The data in this study, obtained from tract-tracing experiments in the monkey, imply that the palisade endings located at the myotendinous junction of eye muscles arise from the motor nuclei in the brain stem.
Abstract
Purpose.
The purpose of this study was to localize the cell bodies of palisade endings that are associated with the myotendinous junctions of the extraocular muscles.
Methods.
Rhesus monkeys received tract-tracer injections (tetramethylrhodamine dextran [TMR-DA] or choleratoxin subunit B [CTB]) into the oculomotor and trochlear nuclei, which contain the motoneurons of extraocular muscles. All extraocular muscles were processed for the combined immunocytochemical detection of the tracer and SNAP-25 or synaptophysin for the visualization of the complete muscle innervation.
Results.
In all muscles—except the lateral rectus—en plaque and en grappe motor endings, but also palisade endings, were anterogradely labeled. In addition a few tracer-labeled tendon organs were found. One group of tracer-negative nerve fibers was identified as thin tyrosine hydroxylase-positive sympathetic fibers, and a second less numerous group of tracer-negative fibers may originate from the trigeminal ganglia. No cellular or terminal tracer labeling was present within the mesencephalic trigeminal nucleus or the trigeminal ganglia.
Conclusions.
These results confirm those of earlier studies and furthermore suggest that the somata of palisade endings are located close to the extraocular motor nuclei—in this case, probably within the C and S groups around the periphery of the oculomotor nucleus. The multiple en grappe endings have also been shown to arise from these cells groups, but it is not possible to distinguish different populations in these experiments.
Unlike skeletal muscle, the extraocular muscles retain several embryologic features and exhibit a more complex anatomy.1–4 They are divided into two distinct layers: The inner global layer inserts into the globe via a well-defined tendon, whereas the outer orbital layer does not reach the globe but inserts into the pulleys, which consist of collagen, elastic fibers, and smooth muscles.5 Based on their location, mitochondrial content, and innervation pattern, six types of muscle fibers have been identified in extraocular muscles.6 Differences in their contractile properties are reflected by their oxidative enzyme content and the expression of different isoforms of myosin heavy chains identified by immunohistochemistry.6,7
Two main categories of muscle fibers can be distinguished: Most of the muscle fibers (80% in the orbital layer, 90% in the global layer) exhibit fast-twitch properties, and they are innervated by a single en plaque nerve ending at the middle third of the muscle fiber, similar to twitch fibers of the skeletal muscle.6 The remaining muscle fibers are contacted by multiple en grappe nerve endings along the length of the muscle fiber. They are nontwitch fibers, which respond with tonic contractions to electrical or pharmacologic stimulation.8,9 This nontwitch extraocular muscle fiber corresponds anatomically to a multiple innervated fiber (MIF): In mammals, this type of fiber is unique to extraocular muscles.10,11 In the orbital layer, the MIFs are more complex and show histochemical and morphologic variation along their length.12 Aside from the multiple innervation by en grappe endings at their proximal and distal poles, they have a central endplate region like that of twitch muscle fibers.12–14 Consequently, the orbital MIFs have mixed physiological properties with a slow-tonic and fast-twitch component.15 It appears that only the global MIFs have pure nontwitch properties. These studies were performed in rats, but the same basic morphology is present in all mammals, including primates.16
At the myotendinous junction of the MIFs in the global layer, a specialized type of nerve ending is present, the palisade ending. According to the terminology of Ruskell,17 the palisade endings—plus a surrounding fibroblast-like cell capsule—are called myotendinous cylinders. Palisade endings arise from nerve fibers that enter the tendon from the central muscle nerve and turn back 180° to re-enter the muscle zone distally, to form a cuff of nerve endings around the tip of the MIFs of the global layer.9,18 So far, palisade endings have been found in all investigated species, including rat,19 rabbit,20 cat,21 sheep,22 dog,23 monkey, and human.24–26
Retrograde tract-tracing studies in primate and rat have shown that the singly innervated fibers (SIFs) and MIFs are supplied by distinct motoneuronal groups, which are anatomically separated and differ in their histochemistry.27–29 Motoneurons within the boundaries of the classic motor nuclei are considered to activate the singly innervated twitch fibers, whereas motoneurons around the periphery of the motor nuclei (oculomotor, trochlear, and abducens) are thought to be the source of the multiple innervation of MIFs.27 In contrast, there are conflicting reports on the location of the somata of palisade endings. Early lesion experiments in monkey suggested the motoneurons as the source, since palisade endings did not degenerate after the sensory trigeminal nerve was sectioned, but did degenerate after the oculomotor nerves were cut.30,31 Furthermore, palisade endings use acetylcholine as a transmitter, as do motoneurons.32–35 Other reports favored the trigeminal ganglion as the obvious source of palisade ending somata, to support the large body of physiological evidence for the presence of some sensory feedback from eye muscles to the brain,18,26,32 and tracer injections into the trigeminal ganglion of the cat led to anterograde labeling of palisade endings in the extraocular muscles.36 These conflicting findings contribute to an ongoing debate on the function of the palisade endings, whether they subserve sensory or motor or even combined sensory–motor function.24,37
In an attempt to identify the location of the cell somata innervating the palisade endings, we performed central tracer injections into the oculomotor nucleus of the monkey and studied the extraocular muscles for the presence of anterogradely labeled nerve endings.
Methods
All experimental procedures conformed to the state and university regulations on Laboratory Animal Care, including the Principles of Laboratory Animal Care (NIH Publication 85-23, revised 1985), and were approved by animal care officers and Institutional Animal Care and Use Committees at Emory University, where all surgical interventions and perfusions were undertaken. Three monkeys were used in total. All were managed and the research was conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Two rhesus monkeys (Macaca mulatta), between 2 and 3 years old, were used for three central tracer injections: (1) tetramethylrhodamine dextran (TMR-DA) into the left side oculomotor nucleus (nIII) (case 1) and (2) cholera toxin subunit B (CTB) in the right side (case 2) of one animal and (3) TMR-DA into the oculomotor nerve (NIII) of a second animal (case 3). For surgery under aseptic conditions, the animals (born in captivity at Yerkes National Primate Research Center, Atlanta, GA) were anesthetized with isoflurane (1.25%–2.5%). Blood pressure, heart rate, blood oxygenation, body temperature, and CO2 in expired air were monitored (SurgiVet monitor; Smiths Medical, Dublin OH) and maintained within physiological limits. Postsurgical analgesia (buprenorphine, 0.01 mg/kg, every 6 hours) and anti-inflammatory (banamine 1.0 mg/kg, every 6 hours) treatments were administered for several days, as recommended by the manufacturer. Using stereotaxic methods a titanium head stabilization post and titanium recording chamber (Crist Instruments, Hagerstown, MD) were implanted (anterior, 2 mm; lateral, 1 mm; tilted 20° away from midline) and aimed such that an electrode track located in the center of the chamber intersected a point near the oculomotor nucleus.
In all animals, the injection sites were identified with single unit recording with tungsten microelectrodes. Motoneurons were recognized by their characteristic burst-tonic responses in association with appropriately directed saccades. For injection, the recording electrode was replaced either by a custom-made micropipette equipped with a bevelled glass tip (20–50-μm diameter) and attached by polyethylene tubing to a picoliter pump (model 830; World Precisions Instruments, Sarasota, FL) (cases 1 and 2) or a thin syringe (Hamilton, Reno, NV) (case 3). Short-duration (50 ms) pressure pulses delivered over several minutes ejected small tracer volumes at each site. The pipette was left in place for 5 to 10 minutes after injection and then gradually removed. In case 2, 0.2 μL nontoxic CTB (C-167, 1% in aqua bidest [ultrapure water]; Sigma/List Biological Laboratories, Campbell, CA;) and in case 1, 0.5 μL TMR-DA (D-3308, 3000 MW; 15% in acetate buffer, pH 3; Invitrogen-Molecular Probes, Eugene, OR) was injected. Case 3 received 1 μL TMR-DA directly through the syringe, which was also left in place for 10 minutes.
