This study provides the first evidence that corneal inflammatory lymphangiogenesis has a critical time window for intervention therapy. A combined blockade of VEGFR-2 and VEGFR-3 suppresses both early- and middle-stage lymphangiogenesis.
Abstract
Purpose.
Lymphangiogenesis (LG) accompanies many corneal diseases after inflammatory, infectious, or chemical insults and is a primary mediator of transplant rejection. The purpose of this study was to investigate whether there is a time window for therapeutic intervention of corneal LG and whether a combined blockade of VEGFR-2 and VEGFR-3 effectively suppresses early-, middle-, or late-stage LG.
Methods.
Corneal inflammatory neovascularization was induced by a standard suture placement model in mice. Neutralizing antibodies against VEGFR-3 and/or VEGFR-2 were administrated systemically with the treatment started at postoperative day 0, day 7, or day 14. Whole mount corneas were sampled for immunofluorescence microscopic studies using LYVE-1 (a lymphatic marker) antibodies. Digital images were analyzed by software.
Results.
Both VEGFR-3 and VEGFR-2 were involved in corneal suture-induced inflammatory LG. Their combined blockade led to a significant inhibition of both early- and middle-stage LG while demonstrating no effect on late-stage LG.
Conclusions.
Corneal inflammatory LG has a discrete time window for intervention therapy. Although it is important to start the treatment as soon as possible, interventions initiated in the middle of the LG process are still effective. These novel findings will shed some light on our understanding of inflammatory LG and the development of new therapeutic protocols for LG-related diseases at different stages.
Lymphatic research has experienced exponential growth in recent years largely because of the advancement of technologies and the discoveries of several lymphatic endothelial specific molecules, including lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1), Prox-1, and VEGFR-3. The lymphatic network penetrates most tissues in the body, and its dysfunction has been found in a broad spectrum of disorders, such as cancer metastasis, inflammation, transplant rejection, obesity, and hypertension.1–6 To date, there is still little effective treatment for lymphatic diseases; it is, therefore, a field with an urgent demand for new therapeutic protocols.
Given its accessible location and transparent nature, the cornea provides an optimal site for lymphatic research. Although the normal cornea does not have lymphatic vessels, lymphangiogenesis (LG; the development of new lymphatic vessels) can be induced in this tissue after inflammatory, traumatic, chemical, or infectious damage.7–9 LG also constitutes the afferent arm of the immune reflex arc of corneal transplantation immunity,7,9,10 and, most recently, it has been demonstrated that LG is a primary mediator of corneal transplant rejection.11
The VEGF family is the backbone of a complex network controlling blood and lymphatic vessel processes. A number of previous studies have shown that VEGFR-3 mediates LG in the cornea and other tissues, and its inhibition suppresses transplant rejection, tumor growth, and metastasis.11–17 The specific role of VEGFR-2 in LG, however, has been much less studied. It is known that this factor is critically involved in hemangiogenesis (HG; the development of new blood vessels) and that its inhibition suppresses HG and tumor growth.18–21 Most recently, it has been indicated that VEGFR-2 also plays a role in corneal LG,12,22,23 though the underlying mechanisms still remain largely unknown.
Although results from previous corneal studies on LG are promising, there is still a large knowledge gap when translating these results to clinic settings. For example, almost all the previous studies focused on how to prevent LG by testing an intervention started right before, or on the same day as, a pathologic stimulation, which happens only infrequently in patients. Indeed, many patients who need LG treatment miss their first chance to receive emergent care, possibly because of difficult living conditions, transportation problems, or insufficient knowledge about the disease process itself. Indeed, LG is a progressive event that can occur insidiously at its early phases.
