A multicenter randomized trial that compared progressive-addition vs. single-vision lenses in slowing 3-year myopia progression in children with high accommodative lag and near esophoria showed a statistically but not clinically significant effect.
Abstract
Purpose.
To determine whether progressive-addition lenses (PALs) relative to single-vision lenses (SVLs) slow the progression of low myopia in children with high accommodative lag and near esophoria.
Methods.
One hundred eighteen children 8 to <12 years of age with spherical equivalent refraction (SER) from −0.75 to −2.50 D and near esophoria ≥2 PD were enrolled in this double-masked multicenter randomized trial. A key additional eligibility criterion was high accommodative lag, initially defined as at least 0.50 D (accommodative response less than 2.50 D for a 3.00-D demand) and later restricted further to at least 1.00 D. One hundred four subjects had accommodative lag of at least 1.00 D, and 14 had lag between 0.50 and 0.99 D. The children were randomized to receive either PALs with a +2.00-D addition or standard SVLs. The clinicians performing the outcome testing, as well as the children and their families, were masked to treatment group. Follow-up visits occurred every 6 months for 3 years. At annual visits, refractive error was assessed in each eye by using cycloplegic autorefraction. The main outcome measure was change from baseline to 3 years in SER by cycloplegic autorefraction.
Results.
The mean change in SER between baseline and the 3-year primary outcome visit was −0.87 D in the PAL group and −1.15 D in the SVL group, for a difference of 0.28 D (95% confidence interval [CI], 0.01–0.55D).
Conclusions.
The PALs used in this study were found to have a statistically but not clinically significant effect of slowing myopia progression in children with high accommodative lag and near esophoria. (ClinicalTrials.gov number, NCT00320593.)
Myopia is a highly prevalent refractive condition, affecting 34% of children in the United States1 and a much higher percentage in some countries in Asia.2,3 Evidence suggests that the prevalence may be increasing over time.1,4,5 Even low degrees of myopia are associated with increased risk of ocular complications, such as retinal detachment and glaucoma.6–9
Single-vision spectacle lenses (SVLs) and contact lenses are commonly prescribed to correct myopia in children. Occasionally other types of spectacle lenses, such as bifocals or progressive-addition lenses (PALs), are prescribed clinically, and many studies have been conducted to evaluate these lenses for slowing the progression of myopia.10–16 Evidence from most well-designed studies shows that these lens therapies for controlling myopia have modest treatment benefits, which are found in the first year and sustained at the same level over time.17 Overall, the use of standard bifocals or PALs versus SVLs for slowing the progression of myopia has produced treatment effects ranging from 0.14 to 0.59 D over 1.5 to 3 years.10–16 The largest treatment trial evaluating PALs versus SVLs was the Correction of Myopia Evaluation Trial (COMET), a multicenter randomized double-masked clinical trial.13 The study's primary finding was that the PALs (Varilux Comfort, Essilor of America, St. Petersburg, FL) slowed 3-year myopia progression by 0.20 D, a result that was statistically significant but not clinically important. Treatment was found to interact significantly with accommodative response and severity of myopia.14 Therefore, additional analyses were conducted that showed statistically significant and more clinically meaningful 3-year treatment effects in subgroups of children with higher lags of accommodation (underaccommodation to near targets) in combination with near esophoria (0.64 D) or lower baseline myopia (0.48 D).14 These results suggest that PALs are a clinically viable spectacle treatment for slowing progression in myopic children with high accommodative lags in conjunction with near esophoria and lower amounts of myopia.
The purpose of the present study was to reinvestigate the effect of PALs relative to SVLs on the progression of myopia in children with low baseline myopia, high accommodative lag, and near esophoria.
Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health and was conducted by the Pediatric Eye Disease Investigator Group (PEDIG) at eight clinical sites consisting of seven optometry colleges and schools and one community-based ophthalmology practice. The study was conducted according to the tenets of the Declaration of Helsinki. The protocol and Health Insurance Portability and Accountability Act (HIPAA)–compliant informed consent forms were approved by institutional review boards, and a parent or guardian (referred to subsequently as “parent”) of each study subject gave written informed consent. Subjects gave assent when required by the local review board of the participating institutions. Study oversight was provided by an independent data and safety monitoring committee. The protocol, which is available on the PEDIG website (www.pedig.net), is summarized in the following sections.
Eligibility Criteria
Major eligibility criteria included age 8 to <12 years and refractive error meeting all the following, obtained by cycloplegic autorefraction: spherical equivalent −0.75 to −2.50 D in both eyes, astigmatism ≤1.50 D in both eyes, and spherical equivalent anisometropia ≤1.00 D. Other major eligibility criteria included near esophoria ≥2 PD, by prism and alternate cover test (PACT) at 33 cm, and high accommodative lag, as assessed by noncycloplegic autorefraction. High accommodative lag was originally defined as lag of at least 0.50 D in response to a target providing an accommodative demand of 3.00 D (33 cm; accommodative response less than 2.50 D for a 3.00-D demand), but was changed 1 year into recruitment to a lag of at least 1.00 D (accommodative response less than 2.00 D for a 3.00-D demand). The more stringent criterion was adopted in light of the results of a pilot study (Gwiazda J, unpublished data, 2005) which showed that the Grand Seiko autorefractor (Binocular Auto Refractor/Keratometer WR-5100; Grand Seiko Co., Ltd., Hiroshima, Japan; also known as Shin-Nippon) used in the present study measured approximately 0.35 D more lag than the autorefractor used in the original COMET study (R-1; Canon, Lake Success, NY). Additional major eligibility criteria included no strabismus, best corrected distance visual acuity at least 20/20, and no current or prior use of PALs, bifocals, or contact lenses in either eye (prior or current use of SVLs was permitted).
Randomization
In a permuted block design stratified by site and by history of previous SVL wear, each subject was randomly assigned with equal probability to receive spectacles that were either PALs with a +2.00-D near addition or SVLs. The clinicians, subjects, and subjects' families were all masked to treatment group for the duration of the study.
Treatment
Each subject received a new pair of spectacles provided by the study. Distance correction was prescribed according to the endpoint of the noncycloplegic subjective refraction (i.e., the minimum minus for best distance visual acuity). Subjects assigned to the SVL group received standard SVLs, whereas the PAL group subjects received Varilux Ellipse lenses (Essilor of America, St. Petersburg, FL) with a +2.00-D near addition. All lenses were polycarbonate and were fabricated at a central laboratory. To help maintain masking of the subjects, both SVLs and PALs were fit by study-certified, unmasked opticians who used a standard PAL fitting protocol. In addition, wearing instructions were identical for both treatment groups: Subjects were encouraged to lower their eyes to read and to tip their chins down, if necessary, to view distant objects clearly. Spectacles were prescribed to be worn during all waking hours.
During follow-up, a change in the distance correction was required if the endpoint of the noncycloplegic subjective refraction differed from the current prescription by 0.50 D or more in spherical equivalent. Prescription changes could be made for smaller differences at investigator discretion if the new prescription improved the subject's visual acuity by at least one line over that in their current correction.
Follow-up Visits
Follow-up visits were conducted every 6 months (±2 weeks) for 3 years, with the 3-year visit specified as the primary outcome visit. At these protocol-specified follow-up visits, all testing procedures were performed by a study-certified optometrist or ophthalmologist who was masked to the subject's lens assignment. To maintain masking of the investigators, the subject saw an unmasked coordinator before the examination who collected the subject's spectacles and told the optometrist or ophthalmologist performing the eye examination what distance refractive correction to use in trial frames for the examination. In addition, anytime a subject reported a problem (e.g., blurry vision or headaches), he or she was evaluated by an unmasked consulting clinician who was aware of the subject's lens assignment. The consulting clinician could update the refraction as needed, but did not perform any study-specific measurements.