After 3 days' survival time, the animals were sedated with ketamine, killed with an overdose of sodium pentobarbital (>90 mg/kg, IV), and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PBS; pH 7.4).
The brain, the orbits, and both trigeminal ganglia were removed. The extraocular muscles were carefully dissected, keeping the myotendinous junction intact. All tissues were equilibrated in 10%, 20%, and 30% sucrose in 0.1 M TBS (Tris-buffered saline) for freeze cutting. The brainstems were cut transversely at 40 μm with a cryostat (HM; Thermo Scientific-Microm, Walldorf, Germany). Eye muscles and trigeminal ganglia were cut longitudinally at 20 μm and directly thaw mounted onto glass slides (Superfrost Plus; M&B Stricker, Oberschleissheim, Germany).
In addition, in a third monkey (case 4), two tracers were injected into the myotendinous junction (the location of palisade and en grappe endings) of the medial rectus (MR; CTB) and the inferior rectus (IR; horseradish peroxidase-conjugated wheat germ agglutinin; WGA-HRP), for analysis of the course of the axons passing through nIII that could be labeled by the injections of cases 1 to 3 and hence contribute to the labeled terminals in the eye muscles. A detailed description of the eye muscle injections and tracer detection is given in a previous report.27
Tracer Detection in the Brain
Brain stem sections were immunocytochemically treated, free-floating withantibodies against CTB (1:20,000; List Biological Laboratories) or TMR-DA (1:6000; Invitrogen-Molecular Probes, Eugene, OR), as described previously29 (Table 1). The antigenic sites were visualized with a reaction in 0.025% diaminobenzidine (DAB) and 0.015% H2O2 in 0.1 M TBS (pH 7.6) for 10 minutes.
Table 1.
List of Antibodies and Their Sources with the Applied Methods Used in the Study
| Antigen Detection | Primary Antibodies | Secondary Antibodies |
|---|---|---|
| Choleratoxin subunit B; immunoperoxidase | Goat anti-choleratoxin 1:20,000; List Biological Laboratories, Campbell, CA | Biotinylated rabbit anti-goat 1:200; Vector Laboratories, Burlingame, CA |
| Choleratoxin subunit B; fluorescence | Goat anti-choleratoxin 1:5 000; List Biological Laboratories | Cy3 donkey anti-goat 1:200; Jackson Immuno Research, West Grove, PA |
| Tetramethylrhodamine dextran; immunoperoxidase | Rabbit anti-tetramehylrhodamine dextran 1:6000; Invitrogen-molecular probes, Eugene, OR | Biotinylated goat anti-rabbit 1:200; Vector Laboratories |
| Tetramethylrhodamine dextran; fluorescence | Rabbit anti-tetramethylrhodamine dextran 1:2000; Invitrogen-molecular | Cy3 donkey anti-rabbit 1:200; Jackson ImmunoResearch |
| Snap 25; fluorescence | Mouse anti-snap-25 1:1000; Sternberger monoclonals Inc., Baltimore, MD | Alexa 488 donkey anti-mouse 1:200; Invitrogen-Molecular Probes |
| Synaptophysin; fluorescence | Mouse anti-synaptophysin 1:20; m0776, Dako, Glostrup, Denmark | Alexa 488 donkey anti-mouse 1:200; Invitrogen-Molecular Probes |
| Myosin heavy chain (slow); immunoperoxidase | Mouse anti-myosin heavy chain (slow) (MHCS) 1:100; Novocastra | Biotinylated horse anti-mouse 1:200; Vector Laboratories |
| Laboratories Ltd, Newcastle-upon-Tyne, UK | ||
| Tyrosine hydroxylase; fluorescence | Rabbit anti-tyrosine hydroxylase 1:100; ab152; Chemicon, Billerica, MA | Alexa 488 donkey anti-rabbit 1:200; Invitrogen-Molecular Probes |
| Choline acetyltransferase; fluorescence | Goat anti-choline acetyltransferase 1:50; ab144p; Chemicon | Alexa 488 donkey anti-goat 1:200; Invitrogen-Molecular Probes |
On-slide immunofluorescence detection of the tracer was used for the trigeminal ganglia. After they were blocked with 2% normal donkey serum in 0.1 M TBS containing 0.3% Triton X-100 for 1 hour, the slides were processed with goat anti-CTB (1:5000; List Biological Laboratories) or rabbit anti-TMR-DA (1:2000; Invitrogen-Molecular Probes) for 48 hours at room temperature. After the CTB- or TMR-DA-containing sections were washed, they were treated with the secondary antibody (Cy3 donkey anti-goat and Cy3 donkey anti-rabbit, respectively; Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature.
Combined Immunofluorescence Labeling in the Extraocular Muscles
To identify the complete innervation and verify the presence of tracer in nerve fibers and terminals the eye muscle sections were stained on-slide for the simultaneous detection of CTB or TMR-DA combined with either mouse anti-synaptosome-associated protein SNAP-25 or mouse anti-synaptophysin (SYN), with double-immunofluorescence (Table 1). After the sections were blocked with 2% NDS in 0.1 M TBS containing 0.3% Triton X-100 for 1 hour, they were processed with a mixture of goat anti-CTB (1:10,000; List Biological Laboratories) or rabbit anti-TMR-DA (1:2000; Invitrogen-Molecular Probes) and either mouse anti-SYN (1:20; DAKO, Glostrup, Denmark) or mouse anti-SNAP-25 (SMI 81; 1:2000; Sternberger Monoclonals Inc., Baltimore, MD) for 48 hours at room temperature. After several buffer washes, a mixture of fluorochrome-tagged secondary antibodies of either Cy3-tagged donkey anti-goat (1:200 for CTB; Jackson ImmunoResearch) or donkey anti-rabbit (1:200 for TMR-DA; Jackson ImmunoResearch) combined with Alexa 488-tagged donkey anti-mouse (1:200; Invitrogen-Molecular Probes) for SYN or SNAP-25 were incubated for 2 hours at room temperature.
Combined immunofluorescence for tyrosine hydroxylase (TH) and CTB was used to specify sympathetic nerve fibers in the eye muscles (Table 1). After immunostaining for CTB, as just described, the sections were incubated in rabbit anti-TH (1:100; Chemicon, Billerica, MA) for 24 hours at room temperature. After the sections were washed, they were treated with donkey anti-rabbit tagged with the fluorescent dye Alexa 488 (1:200; Invitrogen-Molecular Probes) for 2 hours.
All fluorochrome-stained sections were coverslipped with permanent aqueous mounting medium (Gel/Mount; Biomeda, San Francisco, CA) and stored in the dark at 4°C.
