stained with rhodamine-conjugated phalloidin, and visualized by fluorescence microscopy.
Ac-EEED Peptide Reduces TGF-β–Induced CCN and Fibrillar Collagen Gene Expression
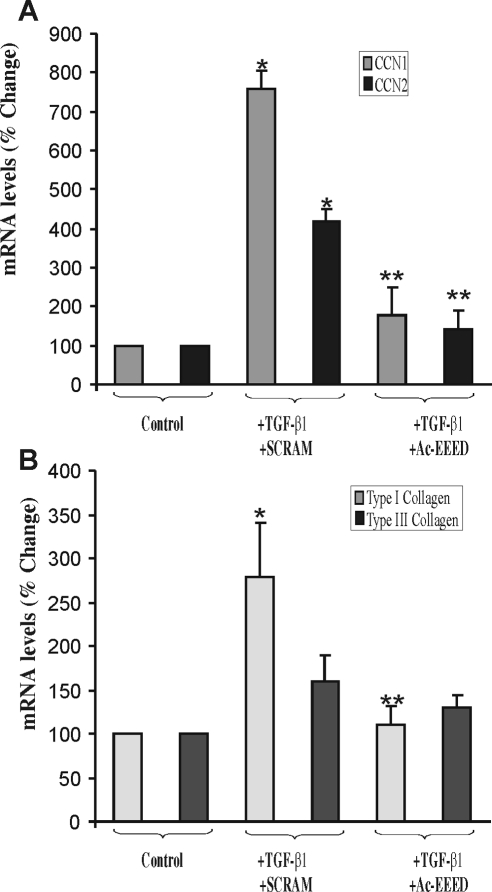
When kept under conventional culture conditions in the presence of TGF-β, fibroblasts are in a state of continuous activation of isoactin gene expression. Since TGF-β1 stimulates a strong fibrogenic response in fibroblasts, we examined the effects of the fusion peptide on the expression of early (e.g., CCN1/Cyr61, CCN2/CTGF) and late (e.g., COL1A2, COL3A1) marker of fibrosis. TGF-β induced a nearly eightfold increase in CCN1 and a more than fourfold increase in CCN2 mRNA in 3T3 fibroblasts (Fig. 2A). These increases were reduced in cells pre-incubated with the Ac-EEED fusion peptide. The presence of the Ac-EEED fusion peptide reduced CTGF/CCN2 and Cyr61/CCN1 expression to basal levels. The level of COL1A2 mRNA expression increased nearly threefold with TGF-β exposure but was significantly reduced in the presence of Ac-EEED peptide (Fig. 2B). There was a similar, albeit smaller, trend in COL3A1 mRNA expression, but the change did not achieve statistical significance. Moreover, the contribution of COL3A1 to the fibrotic reaction is reportedly insignificant compared to that of COL1A2.
Figure 2.
Effects of the Ac-EEED fusion peptide on TGF-β–induced fibrotic gene expression in 3T3 fibroblasts. (A) Cells were incubated with TGF-β1 (10 ng/mL) in the presence of either a scrambled peptide (SCRAM) or Ac-EEED fusion peptide (10 μg/mL) for 1 hour. CCN1 and CCN2 mRNA levels were determined by real-time PCR. To compare data from different experiments, the mRNA levels in untreated cells was set to 100%. *P < 0.001 versus control; **P < 0.005 versus +SCRAM. (B) Cells were incubated with TGF-β1 (10 ng/mL) in the presence of either a scrambled peptide (SCRAM) or Ac-EEED fusion peptide (10 μg/mL) for 16 hours. Type I collagen (COL1A1) and type II collagen (COL3A1) mRNA levels were determined by real-time PCR. *P < 0.001 versus control; **P < 0.005 versus SCRAM.