In this study, we first demonstrate that VEGFR-2 is involved in suture-induced inflammatory LG but to a lesser extent than VEGFR-3. More important, we reveal that there is a discrete time window for therapeutic intervention of corneal inflammatory LG. We show that a combined blockade of VEGFR-2 and VEGFR-3 suppresses both early- and middle-stage LG while demonstrating no effect on late-stage LG. Close correlation between the timing and outcomes of the treatment is also defined. It is hoped that our results will provide a useful guide for understanding of the LG processes and the development of novel therapeutic protocols for LG-related diseases at various stages.
Materials and Methods
Animals
Six- to 8-week-old male BALB/c mice (Taconic Farms, Germantown, NY) were used for these experiments. All mice were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee of the University of California at Berkeley. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure.
Corneal Inflammatory Neovascularization and Pharmaceutical Interventions
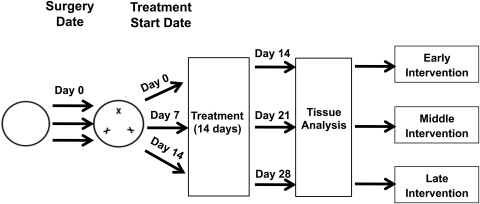
The standard suture placement model was used to induce inflammatory LG and HG, as described previously.24–26 Briefly, three 11–0 nylon sutures (AROSurgical, Newport Beach, CA) were placed into the stroma of the central corneas without penetrating to the anterior chamber. Mice were randomized to receive systemic administration of neutralizing antibodies of VEGFR-2 (800 μg; DC101) and/or VEGFR-3 (700 μg; mF4–31C1; kindly provided by ImClone Systems Incorporated, Eli Lilly and Company, New York, NY), or their isotype controls twice a week for 2 weeks, as illustrated in Figure 1. The experiments were repeated at least twice with a total of 10 mice in each group of the study.
Figure 1.
Schematic illustration of the experimental procedures. Briefly, three sutures were placed in the cornea, and mice were randomized to receive systemic administrations of the VEGFR-2 and/or VEGFR-3 neutralizing antibodies or their isotype controls twice a week for 2 weeks. For early, middle, and late intervention strategies, the treatment was started at postoperative day 0, 7, or 14, respectively. Corneas were sampled by the end of the treatment for immunofluorescence microscopic assays.
Immunofluorescence Microscopic Assay
The experiments were performed according to the standard protocol.24,26–28 Briefly, freshly excised corneas were fixed in acetone for immunofluorescent staining. Nonspecific staining was blocked with donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The samples were stained with purified rabbit anti-mouse LYVE-1 (Abcam, Cambridge, MA) overnight, visualized by a Cy3-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc.). Samples were then covered with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA) and were examined with an epifluorescence microscope (AxioImager M1; Carl Zeiss AG, Göttingen, Germany), and digital images were taken (AxioVision version 4.8; Carl Zeiss AG).
Vascular Quantification
LG was graded using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), as described previously.26,28 Basically, the LG area was normalized to the total corneal area to obtain a percentage coverage score for each sample. The LG area refers to the fraction of the corneal area in which vessels were present, and the total corneal area was measured by outlining the innermost vessels of the limbal arcade. Additionally, corneal HG was examined by a slit lamp with an integrated digital camera system (SL-D4 and DC-3; Topcon Medical Systems, Tokyo, Japan) and was graded according to the standard protocol, as described previously.27,29 Briefly, quantification was based on two primary parameters. One was the circumferential extent of 12 areas around the clock. A score of 1 was given to each area if the vessels were present in the sector. The other was the centripetal growth of the longest vascular frond in each area. A grade between 0 (no growth) and 2 (at suture site) was given to each area. Scores for each area were then summed to derive the final index (range, 0–24).
Statistical Analysis
Data are expressed as the mean ± SEM. The statistical significance of the difference between each group (n = 10) was evaluated using the Mann-Whitney U test, and the comparison between groups was analyzed by one-way ANOVA test statistical analysis software (Prism; GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered significant.