Combined Immunoperoxidase Labeling in Extraocular Muscles
In selected sections, the detection of CTB was combined with immunostaining for the slow isoform of myosin heavy chain (mouse anti-myosin heavy chain [slow] [MHCs]; 1:100; Novocastra Laboratories Ltd., Newcastle-upon-Tyne, UK) (Table 1). After the immunostaining for CTB, the antigenic sites were visualized with 0.025% DAB, 0.2% ammonium nickel sulfate (Riedl-De Haën; Hannover, Germany), and 0.015% H2O2 in 0.1 M TBS (pH 7.4) for 10 minutes, to yield a black stain. After residual peroxidase activity was blocked with 1% H2O2 in 0.1 M TBS (pH 7.4) for 30 minutes, the sections were blocked with 2% normal horse serum in 0.1 M TBS (pH 7.4) containing 0.3% Triton X-100 for 1 hour and subsequently processed with mouse anti-MHCs (1:100; Novocastra Laboratories) for 2 days at 4°C. After several buffer washes, the sections were treated with biotinylated horse anti-mouse (1:200; Vector Laboratories; Burlingame, CA) for 1 hour at room temperature. The antigenic sites were visualized with 0.025% DAB and 0.015% H2O2 in 0.1 M TBS (pH 7.4) for 10 minutes, to yield brown staining of the MIFs.
Tracer Injection Sites
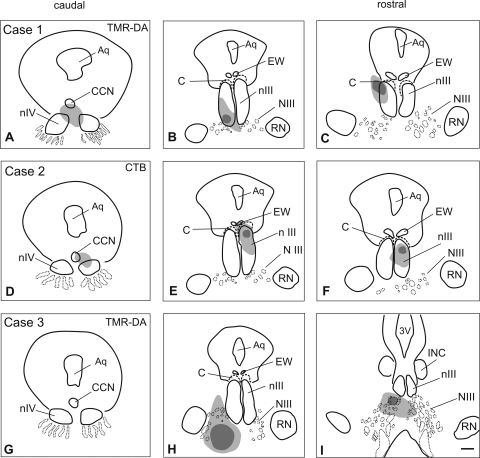
In two cases (cases 1 and 2) the tracer injection site was centered in nIII (Fig. 1; Table 2). In case 1, the injection (TMR-DA) was centered ventrally in the MR A-group of the left nIII (Figs. 1A–C). The tracer uptake area involved the subgroups of the IR, superior rectus (SR), and inferior oblique (IO) muscles in the left nIII as well as the trochlear nucleus (nIV) and the central caudal nucleus (CCN), containing the levator palpebrae (LP) motoneurons38 (Table 2; Figs. 1A–C).
Figure 1.
Reconstructions of transverse brain stem sections (from caudal to rostral) of three monkey cases showing the location (dark gray) of the tracer injections and the extent of the uptake area (light gray). (A–C) Case 1: The Tetramethylrhodamine dextran (TMR-DA) injection involved the oculomotor (nIII) and trochlear nucleus (nIV), but spared the C-group (C). (D–F) Case 2: CTB injection involved nIII, EW and the C-group and, to a minor extent, nIV. (G–I) Case 3: The TMR-DA injection was placed into the oculomotor nerve (NIII), and did not involve the motoneurons in nIII and nIV. Aq, aqueduct; CCN, central caudal nucleus; RN, red nucleus; 3V, third ventricle; INC, interstitial nucleus of Cajal. Scale bar, 500 μm.
Table 2.
Overview of the Tracer Injection Cases, Indicating the Injection Sites and Specifying the Involved Motoneuronal Groups
| Case | Tracer Injection | Tracer Uptake |
|---|---|---|
| 1 | Tetramethylrhodamine dextran injection in the left nIII | MR, IR, IO region in left nIII; SR region right, trochlear nucleus, both sides |
| 2 | Choleratoxin subunit B injection in the right nIII | IR, MR, IO in right nIII; SR region in nIII bilateral, C-group, EW; trochlear nucleus right |
| 3 | Tetramethylrhodamine dextran injection in the left NIII | Nerve fibers mainly left side; also contamination of a few fibers on the right side |
| 4 | WGA-HRP injection in the distal part of IR; choleratoxin subunit B injection in the distal part of the MR | Myotendinous area of injected IR and MR |
In case 2, the tracer injection (CTB) was centered more dorsally in the right nIII involving the subdivisions of the IR, MR, SR, and IO (Figs. 1D–F). Additional uptake was noted in the left nIII covering the SR area, the right nIV, and CCN. Tracer uptake also occurred from the C-group and the preganglionic neurons in the Edinger-Westphal (EW) nucleus, as indicated by the retrograde labeling in the olivary pretectal nucleus.39
In case 3, the injection site (TMR-DA) was placed ventrolateral to nIII, hitting the traversing axons of the third nerve as they leave nIII (Figs. 1G–I; Table 2.). Strongly labeled tracer-filled axons within the fascicles of the third nerve indicated that tracer uptake had occurred from the injured axons of the nerve. Case 3 was the only case that was not analyzed in detail.
Analysis
The slides were examined with one of two microscopes (DMRB; Leica, Bensheim, Germany, or Axioplan; Carl Zeiss MicroImaging, Oberkochen, Germany), equipped with appropriate filters for red fluorescent Cy3 (DMRB: N2.1; excitation filter, BP 515–560 nm; dichromatic mirror, 580 nm; suppression filter, LP 590 nm; Axioplan: excitation filter, BP 546 nm; dichromatic beam splitter, FT 580 nm; barrier filter, LP 590 nm) and green fluorescent Cy2 or Alexa 488 (DMRB: I3: excitation filter, BP 450–490 nm, dichromatic mirror: 510 nm, suppression filter LP 515 nm; Axioplan: excitation filter, BP 475 nm; dichromatic beam splitter, FT 500 nm; barrier filter, LP 530 nm). Micrographs were taken with a digital camera (Pixera Pro 600 ES; Klughammer, Markt Indersdorf, Germany), captured on a computer (Pixera Viewfinder software; Klughammer), and processed in image-analysis software (Photoshop 7.0; Adobe Systems, Mountain View, CA).
Confocal images were obtained on a confocal laser scanning microscope (TCS SP; Leica) with a 40× oil objective (NA 1.4, resolution 200 nm/pixel). The double-immunofluorescence slides with Cy3, Cy2, or Alexa 488 dyes were recorded at a 543- or 488-nm excitation wave length. The sharpness, contrast, and brightness were adjusted to reflect the appearance of the labeling seen through the microscope. The images were arranged and labeled for display (CorelDraw 11.0; Corel, Ottawa, ONT, Canada).
The axonal course of retrogradely labeled motoneurons was reconstructed (Neurolucida software, ver. 6; MicroBrightField, Inc., Williston, VT).
Results
Anterograde Tracer Labeling in Extraocular Muscles: Cases 1, 2, and 3
All tracer injections involving either nIII (cases 1 and 2) or nerve bundles of the oculomotor nerve (NIII) (case 3) revealed distinct anterograde labeling of nerve endings in the respective extraocular muscles, depending on the injection site. Accordingly, in all cases the lateral rectus muscles, whose motoneurons lie in the abducens nucleus at pontomedullary level, were tracer negative.