At each follow-up visit, the coordinator discussed spectacle compliance with the subject and family and estimated how often the spectacles were being worn during school, after school, and on weekends, by using a five-point Likert scale (always, 5; often, 4; sometimes, 3; rarely, 2; never, 1) for each period.
Testing Procedures and Assessments
At annual visits, the primary outcome of cycloplegic refractive error was assessed in each eye with the Grand Seiko autorefractor 30 minutes after administration of 2 drops of 1% tropicamide. Five readings (sphere, cylinder, and axis) were taken in each eye, with the room lights out while the subject fixated a row of 20/100 letters on an ETDRS chart in a light box at 4 m.
Additional procedures performed at all protocol-specified visits consisted of subjective refraction of both eyes and an oculomotor assessment using the cover–uncover test and the PACT. Accommodative response was measured with the Grand Seiko autorefractor with the room lights off. The accommodative target was an illuminated row of 20/100 letters positioned 33 cm from the subject and calibrated for that distance. The subject's right eye viewed the target through a lens placed in a trial frame that was equal to the spherical equivalent of the endpoint of the subjective refraction, and the left eye was occluded. Five readings (sphere, cylinder, and axis) were taken. To determine the accommodative lag or accommodative lead, the accommodative measurement was added to the 3.00-D demand to result in the accommodative lag or lead. If the value was positive (i.e., underaccommodation), it indicated accommodative lag; if the number was negative (i.e., overaccommodation), it indicated accommodative lead. The median of the five baseline accommodative lag/lead readings for each subject was used in the analysis.
At one visit, the parental history of myopia was assessed by asking each subject's parents, “Are you nearsighted? In other words, do you (or did you ever) wear glasses or contact lenses primarily to see far away or that are equally important for seeing far away and up close?”
Statistical Methods
The sample size was computed to be 100 subjects overall (50 per treatment group) to reach a 90% power and a type 1 error rate of 5%, so that a difference in 3-year myopia progression between PAL and SVL treatment groups would be detected if the true difference was at least 0.60 D, assuming a standard deviation of 0.85 D in each treatment group and allowing for a 10% loss to follow-up.
The primary analysis was a comparison of treatment groups using an analysis of covariance (ANCOVA) model in which myopia at 3 years was adjusted for myopia at baseline and prior SVL wear (currently or in the past versus never). The primary outcome measure was a change in spherical equivalent refractive error (SER) in diopters from baseline to the 3-year visit, as assessed by cycloplegic autorefraction. Each autorefraction reading was converted to an SER, and the medians of the five SERs for each eye were averaged to produce a subject-level SER for analysis (Pearson correlation for SER between right and left eyes at 3 years = 0.89). All analyses followed the intent-to-treat principle. The Monte Carlo Markov Chain (MCMC)18 method of multiple imputation was used to impute data for subjects who did not complete the 3-year visit. To evaluate the effect of imputation on the primary results, we also performed the primary analysis (1) using the last-observation-carried-forward method and (2) using only data from subjects who completed the 3-year visit. In addition, an adjusted analysis was performed by including in the ANCOVA model the following covariates, which are known to be related to myopia progression: age, sex, ethnicity, accommodative lag, and magnitude of near esophoria. Interaction between baseline factors and treatment effect was formally assessed by including interaction terms in the ANCOVA model, although it is acknowledged that the statistical power is low for detecting such interactions. For the adjusted model and for models testing for interactions, the baseline factors of refractive error, age, accommodative lag, and near esophoria size were treated as continuous variables, unless there was evidence of nonlinear associations between the factor and the outcome (for adjusted models) or between the factor and the treatment effect (for interaction models).
Secondary analyses were conducted to assess the treatment effect on myopia at interim time points of 1 and 2 years, using the same method as was used in the primary analysis, except that only complete cases were included.