Ac-EEED Peptide Reduces Adhesion and VEGF-Induced Growth in Retinal Endothelial Cells
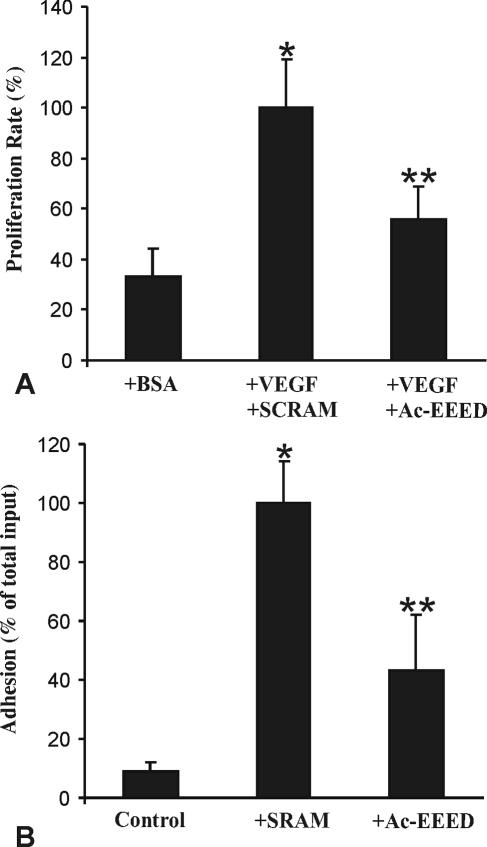
Cytoskeletal proteins that form stress fibers in endothelial cells are composed essentially of intermediate filaments, actin microfilaments, and tubulin microtubules. These structures act in concert to control cell morphology and biomechanics during sprouting angiogenesis. To determine whether the Ac-EEED fusion peptide modulates the motor function of actin in general, we determined its impact on endothelial cell adhesion and growth. Pre-incubation with the Ac-EEED peptide reduced endothelial cell adhesion to collagen-coated substrate by nearly 60% compared with the scrambled peptide (Fig. 3). Similarly, the proliferation rate of retinal endothelial cells stimulated with VEGF-A was significantly diminished on pretreatment with the Ac-EEED fusion peptide. The effects of the Ac-EEED peptide have not been tested on choroidal endothelial cells. However, the peptide exhibited similar effects on umbilical endothelial cells suggesting that its effects are not specific to a particular endothelial tissue bed.
Figure 3.
Effects of the Ac-EEED fusion peptide on VEGF-induced growth and adhesion of rat retinal endothelial cells. (A) Rat retinal endothelial cells were plated in type I collagen–coated wells for 24 hours. Cells were then incubated with VEGF (10 ng/mL) in the presence of either a scrambled peptide (SCRAM) or Ac-EEED fusion peptide (10 μg/mL) for 16 hours. Cell growth was measured using proliferation assay. (B) Cells were plated on type I collagen-coated wells in the presence of either a scrambled peptide (SCRAM) or Ac-EEED fusion peptide (10 μg/mL). After incubation at 37°C for 30 minutes, attached cells were fixed with paraformaldehyde and stained with crystal violet, solubilized in SDS 2%, and quantified by densitometric measurement. *P < 0.001 versus control; **P < 0.005 versus SCRAM.
Ac-EEED Peptide Reduces Experimentally Induced CNV
Choroidal neovascular lesions resulting from laser rupture of Bruch's membrane may vary not only in circumference but also in depth. Measuring total neovascular lesion volume is a more accurate parameter than lesion area when quantifying the effectiveness of a test agent. Images of CNV lesions (as z-stack compressions) from untreated and Ac-EEED-treated (200 ng) mouse eyes are shown in Figures 4A and 4B, respectively. Ac-EEED-treated CNV lesions showed a reduction in size and in fluorescence intensity based on quantitative volumetric morphometry, and this decrease was concentration dependent (Fig. 4C). However, it must be noted that the highest dose tested (500 ng) resulted in microophthalmia and friable eyes with evidence of anatomic damage. Nevertheless, there was more than twofold reduction in lesion volume with the highest safe dose compared to vehicle or SCRAM control.
Figure 4.
Ac-EEED peptide reduces experimentally induced CNV lesion volume in a concentration-dependent manner. (A–E) Typical and representative z-stack compressions of confocal image captures of CNV lesions from mice receiving vehicle, scrambled peptide, or 20 ng, 200 ng, or 500 ng Ac-EEED peptide, respectively. Red fluorescence results from TRITC-conjugated R. communis agglutinin I that binds to endothelium. Scale bar, 100 μm. (F) Summary of the quantitative changes in CNV lesion volume resulting from increasing concentrations of Ac-EEED (n = 5 animals per condition). There was marked observable toxicity to the eyes with the highest dose tested. *P < 0.05 versus vehicle treatment; **P < 0.01 versus vehicle treatment.