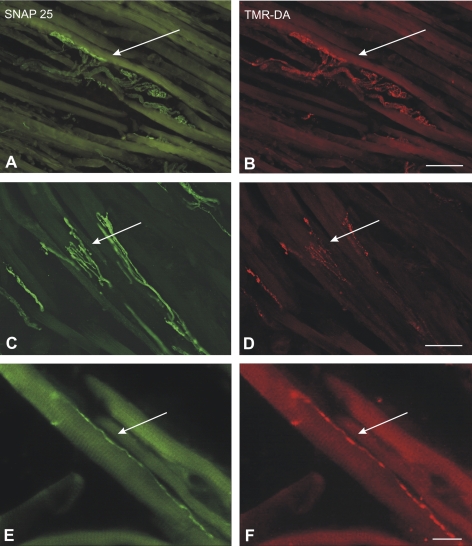
The anterogradely labeled terminals comprised en plaque endings concentrated around the middle third of the muscle fibers in the global and in the orbital layer (Figs. 2A, 2B) and small en grappe endings, which were distributed along single muscle fibers but were concentrated at the distal and proximal ends (Figs. 2E, 2F). In addition, all extraocular muscles contained numerous anterogradely tracer-labeled palisade endings at the myotendinous junctions (Figs. 2C, 2D; 3F, 3G).
Figure 2.
Combined immunofluorescence of the tracer TMR-DA (red) and SNAP-25 (green) identifying en plaque (A, B), en grappe (E, F), and palisade endings (C, D) in the medial rectus muscle of the monkey in case 1. Each pair of neighboring photographs shows the same section with different fluorescence filters. (A, B, E, F) Micrographs were obtained with a fluorescence microscope (DMRB; Leica, Bensheim, Germany) and (C, D) with a confocal microscope (TCS SP; Leica). Scale bar: (A–D) 50 μm; (E, F) 100 μm.
Figure 3.
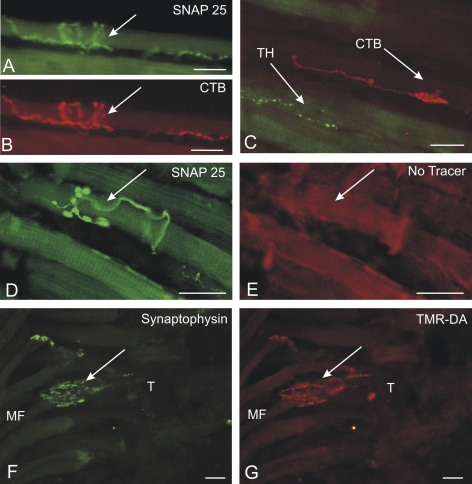
Different nerve endings in the extraocular muscles. (A, B) An identified SNAP 25-positive spiral ending (A; green) was anterogradely labeled with CTB (B; red) after injection into the oculomotor nucleus tracer (case 2). (C) CTB-labeled en plaque endings (red) and TH-positive nerve ending (green), which does not contain tracer (case 2). (D, E) Tracer-negative (D) small nerve ending in the eye muscles stained for SNAP-25 (E) (case 1). (F, G) The synaptic endings of an anterogradely TMR-DA-labeled palisade ending (G; red) are visualized by immunostaining for synaptophysin (F; green) (case 1). (All images obtained with a DMRB fluorescence microscope; Leica, Bensheim, Germany). Scale bar, 20 μm.
The systematic analysis of combined immunostaining for tracer and either SNAP-25, a marker for the complete innervation pattern19 (Figs. 2; 3A, 3B, 3D, 3E), or SYN (Figs. 3F, 3G), a marker for all synaptic endings, permitted an estimation of the percentage of tracer-labeled nerve endings and a judgment of their types (Table 3). Slides from the orbital and global layer of cases 1 and 2 were used for the semiquantitative analysis, It is important to note that this was not a quantitative analysis of the number of nerve terminals. The focus of interest was on the presence or absence of labeling in palisade endings and the accompanying terminals in the global layer
Table 3.
Number of En Plaque, En Grappe, and Palisade Endings in Different Extraocular Muscles of Cases 1 and 2 and Their Tracer Labeling
| Extraocular Muscles | % of Tracer-Positive En Plaque Endings | Counted En Plaque Endings | Tracer-Positive En Plaque Endings | % of Tracer-Positive En Grappe Endings | Counted En Grappe Endings | Tracer-Positive En Grappe Endings | % of Tracer-Positive Palisade Endings | Counted Palisade Endings | Tracer-Positive Palisade Endings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 TMR-DA | MR le | 100 | 2497 | 2487 | 100 | 20 | 20 | 100 | 23 | 23 |
| IR le | 20 | 1610 | 325 | 6 | 18 | 1 | 0 | 3 | 0 | |
| IO le | 76 | 1202 | 910 | 46 | 11 | 5 | 100 | 1 | 1 | |
| SO ri | 88 | 1248 | 1096 | 71 | 51 | 36 | 100 | 6 | 6 | |
| SO le | 90 | 1072 | 968 | 94 | 36 | 34 | 95 | 19 | 18 | |
| SR ri | 86 | 1034 | 878 | 73 | 26 | 19 | 100 | 4 | 4 | |
| SR le | 0 | NC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LR le | 0 | NC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Case 2 CTB | MR ri | 50 | 1765 | 875 | 29 | 14 | 4 | 80 | 15 | 9 |
| IR ri | 18 | 932 | 167 | 13 | 8 | 1 | 0 | 2 | 0 | |
| IO ri | 46 | 811 | 374 | 50 | 6 | 3 | 100 | 2 | 2 | |
| SR le | 16 | 910 | 137 | 5 | 20 | 1 | 33 | 6 | 2 | |
| SR ri | 14 | 1744 | 241 | 8 | 37 | 3 | 25 | 4 | 1 | |
| SO le | 1 | 1106 | 13 | 0 | 67 | 0 | 0 | 4 | 0 | |
| SO ri | 0 | NC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LR ri | 0 | NC | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
In the global and adjacent part of the orbital layer, every 15th slide was counted (except in the MRs, where every 10th slide was counted. The results are therefore not representative of whole muscle counts. NC, not counted. ri, right, le, left.
The TMR-DA injection in case 1 resulted in strong anterograde terminal labeling in the corresponding extraocular muscles (i.e., ipsilateral MR, IR, and IO and contralateral SR; Figs. 1A–C). The detailed analysis revealed that in the left MR almost all (99.6%) en plaque, en grappe, and palisade endings appeared to be tracer labeled (Fig. 2; Table 3). In the ipsilateral IR, 20% were labeled; in the inferior oblique, 75%; and in the contralateral SR, 85%. The strong labeling of all nerve ending types in the SO of both sides (ipsilateral, 91%; contralateral, 87%) was attributed to tracer uptake of the injured trochlear nerve, which was damaged by the injection needle.
In case 2 CTB-positive nerve terminals were found in the ipsilateral IR (18%), MR (50%) and IO (46%), as well as a few in the contralateral SO (1%) and SR of both sides (ipsilateral, 14%; contralateral, 15%; Figs. 1D–F, Table 3). The partial tracer labeling of the complete nerve ending population in the respective eye muscles was attributed to the incomplete coverage of the nIII with partial inclusion of the nIV by the CTB-injection (Figs. 1D–F).
In both cases, it should be pointed out that in all muscles—irrespective of the amount of tracer labeling—all types of nerve endings (en plaque, en grappe, and palisade) were labeled. In addition every muscle contained a few tracer-positive spiral endings around single muscle fibers (Figs. 3A, 3B).
The TMR-DA injection in case 3 (Figs. 1G–I), which involved only the oculomotor nerve revealed a similar pattern of anterograde labeling in the ipsilateral IR, MR, and SR, as already described. A fraction of all nerve ending types including palisade endings were tracer labeled.