Results
Both VEGFR-3 and VEGFR-2 Are Involved in Corneal Inflammatory LG
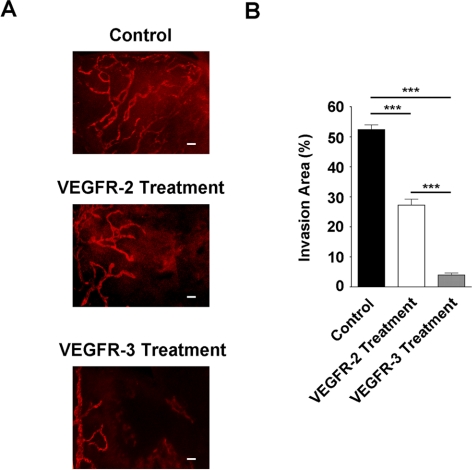
We first set out to study whether VEGFR-2 is involved in suture-induced inflammatory LG in the cornea using VEGFR-3 as a reference control. To approach this, mice after suture placement (day 0) were randomized to receive systemic administration of VEGFR-2 or VEGFR-3 neutralizing antibodies or their isotype control antibodies twice a week for 2 weeks. Corneas were sampled by the end of the treatment for immunofluorescence microscopic assays. As shown in Figure 2, our results showed that single blockade of VEGFR-3 or VEGFR-2 suppressed LG in terms of the invasion area, indicating that both factors are involved in the process. Nevertheless, the effect of the VEGFR-2 blockade was less significant than that of the VEGFR-3 blockade.
Figure 2.
VEGFR-2 and VEGFR-3 are both involved in corneal inflammatory LG. (A) Representative immunofluorescence micrographs showing that VEGFR-2 or VEGFR-3 single blockade significantly inhibited corneal LG. Treatment started at day 0 of the suture placement (early intervention). Scale bars, 100 μm. (B) Summarized data from repetitive experiments showing the blockade of VEGFR-3 led to a more significant suppression of corneal LG than that of VEGFR-2. ***P < 0.001.
Combined Blockade of VEGFR-2 and VEGFR-3 Suppresses Early-Stage LG
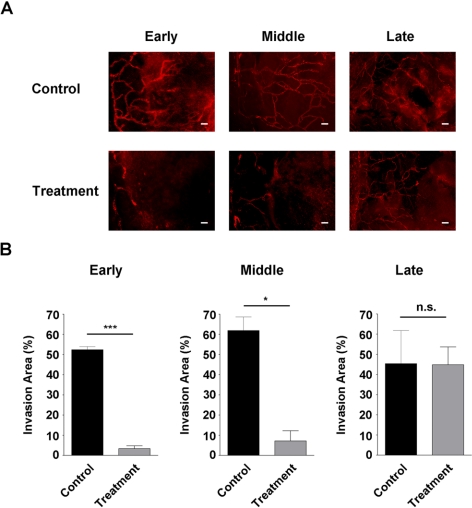
We next investigated the effect of the combined blockade of VEGFR-2 and VEGFR-3 on early-stage LG. To approach this, the experiments were preformed as described except that combined antibodies of VEGFR-2 and VEGFR-3, or their isotype control antibodies, were administered to the mice. As shown in Figures 3A and 3B (left panels), this combined blockade led to a significant reduction of corneal LG when the treatment started on day 0 of the suture placement.
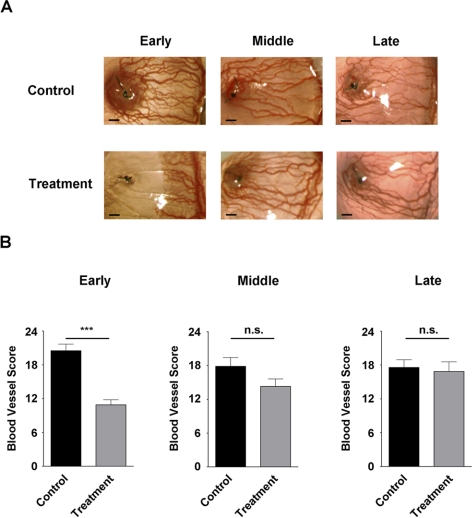
Figure 3.