Secondary outcomes evaluated were main axis astigmatism (J0, dioptric power of a Jackson cross cylinder with axis at 0°) and oblique astigmatism (J45, dioptric power of a Jackson cross cylinder with axis at 45°) by using the power vector approach.19 For J0 and J45, the average of the medians of the five autorefraction readings for each measure was used for analysis. Because oblique astigmatism is often mirror symmetric in the two eyes, the average J45 values were calculated by transforming the axis values between 91° and 180° to values between 0° and 90° for each eye, then averaging the median value between the two eyes. J0 and J45 were compared between treatment groups at annual visits by using separate ANCOVA models in which the outcome was adjusted for their respective baseline values.
Results
Between April 2005 and March 2007, 118 subjects were enrolled in the study at eight clinical centers. The number of subjects enrolled per site ranged from 6 to 25 (median = 15). As part of the screening for the randomized trial, 67 (57%) of the 118 subjects participated in an ancillary study that was an evaluation of the methods of measuring accommodation.20 The average age of the 118 subjects was 10.1 (±1.1) years, 64 (54%) were girls, and 59 (50%) were white. One hundred four children had accommodative lags of 1.00 D or more (accommodative response less than 2.00 D for a 3.00-D demand), and 14 had lags between 0.50 and 0.99 D. The mean SER at baseline was −1.48 (±0.46) D. All baseline characteristics that were evaluated were well balanced between treatment groups (Table 1).
Table 1.
Baseline Characteristics According to Treatment Group
| Characteristic | SVL (n = 59) n (%) | PAL (n = 59) n (%) |
|---|---|---|
| Sex, female | 28 (47) | 36 (61) |
| Race/ethnicity | ||
| White | 31 (53) | 28 (47) |
| African American | 10 (17) | 14 (24) |
| Hispanic or Latino | 12 (20) | 10 (17) |
| Asian | 4 (7) | 5 (8) |
| Other | 2 (3) | 2 (3) |
| Age, y | ||
| Mean (SD) | 10.0 (1.1) | 10.2 (1.1) |
| Range | 8.1 to 12.0 | 8.0 to 11.9 |
| 8 to <9 | 14 (24) | 8 (14) |
| 9 to <10 | 17 (29) | 15 (25) |
| 10 to <11 | 14 (24) | 18 (31) |
| 11 to <12 | 14 (24) | 18 (31) |
| Spectacle wear (SVLs) | ||
| Currently | 33 (56) | 33 (56) |
| In past but not currently | 2 (3) | 4 (7) |
| Never | 24 (41) | 22 (37) |
| Average spherical equivalent between the eyes, D* | ||
| Mean (SD) | −1.45 (0.47) | −1.50 (0.45) |
| Range | −0.75 to −2.40 | −0.78 to −2.38 |
| −0.75 to −0.99 | 12 (20) | 8 (14) |
| −1.00 to −1.49 | 23 (39) | 22 (37) |
| −1.50 to −1.99 | 14 (24) | 18 (31) |
| −2.00 to −2.50 | 10 (17) | 11 (19) |
| Average J0 between the eyes, D* | ||
| Mean (SD) | −0.02 (0.15) | −0.02 (0.18) |
| Range | −0.53 to 0.27 | −0.47 to 0.54 |
| Average J45 between the eyes, D* | ||
| Mean (SD) | 0.10 (0.07) | 0.11 (0.07) |
| Range | 0.00 to 0.27 | 0.00 to 0.33 |
| Anisometropia, D | ||
| Mean | 0.21 (0.16) | 0.22 (0.20) |
| Range | 0.00 to 0.93 | 0.00 to 1.00 |
| Near esophoria, PD | ||
| Mean (SD) | 5.9 (3.4) | 5.8 (3.2) |
| Range | 2–20 | 2–16 |
| 2 to 5 | 34 (58) | 33 (56) |
| 6 to 10 | 21 (36) | 22 (37) |
| 11 to 15 | 3 (5) | 3 (5) |
| 16 to 20 | 1 (2) | 1 (2) |
| Accommodative lag, D† | ||
| Mean (SD) | 1.40 (0.48) | 1.47 (0.53) |
| Range | 0.6–3.7 | 0.5–2.9 |
| 0.50 to 0.99 | 6 (10) | 8 (14) |
| 1.00 to 1.49 | 33 (56) | 26 (44) |
| 1.50 to 1.99 | 16 (27) | 13 (22) |
| 2.00 or more | 4 (7) | 12 (20) |
| Parental history of myopia‡ | ||
| Neither parent | 5 (14) | 10 (23) |
| One parent | 10 (29) | 16 (36) |
| Both parents | 20 (57) | 18 (41) |
N = 118. Rounded percentages may not sum to 100%.