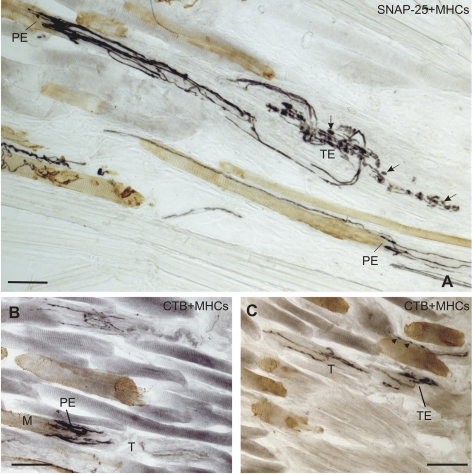
A surprising result was the discovery of several anterogradely labeled neurotendinous ending complexes, which correspond to the tendon organs described by Ruskell40 in the myotendinous junction of MR, in both the CTB and the TMR-DA tracer cases (cases 1 and 2), as well as in the SO of the TMR-DA tracer case (Fig. 4C, case 1). They were often seen associated with a tracer-labeled palisade ending (Fig. 4B), but evidence for a definite neural continuity between a tendon organ and a palisade ending was not visible in these tracer sections. However, evidence of a neural continuity between tendon organs and palisade endings was clearly shown in a series of sections of the SO stained for SNAP-25 (to visualize the nerve terminals) and the slow isoform of myosin heavy chain (MHCs) to identify the MIFs (Fig. 4A).41
Figure 4.
Bright-field immunoperoxidase staining for MHCs (brown) combined with either SNAP-25 (black) or CTB (black). (A) A SNAP-25-positive palisade ending (PE) (black) contacts an MHCs-positive MIF in the top left corner; its axon is seen to be continuous with a tendon ending (TE) in the superior oblique tendon (T). (B, C) CTB labeled palisade endings (black) and labeled tendon ending (black) in the MR muscle, after tracer injection. (case 2). (All images obtained with a DMRB microscope; Leica, Bensheim, Germany). Scale bar, 50 μm.
Tracer-Negative Nerve Endings in Extraocular Muscles
In all central injection in cases 1, 2, and 3, two distinct nerve fiber types remained tracer negative and were visualized only by SNAP-25 immunoreactivity. One group gave rise to very small terminals, which innervated one muscle fiber via a single contact. These terminals were found throughout the muscle, with an incidence of two to three per section (Figs. 3D, 3E).
Another tracer-negative type represents fine fibers with small endings resembling that of en grappe, but meandering across several muscle fibers and often associated with blood vessels. Combined immunostaining for TH and the tracer indicated that they were sympathetic nerve fibers (Fig. 3C).
Analysis of the Trigeminal Ganglia and the Mesencephalic Trigeminal Nucleus
The systematic analysis of the trigeminal ganglia (TG) and the mesencephalic trigeminal nucleus (MesV) of both sides in cases 1 and 2 did not reveal any tracer-labeled cell bodies or terminals.
Course of Axons Arising from Motoneurons in the C-Group: Case 4
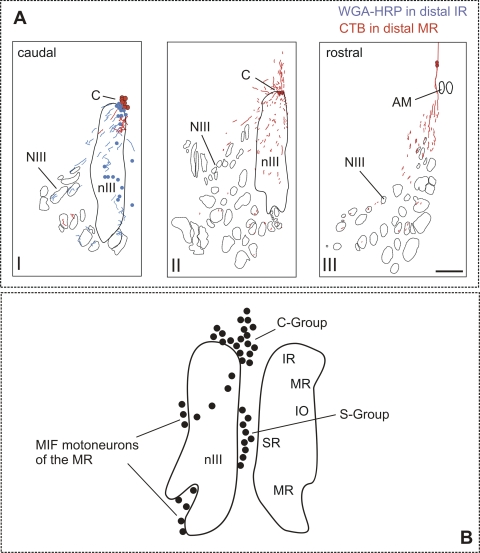
Injections were made into the nIII-labeled axons, passing through the nucleus, as well as the motoneurons. To investigate how much and which terminal labeling in the eye muscles in cases 1, 2 and 3 could be attributed to axons passing through nIII, retrograde tracer substances were injected into in the distal muscle ends of the MR (CTB) and IR (WGA-HRP) in one monkey (case 4). Retrogradely filled cells were located only in the peripheral oculomotor C-group and not in the classic MR (A and B groups) and IR motoneuron subgroups within nIII (Fig. 5). The course of the C-group axons in nIII is plotted in Figure 5A. They took a ventrolateral course through nIII thereby traversing the other motoneuron subgroups before leaving nIII (Fig. 5A). These axons are involved in the oculomotor injections and contribute to the terminal labeling seen in cases 1, 2 and 3.
Figure 5.
(A) Reconstruction of caudorostrally arranged transverse brain stem sections demonstrating motoneurons (dots) in the C-group (C) that have been tracer-labeled by injections into the myotendinous junction of the medial rectus (MR; red) and inferior rectus (IR, blue). The respective tracer-labeled axons of both populations travel through the oculomotor nucleus (nIII) and would be labeled by injections into nIII. Scale bar, 500 μm. NIII, oculomotor nerve; AM, anteromedian nucleus. (B) A summary diagram of the total population of neurons around and within the oculomotor nucleus (nIII) after injection into the myotendinous junction of all eye muscles, including the C-group and S-group.21 Present results suggest that they contain both MIF-motoneurons and palisade ending somata.
Discussion
Central tracer injections into the midbrain targeting nIII, nIV, or the oculomotor nerve, led to anterograde labeling of classic motor endings in the eye muscles, en plaque and en grappe terminals, and spiral endings. Furthermore, the palisade endings and nerve terminals within the tendon at the distal myotendinous junction were tracer positive. The results support the conclusion that the cell bodies and traversing axons of all these structures were involved in the injection uptake areas.
The Different Types of Labeled Terminals in the Eye Muscles
The extent of the labeling of en plaque endings of SIFs in each eye muscle depended on the involvement of the motoneuronal subgroups in nIII in the tracer uptake area. Only the relatively small uptake of tracer in the trochlear nucleus of case 1 did not correlate with the high number of labeled terminals in the SO muscles, and the cause remains unclear. A possible explanation could be a polyneuronal innervation of the muscle fibers as described in cat inferior oblique muscle.42
Hence, the en grappe terminals on the MIFs (which form approximately 10%–20% of the muscle fibers) were also labeled, even though the motoneurons thought to supply them in the peripheral C and S groups were not within the uptake area (see case 1). Injections of retrograde tracers into the myotendinous junctions of MR and IR muscle targeted the en grappe innervated tips of the MIFs (case 4) and retrogradely labeled only the peripheral C-group and their axons, which traveled ventrally through nIII before joining the oculomotor nerve (Fig. 5A, case 4). Therefore, the labeling of the en grappe terminals in case 1 could be explained by tracer uptake from their axons passing through nIII. Table 3 shows the number of tracer-positive en grappe terminals counted.
Slides from the orbital and global layer of cases 1 and 2 were used for the semiquantitative analysis. As mentioned earlier, it is important to note that this is not a quantitative analysis of the number of nerve terminals: The focus of interest was on the presence or absence of labeling in palisade endings and the accompanying terminals in the global layer. Therefore, the number of counted en grappe endings in Table 3 does not reflect the true proportion of en grappe to en plaque found in eye muscles. The data in Table 3 must be interpreted with this in mind.
The major finding of this study is the anterograde tracer labeling of palisade endings and neurotendinous terminals by injection into nIII.