Effect of combined treatment on early-, middle-, and late-stage LG. (A) Representative immunofluorescence micrographs demonstrating VEGFR-2 and VEGFR-3 combined blockade significantly inhibited both early- and middle-stage but not late-stage LG. Scale bars, 100 μm. (B) Summarized data from repetitive experiments. ***P < 0.001; *P < 0.05. n.s., not significant.
Combined Blockade of VEGFR-2 and VEGFR-3 Suppresses Middle-Stage LG
We next examined whether the combined blockade was also effective in inhibiting middle-stage LG, which mimics a patient's condition when the chance for emergent care has been missed and the cornea is already invaded by some vessels. To study this, the combined antibodies or their isotype controls were administered systemically twice a week for 2 weeks with the treatment started on day 7 after suture placement. At this time with the suture placement model, the cornea was already occupied by a certain degree of LG.30 As shown in Figures 3A and 3B (middle panels), surprisingly, it was found that the combined strategy was also effective in suppressing middle-stage LG, though to a slightly less extent.
Combined Blockade of VEGFR-2 and VEGFR-3 Yields No Effect on Late-Stage LG
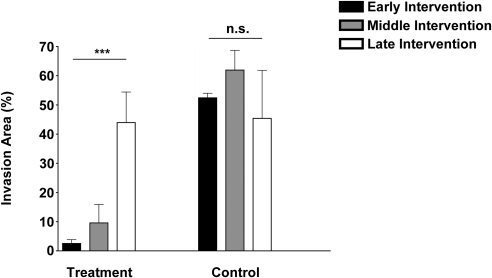
To further investigate whether the combined blockade of VEGFR-2 and VEGFR-3 can interfere with late-stage LG, we performed another experiment and started the treatment on day 14 after suture placement. Results from this study showed that this significant delay in treatment abolished the inhibitory effect of the intervention, as shown in Figures 3A and 3B (right panels). Taken together, our data from all three stage studies have revealed that timing is a crucial factor in determining the treatment outcome of corneal LG. This trend of decreased treatment efficacy with increased time delay is presented in Figure 4.
Figure 4.
Effect of timing on treatment outcomes of LG. Summarized data from a series of studies showing a correlation between the timing and outcomes of treatment. Treatment efficacy decreased as the delay time increased. ***P < 0.001. n.s., not significant.
Combined Blockade of VEGFR-2 and VEGFR-3 Suppresses Only Early-Stage HG
Finally, we investigated whether corneal HG, a process also induced by inflammation, is affected by the combined treatment and how it differs from LG. As demonstrated in Figure 5, it was revealed that, in contrast to LG, HG responded to the combined intervention only at its early stage. No significant difference was observed for middle- or late-stage HG between the treatment and control groups.
Figure 5.
Effect of combined treatment on early-, middle-, and late-stage HG. (A) Representative slit-lamp micrographs at the end of the study demonstrating VEGFR-2 and VEGFR-3 combined blockade only inhibited early HG. Scale bars, 200 μm. (B) Summarized data from repetitive experiments. ***P < 0.001. n.s., not significant.
Discussion
In this study, we provide the first evidence showing that corneal inflammatory LG has a discrete time window for intervention therapy. At least two important conclusions can be drawn from our data: combined blockade of VEGFR-2 and VEGFR-3 is effective in suppressing corneal LG at its early and middle stages; though it is important to initiate treatment as soon as possible, any interventions falling within the therapeutic time window should still be effective. However, a significant time delay will result in a loss of treatment efficacy.