Ac-EEED Peptide Reduces Collagen Deposition in Experimental CNV
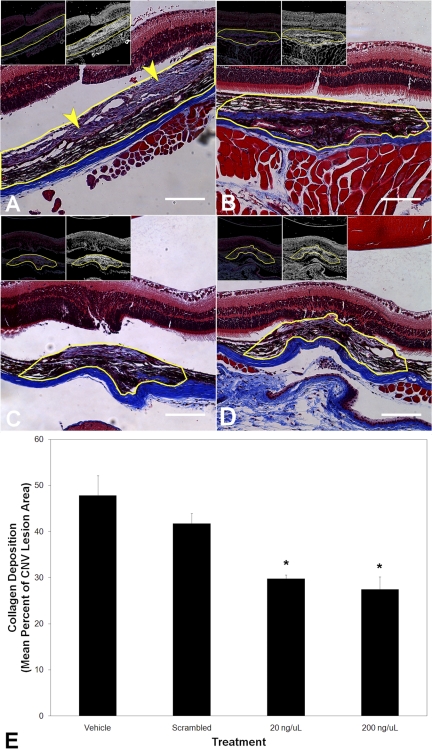
A major pathophysiological component of CNV is fibrosis, as measured by collagen deposition, which can increase the overall size of the lesions as well as contribute to impaired resolution. After laser rupture of Bruch's membrane to induce CNV, Masson's trichrome stain demonstrates collagen deposition within and surrounding the lesions (Figs. 5A and 5B). A reduction in the amount of collagen staining was observed in the Ac-EEED-treated versus SCRAM-treated eyes. Results from quantitative color threshold morphometry are summarized in Figure 5C and show a concentration-dependent reduction in collagen deposition within CNV lesions with Ac-EEED treatment. Similar to its effect on reducing CNV lesion volume, the highest safe dose of Ac-EEED resulted in a nearly twofold decrease in collagen deposition compared to treatment with vehicle alone.
Figure 5.
Ac-EEED peptide reduces fibrosis associated with injury-induced CNV. Masson's trichrome stain was used to detect collagen in histology sections from mouse eyes subjected to laser rupture of Bruch's membrane and then left untreated or injected into the vitreous with either SCRAM peptide or Ac-EEED peptide. (A, D) Typical bright field photomicrographs showing the lesion area in cross section, with collagen stained light to medium purple, from eyes receiving vehicle, scrambled peptide, or 20 ng or 200 ng Ac-EEED, respectively. The lesion area in each image is encircled in yellow with the actual morphometric region of interest used to determine staining density. The lesion in (A) actually extended beyond the border of the field depicted, and its analysis was continued across multiple fields to encompass the entire lesion. The arrowheads in (A) point to the collagen deposition stained bluish to light purple by Masson's trichrome. The insets in each panel depict the color threshold applied to that image (left inset), as well as the binary threshold image that was used for final morphometry (right inset). Note both a decrease in lesion cross-sectional area and the area of collagen. Scale bar, 50 μm. (E) Summary of computer-assisted quantitative measurements by color threshold morphometry of collagen area within the lesions (n = 5 animals per treatment condition). *P < 0.05 versus vehicle (PBS) treatment.
Discussion
In this study we demonstrate that the fusion peptide Ac-EEED has the ability to reduce neovessel outgrowth in a mouse model of CNV. The fusion peptide was designed to act as a dominant negative of smooth muscle α-actin, inducing actin depolymerization and reducing stress fiber formation by interfering with the interaction of actin and actin-binding proteins. Actin filaments are highly dynamic structures that are being constantly assembled, disassembled, and reorganized as the cells change their shape, divide, or adhere to a substratum. They assume the role of a scaffolding structure on which proteins of the signaling machinery and other cytoplasmic structures are docked and become activated.
The Ac-EEED fusion peptide likely interferes with adhesion, mitogenic, and differentiation signals in the cells, allowing it to inhibit choroidal neovessel growth. Interestingly, choroidal neovessels were previously shown to be covered with enlarged pericytes,11 a potential indication of an expanded cytoskeleton, whereas new sprouts either had no pericytes or the pericytes were of unusual morphology. In cultured SMC and pericytes, Ac-EEED–induced changes in the actin cytoskeleton morphology by inhibiting polymerization of G-actin and changing the amount and physical appearance of F-actin and stress fibers. Pericytes, in particular, are anchorage-dependent cells, and their survival and function require the interaction between ECM proteins and cytoskeletal actin via integrins.8 Ac-EEED peptide-induced cytoskeletal alterations in pericytes in the CNV model would compromise adhesion, migration, and survival of growing pericytes and SMCs. Furthermore, when pretreated with the Ac-EEED peptide, cultured endothelial cells become less responsive to the mitogenic and proadhesive effects of VEGF. Thus, Ac-EEED affects the broad mechanical properties of cytoskeletal actin and associated signaling cascades in response to exogenous stimuli in various cell types.