Location of the Palisade Ending Cell Bodies in the C-Group
A central brain stem location of the cell bodies of palisade endings was proposed in 1910 by Tozer and Sherrington,30 who observed degenerated motor endplates and palisade endings after transecting the third, fourth, and sixth cranial nerves in monkeys. Similar observations were made later by Sas and Scháb31 after lesions in the area of the eye muscle motor nuclei in the cat. Our data support these findings, rather than those of more recent studies, which suggest that the palisade ending somata lie in the trigeminal ganglion. In one study, tracer injections into the trigeminal ganglion of cat anterogradely labeled palisade endings, but not after tracer injections into the extraocular motor nuclei.36 The reason for the discrepancy between findings in this study and our results remains unclear.
It is well established by previous studies that tracer injections into the myotendinous junction of extraocular muscle—the location of palisade endings and tips of MIFs—lead to retrogradely labeled neurons around the periphery of the classic motor nuclei within the brain stem, in the C and S groups, the at dorsal cap of nIV, and in the periphery of nVI, but not within the classic motoneurons.27 A few retrogradely filled neurons were found in nIII, but they tended to lie between and around the subgroups, but did not intermingle with the classic motoneurons.27 More specifically, injections into the MR and IR labeled the C-group (as in case 4), but IO and SR injections labeled the S-group. So far, these peripheral cell groups have generally been regarded as the motoneurons supplying the MIFs via multiple en grappe endings, since these fibers enter more deeply into the myotendinous junction than the twitch muscle fibers.27,43 Büttner-Ennever et al.27 also considered the palisade endings innervating the tips of MIFs as a second possible source, but the muscle injection experiments could not distinguish between the different populations supplying palisade endings and MIF endplates. We conclude on the basis of our anterograde tracer labeling that the palisade ending cells of origin must lie within the peripheral cell groups of nIII, including the C and S groups. All the evidence taken together makes it clear that the C and S groups innervate MIFs via endplates and palisade endings, but it is not clear whether the peripheral C and S groups are a homogeneous population. On the basis of the analysis of cell diameters, it seems likely that there are different populations of cells within the peripheral C and S groups,27 but these remain to be identified.
Other Cell Groups Labeled by Muscle Injections: MesV, TG
Previous studies, in sheep44 and cat,36,45 but also in macaque monkeys, have shown that, after tracer injections into extraocular muscles, retrogradely labeled neurons are found not only within the eye muscle motor nuclei,27,46 but also in the mesencephalic trigeminal nucleus, to various degrees in different species.44,45,47 Neurons in the MesV have been thought to supply the sensory innervation of the few muscle spindles present in the monkey,47 but they can be ruled out as a source of somata for palisade endings on account of their transmitter, which is not acetylcholine(Lienbacher K, unpublished observations, 2008). 48 Palisade endings are known to be cholinergic.32,33 In other studies of tracer injections in the eye muscles, retrogradely labeled neurons were found in the trigeminal ganglia which expressed different histochemical properties (e.g., substance P or parvalbumin).47,49,50 These groups of trigeminal ganglia cells supplying the eye muscles may subserve different functions involving pain transmission.50 Trigeminal neurons with a central course of axons near the oculomotor nucleus were not seen, but could also be ruled out as source of palisade endings by the lack of tracer-labeled neurons in the trigeminal ganglia and MesV in the present study.
Muscle Spindles
In the present study, we did not see any muscle spindles in the extraocular muscles in the monkey (Macaca mulatta). This result is in agreement with those in other studies of nonhuman primates.18 A small number may have escaped detection, since only a subset of muscle sections, mainly of the global layer, were systematically analyzed, and detection of muscle spindles in the longitudinal plane is more difficult than in transverse sections. The classic sensory receptors of skeletal muscles such as spindles and Golgi tendon organs are reported to be also present in the extraocular muscles, but these endings differ considerably in their morphology and in their quantity, depending on the species.18,37,51 Numerous well-developed muscle spindles have been found in the orbital layer of human eye muscles,52–54 in contrast to macaque monkey extraocular muscles, in which they are described as few and poorly developed.18,55,56
Motor Terminals of Palisade Endings
Ever since the description of palisade endings by Dogiel,57 there has been evidence that the axons giving rise to them form additional multiple contacts along the muscle fiber. Richmond et al.25 confirmed the finding in humans, and recently it was shown in monkeys that only about one third of palisade endings have any contact at all to MIFs; and in this one third, only 10% of the palisade ending terminals had motor properties. More specifically, they bound α-bungarotoxin.33 The ultrastructural profile showed that these synapses have no basal lamina in the synaptic junction and thus resemble the motor terminals on intrafusal fibers, not those of en plaque terminals on SIFs.34,52,58,59 Most palisade terminals do not contact muscle fibers, but terminate among the collagen bundles of the tendon. These properties of palisade endings do not permit the conclusion that they are simply motor.
Neurotendinous Terminals
Dogiel57 showed branches from the palisade ending axons forming elaborate terminals in the tendon, often extending deep into the tendon and far from the palisade terminal itself. We have found that these are particularly well developed in the superior oblique muscle, not studied by Dogiel or Ruskell40,57 (Fig. 4A). In our sections, clear groups of similar tendon endings were anterogradely labeled in the MR in cases 1 and 2. Unlike the classic Golgi tendon organs described by Ruskell,40 the labeled neurotendinous ending complexes were not encapsulated and did not form neuromuscular junctions (Figs. 4A, 4C). Our results emphasize the unity of tendinous terminals with palisade endings, showing not only that they can be part of one structure, but also that both are derived from cell bodies lying around the periphery of the nIII.
Unlabeled Terminals in the Eye Muscles
One nerve fiber type lacking tracer labeling was TH positive and therefore was interpreted as postganglionic noradrenergic sympathetic fibers, often encircling small blood vessels.60 This finding is in accordance with the fact that TH-positive sympathetic nerves do not originate from nIII, but join the extraocular motor nerves only in the orbit on their way from the superior cervical ganglion to supply the arteries to the eye muscles and control the regulation of blood flow to the muscles.61,62 The other tracer-negative type of small nerve endings with only a single contact per muscle fiber may be nociceptive afferents originating from the trigeminal ganglion.49,50 Whether these nerve endings correspond to the compact or complex endings seen in the cat after trigeminal ganglion tracer injections is not clear, since the morphology in the monkey differs.36
Functional Considerations
Palisade endings are unique to eye muscles. Their location, histochemistry, fine structure, and connectivity have been well studied, but their function is unknown.18,26 Recent studies by Wang and May47 have rekindled interest in the presence of proprioception in eye muscles, and as a result, palisade endings, which are a constant feature of eye muscles, have been often suggested as possible sensory receptors.18,26 Some of their properties are typical of sensory endings, such as the terminals in the collagen tendon and the lack of a basal lamina at synaptic junctions with muscle.5,34,52,53 Other properties of palisade endings are typical of motor structures such as their cholinergic transmitter, the location of the cell bodies in the C-group, and the 10% of terminals in the myotendinous junction that bind α-bungarotoxin. One possibility is that we are looking at two relatively independent populations in the C and S groups—one motor and one sensory; or perhaps as Lukas et al.24 and Blumer et al.33 suggest, palisade endings may have a sensory–motor function.