VEGFR-2 is an emerging factor for LG research.12,22,23 Our data derived from the suture placement model are consistent with recent findings showing that corneal LG induced by VEGF-C or VEGF-D pellets is regulated by VEGFR-2.12,22 Given that the doses of the growth factors used in the micropocket assays are much higher than physiological levels, results from the present study should more closely mimic patients' conditions and thus provide additional data supporting VEGFR-2 interference as an adjunctive treatment for LG. Additionally, because the VEGFR-2 pathway also plays a major role in HG,12,18–21 a process accompanies LG and contributes to tissue injury after myriad corneal insults, combined blockade of VEGFR-2 and another key lymphatic receptor, such as VEGFR-3, will maximize the treatment effect when significant inhibition on both processes is pursued.
Moreover, this study also provides a new guideline for modulating corneal LG. Because LG is a progressive process, it is imperative to determine appropriate strategies for intervention therapy. Our finding that the combined blockade strategy is almost equally effective in suppressing early- and middle-stage LG bears broad implications. Most important, it will help to define a suitable population for treatment, which is larger than previously assumed. As indicated by this study, this population should include patients not only immediately after corneal damage but also those who have missed the chance for emergent care and are still in the middle of LG processes. Although it is yet to be determined how the combined treatment suppresses middle-stage LG, it is plausible to hypothesize that the outcome is a net effect of a prevention of LG progression, a disturbance of LG maturation or induction of LG regression, or both. Further analysis of this exciting finding may, therefore, yield crucial insight into the mechanisms of various aspects of LG.
Last, LG is a primary mediator of corneal transplant rejection. In the high-risk setting in which grafting is performed in the inflamed and lymphatic-rich beds, the rejection rate can be as high as 90%.7,9,10 At this stage, there is little effective treatment. Unfortunately, many patients who are in need of corneal transplantation fall into this category. Our study indicates that we may be able to modify or normalize the high-risk grafting beds, particularly when they are still at the early or middle stages of LG, by the combination strategy to improve the survival rate of high-risk transplants; this warrants further investigation.
Acknowledgments
The authors thank Jeffrey LeDue and Steven Ruzin (University of California at Berkeley) for their excellent technical assistance with the immunofluorescence microscopic studies.
Footnotes
Supported in part by research grants from the National Institutes of Health, the Department of Defense, and the University of California at Berkeley (LC).
Disclosure: D. Yuen, None; B. Pytowski, ImClone Systems (E); L. Chen, None
References
- 1. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476 [DOI] [PubMed] [Google Scholar]
- 2. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953 [DOI] [PubMed] [Google Scholar]
- 4. Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458 [DOI] [PubMed] [Google Scholar]
- 5. Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538 [DOI] [PubMed] [Google Scholar]
- 6. Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145 [DOI] [PubMed] [Google Scholar]
- 7. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009;42:66–76 [PMC free article] [PubMed] [Google Scholar]
- 8. Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2009;207:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281 [DOI] [PubMed] [Google Scholar]
- 10. Chong EM, Dana MR. Graft failure, IV: immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222 [DOI] [PubMed] [Google Scholar]
- 11. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung ES, Saban DR, Chauhan SK, Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Vis Sci. 2009;50:1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815 [DOI] [PubMed] [Google Scholar]
- 14. He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825 [DOI] [PubMed] [Google Scholar]
- 15. Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425 [DOI] [PubMed] [Google Scholar]
- 16. Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao Y, Linden P, Farnebo J, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1998;95:14389–14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Witzenbichler B, Asahara T, Murohara T, et al. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol. 1998;153:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller DW, Vosseler S, Mirancea N, et al. Rapid vessel regression, protease inhibition, and stromal normalization upon short-term vascular endothelial growth factor receptor 2 inhibition in skin carcinoma heterotransplants. Am J Pathol. 2005;167:1389–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218 [PubMed] [Google Scholar]
- 22. Chung ES, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Hu X, Tse J, Tilahun F, Qiu M, Chen L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci. 2011;52:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007;125:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494 [PubMed] [Google Scholar]
- 30. Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447 [DOI] [PubMed] [Google Scholar]