In fibroblasts, our data showed that the Ac-EEED significantly reduced the fibrogenic effects of TGF-β. TGF-β-induced expression of both early and late markers of fibrosis (e.g., CTGF, Cyr61, COL1A2, COL3A1) was significantly decreased on pretreatment with the fusion peptide. Fibrosis is mediated in vivo by activated fibroblasts also known as myofibroblasts, which synthesize and remodel newly created extracellular matrix. While we did not directly measure the contribution of myofibroblast within these CNV lesions, we suspect that these cells are likely involved in the fibrotic reaction we observe. Myofibroblasts express the highly contractile protein α-smooth muscle actin (α-SMA), which is organized into contractile microfilaments. The origin of myofibroblasts is unclear but may result from growth factor-mediated differentiation of resident mesenchymal cells or recruitment of microvascular pericytes.12 Thus, the utilization of the fusion peptide may not only impede neovessel growth but also curtail the fibrotic reactions/scarring associated with the involutional stage of CNV.
The etiology of several vascular diseases is linked to cellular signals that regulate the actin cytoskeleton. In particular, the tone and contractility of vascular smooth muscle cells rely on actin-myosin filaments and are important determinants of their function. Similarly, signaling pathways activated by hypoxia modify cytoskeletal and contractile proteins and alter the biomechanical properties of endothelial cells.13 Cytoskeletal actin is one of the most abundant proteins in a cell and is present as a monomer or polymer. The conversion of monomers of actin into dimers and trimers is the limiting step in the formation of actin polymers and stress fibers. Once dimers and/or trimers are formed, the polymerization becomes dependent on the concentration of free actin. During actin monomer assembly, actin binds to ATP and become incorporated into the polymer. Hydrolysis of ATP into ADP ensues. However, a large proportion of actin in cells is sequestered by actin-binding proteins and thus is not free to polymerize to form stress fibers. Actin monomer-binding proteins such as profilin and β-thymosin regulate actin polymerization by interacting with actin monomers, rendering them unavailable for polymerization.14 A large number of other proteins bind to actin and participate in essential cellular functions, including cell motility, cytokinesis, maintenance of cell structure, and organelle movement.15 The direct interaction between angiogenin, a potent angiogenic factor, and actin has been implicated in remodeling of the ECM and the degradation of basement membrane, therefore promoting cell invasion into the perivascular tissue.16 Binding of angiogenin to preformed F-actin does not cause depolymerization of actin filaments though it causes their stiffening.17
In conclusion, our data not only provided new insights into the effects of the Ac-EEED on CNV and relevant cellular types, but also presented a strategy for developing a hitherto unexplored approach to control tissue remodeling during normal and pathologic angiogenesis and fibrotic diseases. Although the CNV mouse model used in our studies recapitulates many of the clinical manifestations of CNV in humans, the time to develop, course of progression, size, and appearance of the lesion are different. It will be important in future studies to determine the effects of the Ac-EEED peptide on neovessel formation in models of not only choroidal but also retinal neovascularization and identify key signaling partners.
Footnotes
Supported in part by NIH Grants EY012601, EY007739, EY018358 (MBG) and EY019387-0A1 (BC).
Disclosure: S. Caballero, None; R. Yang, None; M.B. Grant, None; B. Chaqour, None
References
- 1. Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15:657–664 [DOI] [PubMed] [Google Scholar]
- 2. Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–508 [DOI] [PubMed] [Google Scholar]
- 4. Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55 [DOI] [PubMed] [Google Scholar]
- 5. Bryan BA, Dennstedt E, Mitchell DC, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou D, Herrick DJ, Rosenbloom J, Chaqour B. Cyr61 mediates the expression of VEGF, alphav-integrin, and alpha-actin genes through cytoskeletally based mechanotransduction mechanisms in bladder smooth muscle cells. J Appl Physiol. 2005;98:2344–2354 [DOI] [PubMed] [Google Scholar]
- 7. Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608–20622 [DOI] [PubMed] [Google Scholar]
- 8. Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149:1666–1677 [DOI] [PubMed] [Google Scholar]
- 9. Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci. 2005;46:343–348 [DOI] [PubMed] [Google Scholar]
- 10. Caballero S, Swaney J, Moreno K, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res. 2009;88:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Killingsworth MC. Angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1995;233:313–323 [DOI] [PubMed] [Google Scholar]
- 12. Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680 [DOI] [PubMed] [Google Scholar]
- 13. Liu T, Guevara OE, Warburton RR, Hill NS, Gaestel M, Kayyali US. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am J Physiol Cell Physiol. 2010;299:C363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. dos Remedios CG, Chhabra D, Kekic M, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473 [DOI] [PubMed] [Google Scholar]
- 15. Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu GF, Strydom DJ, Fett JW, Riordan JF, Vallee BL. Actin is a binding protein for angiogenin. Proc Natl Acad Sci U S A. 1993;90:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pyatibratov MG, Tolkatchev D, Plamondon J, Xu P, Ni F, Kostyukova AS. Binding of human angiogenin inhibits actin polymerization. Arch Biochem Biophys. 2010;495:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]