A similar variety of sensory and motor terminals are found in muscle spindles, on intrafusal fibers.63,64 Indeed the MIFs of the eye muscles have several features in common with intrafusal fibers, except for their giant size. They have a similar histochemical and ultrastructural profile and receive both an en grappe motor innervation.64,65 Another similarity is that in sheep, where muscle spindles in eye muscles are well developed, the intrafusal nuclear-bag muscle fibers can be branches of orbital MIFs. Furthermore, the innervation of the intrafusal nuclear bag fibers can arise from nerve branches of axons supplying the orbital MIFs.64 The casual suggestion by David A. Robinson that “the unit built by 'palisade endings plus MIFs resembles a giant inverted muscle spindle”66 no longer seems outrageous. The neurons innervating the intrafusal muscle fibers modulate the sensitivity of muscle spindles to stretch and are called gamma motoneurons.67,68 It seems likely that the peripheral cell groups around nIII (Fig. 5), nIV, and nVI described in an earlier study69 contain two or more different populations. One may innervate the MIFs and perform a gamma motoneuron function, and another population may innervate the palisade endings. If palisade endings are sensory, then the peripheral cell groups around nIII, including the adjacent EW nucleus, would provide the ideal situation for interaction with the near response (i.e., convergence, lens accommodation, and pupillary constriction).
In conclusion, the present study demonstrates that palisade endings, along with their tendon endings and endplates on MIFs, may arise from neurons around the periphery of the motor nuclei of extraocular muscles, such as the C-group, but the role of palisade endings in eye muscle control remains unclear.
Acknowledgments
The authors thank Sergei Yakushin (Mount Sinai Hospital, New York, NY) for generous supply of primate tissue, Ahmed Messoudi for excellent technical assistance and Olga Alexandrova (Faculty of Biology, Department II, Neurobiology, Ludwig-Maximilian University, Munich) for help with the confocal microscope.
Note Added in Proof
Since the submission of this article, a study with central tracer injections into the abducens nucleus of a macaque monkey has been published, which demonstrates anterograde labeling of palisade endings in the lateral rectus muscle.70
Footnotes
Supported by Deutsche Forschungsgemeinschaft Grant DFG HO 1639/4-3 and National Institutes of Health (NIH) Grants EY013308, EY020744, and RR00166, as well as NIH EY019347 and Research to Prevent Blindness core grants.
Disclosure: K. Lienbacher, None; M. Mustari, None; H.S. Ying, None; J.A. Büttner-Ennever, None; A.K.E. Horn, None
References
- 1. McLoon LK, Wirtschafter JD. N-CAM is expressed in mature extraocular muscles in a pattern conserved among three species. Invest Ophthalmol Vis Sci. 1996;37:318–327 [PubMed] [Google Scholar]
- 2. Wieczorek DF, Periasamy M, Butler Browne GS, Whalen RG, Nadal Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischer MD, Corospe JR, Felder E, et al. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol Genom. 2002;9:71–84 [DOI] [PubMed] [Google Scholar]
- 4. Horton RM, Manfredi AA, Conti-Tronconi BM. The “embryonic” gamma subunit of the nicotinic acetylcholine receptor is expressed in adult extraocular muscle. Neurology. 1993;43:983–986 [DOI] [PubMed] [Google Scholar]
- 5. Demer JL, Yeul Oh S, Poukens V. Evidence for active control of rectus muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290 [PubMed] [Google Scholar]
- 6. Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80 [DOI] [PubMed] [Google Scholar]
- 7. Kjellgren D, Thornell L-E, Andersen J, Pedrosa-Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425 [DOI] [PubMed] [Google Scholar]
- 8. Lennerstrand G. Electrical activity and isometric tension in motor units of the cat's inferior oblique muscle. Acta Physiol Scand. 1974;91:458–474 [DOI] [PubMed] [Google Scholar]
- 9. Chiarandini DJ, Stefani E. Electrophysiological identification of two types of fibres in rat extraocular muscles. J Physiol. 1979;290:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan DL, Proske U. Vertebrate slow muscle: its structure, pattern of innervation, and mechanical properties. Physiol Rev. 1984;64:103–138 [DOI] [PubMed] [Google Scholar]
- 11. Shall MS, Goldberg SJ. Extraocular motor units: type classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res. 1992;587:291–300 [DOI] [PubMed] [Google Scholar]
- 12. Pachter BR. Rat extraocular muscle: 3, histochemical variability along the length of multiply-innervated fibers of the orbital surface layer. Histochem. 1984;80:535–538 [PubMed] [Google Scholar]
- 13. Davidowitz J, Chiarandini DJ, Philips G, Breinin GM. Morphological variation along multiply innervated fibers of rat extraocular muscles. In: Lennerstrand G, Zee DS, Keller EL. eds. Functional Basis of Ocular Motility Disorders.Oxford, UK: Pergamon Press; 1982:17–26 [Google Scholar]
- 14. Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol. 1989;61:116–125 [DOI] [PubMed] [Google Scholar]
- 15. Lynch GS, Frueh BR, Williams DA. Contractile properties of single skinned fibres from the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol. 1994;475:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer RF, Porter JD. Structural organization of the extraocular muscles. In: Büttner-Ennever JA. ed. Neuroanatomy of the Oculomotor System. Amsterdam: Elsevier; 1988:33–79 [PubMed] [Google Scholar]
- 17. Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles in rhesus monkey. J Neurocytol. 1978;7:693–708 [DOI] [PubMed] [Google Scholar]
- 18. Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999;18:269–291 [DOI] [PubMed] [Google Scholar]
- 19. Eberhorn AC, Horn AKE, Eberhorn N, et al. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J Anat. 2005;205:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumer R, Wasicky R, Hötzenecker W, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp Eye Res. 2001;73:787–796 [DOI] [PubMed] [Google Scholar]
- 21. Alvarado-Mallart RM, Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–584 [DOI] [PubMed] [Google Scholar]
- 22. Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci Lett. 1998;248:49–52 [DOI] [PubMed] [Google Scholar]
- 23. Rungaldier S, Pomikal C, Streicher J, Blumer R. Palisade endings are present in canine extraocular muscles and have a cholinergic phenotype. Neurosci Lett. 2009;465:199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lukas JR, Blumer R, Denk M, Baumgartner I, Neuhuber W, Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000;41:2422–2431 [PubMed] [Google Scholar]
- 25. Richmond FJR, Johnston WSW, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscle. Invest Ophthalmol Vis Sci. 1984;25:471–476 [PubMed] [Google Scholar]
- 26. Donaldson IML. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond Biol. 2000;355:1685–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Büttner-Ennever JA, Horn AKE, Scherberger H, D'Ascanio P. Motoneurons of twitch and non-twitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438:318–335 [DOI] [PubMed] [Google Scholar]
- 28. Eberhorn AC, Ardelenanu P, Büttner-Ennever JA, Horn AKE. Histochemical differences between motoneurons supplying multiply and singly innervated extraocular muscle fibers. J Comp Neurol. 2005;491:352–366 [DOI] [PubMed] [Google Scholar]
- 29. Eberhorn AC, Büttner-Ennever JA, Horn AKE. Identification of motoneurons innervating multiply- or singly-innervated extraocular muscle fibres in the rat. Neurosci. 2006;137:891–903 [DOI] [PubMed] [Google Scholar]
- 30. Tozer FM, Sherrington CS. Receptors and afferents of the third, fourth and sixth cranial nerves. Proc R Soc London Ser. 1910;82:451–457 [Google Scholar]
- 31. Sas J, Scháb R. Die sogenannten “Palisaden-Endigungen” der Augenmuskeln. Acta Morph Acad Sci (Hungary). 1952;2:259–266 [Google Scholar]
- 32. Konakci KZ, Streicher J, Hoetzenecker W, et al. Palisade endings in extraocular muscles of the monkey are immunoreactive for choline acetyltransferase and vesicular acetylcholine transporter. Invest Ophthalmol Vis Sci. 2005;46:4548–4554 [DOI] [PubMed] [Google Scholar]
- 33. Blumer R, Konakci KZ, Pomikal C, Wieczorek G, Lukas JR, Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009;50:1176–1186 [DOI] [PubMed] [Google Scholar]
- 34. Konakci KZ, Streicher J, Hoetzenecker W, Blumer MJF, Lukas J-R, Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci. 2005;46:155–165 [DOI] [PubMed] [Google Scholar]
- 35. Blumer R, Konakci KZ, Streicher J. Proprioception in the extraocular muscles of mammals and man. Strabismus. 2006;14:101–106 [DOI] [PubMed] [Google Scholar]
- 36. Billig I, Buisseret-Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat Rec. 1997;248:566–575 [DOI] [PubMed] [Google Scholar]
- 37. Büttner-Ennever JA, Konakci KZ, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2006;151:81–93 [DOI] [PubMed] [Google Scholar]
- 38. Porter JD, Burns LA, May PJ. Morphological substrate for eyelid movements: innervation and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol. 1989;287:64–81 [DOI] [PubMed] [Google Scholar]
- 39. Büttner-Ennever JA, Cohen B, Horn AKE, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359 [DOI] [PubMed] [Google Scholar]
- 40. Ruskell GL. The incidence and variety of Golgi tendon organs in extraocular muscles of the rhesus monkey. J Neurocytol. 1979;8:639–653 [DOI] [PubMed] [Google Scholar]
- 41. Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci. 2000;41:3391–3398 [PubMed] [Google Scholar]
- 42. Dimitrova DM, Allman BL, Shall MS, Goldberg SJ. Polyneuronal innervation of single muscle fibers in cat eye muscle: inferior oblique. J Neurophysiol. 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mayr R, Gottschall J, Gruber H, Neuhuber W. Internal structure of cat extraocular muscle. Anat Embryol. 1975;148:25–34 [DOI] [PubMed] [Google Scholar]
- 44. Bortolami R, Lucchi ML, Pettorossi VE, Callegari E, Manni E. Localization and somatotopy of sensory cells innervating the extraocular muscles of lamb, pig and cat: histochemical and electrophysiological investigation. Arch Ital Biol. 1987;125:1–15 [PubMed] [Google Scholar]
- 45. Alvarado-Mallart MR, Batini C, Buisseret-Delmas C, Corvisier J. Trigeminal representations of the masticatory and extraocular proprioceptors as revealed by horseradish peroxidase retrograde transport. Exp Brain Res. 1975;23:167–179 [DOI] [PubMed] [Google Scholar]
- 46. Spencer RF, Porter JD. Innervation and structure of extraocular muscles in the monkey in comparison to those of the cat. J Comp Neurol. 1981;198:649–665 [DOI] [PubMed] [Google Scholar]
- 47. Wang N, May PJ. Peripheral muscle targets and central projections of the mesencephalic trigeminal nucleus in macaque monkeys. J Comp Neurol. 2008;291:974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59 [DOI] [PubMed] [Google Scholar]
- 49. Porter JD, Donaldson IML. The anatomical substrate for cat extraocular muscle proprioception. Neurosci. 1991;43:473–481 [DOI] [PubMed] [Google Scholar]
- 50. Fackelmann K, Nouriani A, Horn AK, Büttner-Ennever JA. Histochemical characterisation of trigeminal neurons that innervate monkey extraocular muscles. Prog Brain Res. 2008;171:17–20 [DOI] [PubMed] [Google Scholar]
- 51. Cooper S, Daniel PM, Whitteridge D. Muscle spindles and other sensory endings in the extrinsic eye muscles; the physiology and anatomy of these receptors and of their connexions with the brain-stem. Brain. 1955;78:564–583 [DOI] [PubMed] [Google Scholar]
- 52. Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989;167:199–214 [PMC free article] [PubMed] [Google Scholar]
- 53. Blumer R, Lukas JR, Aigner M, Bittner R, Baumgartner I, Mayr M. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci 1999;40:55–64 [PubMed] [Google Scholar]
- 54. Wicke W, Wasicky R, Brugger PC, Kaminski S, Lukas JR. Histochemical and immunohistochemical study on muscle fibers in human extraocular muscle spindles. Exp Eye Res. 2007;84:670–679 [DOI] [PubMed] [Google Scholar]
- 55. Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974;143:397–408 [DOI] [PubMed] [Google Scholar]
- 56. Greene T, Jampel R. Muscle spindles in the extraocular muscles of the Macaque. J Comp Neurol. 1966;126:547–550 [PubMed] [Google Scholar]
- 57. Dogiel AS. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Säugetieren. Arch Mikroskop Anat. 1906;68:501–526 [Google Scholar]
- 58. Kubota M. Ultrastructural observations on muscle spindles in extraocular muscles of pig. Anat Anz. 1988;165:205–228 [PubMed] [Google Scholar]
- 59. Blumer R, Konakci KZ, Brugger PC, et al. Muscle spindles and Golgi tendon organs in bovine calf extraocular muscle studied by means of double-fluorescent labeling, electron microscopy, and three-dimensional reconstruction. Exp Eye Res. 2003;77:447–462 [DOI] [PubMed] [Google Scholar]
- 60. Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967;6:773–776 [DOI] [PubMed] [Google Scholar]
- 61. Thakker MM, Huang J, Possin DE, et al. Human orbital sympathetic nerve pathways. Ophthalmic Plast Reconstr Surg. 2008;24:360–366 [DOI] [PubMed] [Google Scholar]
- 62. Hayakawa T, Itoh M, Miki T, Kaneto T, Tomiyama H, Takeuchi Y. Sympathetic fibers innervating the extraocular muscles: Cells of origin in the cat superior cervical ganglion. Okajimas Folia Anat Jpn. 2000;77:119–124 [DOI] [PubMed] [Google Scholar]
- 63. Harker DW. The structure and innervation of sheep superior rectus and levator palpebrae extraocular eye muscles: II, muscle spindles. Invest Ophthalmol Vis Sci. 1972;11:970–979 [PubMed] [Google Scholar]
- 64. Barker D. The morphology of muscle receptors. In: Barker D, Hunt CC, McIntyre AK. eds. Muscle receptors. Berlin: Springer-Verlag; 1974:1–190 [Google Scholar]
- 65. Pedrosa-Domellof F, Soukup T, Thornell LE. Rat muscle spindle immunocytochemistry revisited. Histochemistry. 1991;96:327–338 [DOI] [PubMed] [Google Scholar]
- 66. Robinson DA. Overview. In: Carpenter RHS. ed. Eye movements: Vol. XIII. Vision and visual dysfunction. London: Macmillan; 1991:320–331 [Google Scholar]
- 67. Burke R. Spinal cord: ventral horn. In: Shepherd GM. ed. The synaptic organization of the brain. New York: Oxford University Press; 2004:79–123 [Google Scholar]
- 68. Kuffler SW, Hunt CC, Quilliam JP. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951;14:29–54 [DOI] [PubMed] [Google Scholar]
- 69. Büttner-Ennever JA. The extraocular motor nuclei: organization and functional neuroanatomy. Prog Brain Res. 2006;151:95–125 [DOI] [PubMed] [Google Scholar]
- 70. Zimmermann L, May P, Pastor AM, Streicher J, Blumer R. Evidence that the extraocular motor nuclei innervate monkey palisade endings. Neurosci Lett. 2011;489:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]