Spherical equivalent, J0, and J45 are averages of the median values from the five readings of cycloplegic autorefraction in each eye.
Accommodative lag is the average of the median accommodative lag of the five readings of the right eye.
Thirty-nine subjects had missing data or unknown parental history of myopia for at least one parent—15 subjects had missing data for both parents, 1 subject had missing data for one parent and uncertain data for the other, 19 subjects had missing data for one parent and known data for the other, and 4 subjects had data for one parent and uncertain data for the other.
Study and Visit Completion
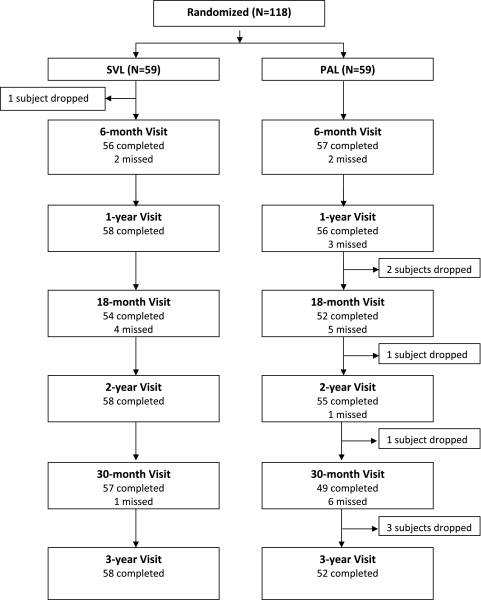
Of the 118 subjects enrolled in the study, 110 (93%) completed the 3-year visit. One (2%) SVL group subject and seven (12%) PAL group subjects withdrew from the study early and did not complete the 3-year visit (Fig. 1). Of the eight subjects who did not complete the study, three were lost to follow-up, one moved to another state, three withdrew, and one was withdrawn by the clinical site. Compared with the 110 subjects who completed the study, the eight subjects who did not had less baseline myopia (mean SER −1.20 D vs. −1.50 D) and less baseline accommodative lag (mean, 1.16 D vs. 1.46 D). Overall, the 3-year visits were completed within the ±2-week window in 74 (67%) of the subjects, 1 to 30 days early or late in 22 (20%), and more than 30 days early or late in 14 (13%).
Figure 1.
Flowchart of subjects throughout the study.
Effect of Treatment on Change in Myopia
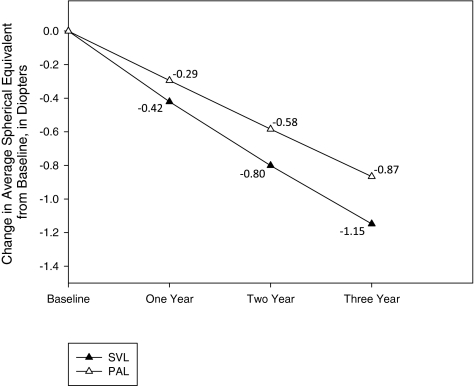
From baseline to the 3-year visit, the mean change in SER was −0.87 D in the PAL group versus −1.15 D in the SVL group, for a difference of 0.28 D (95% confidence interval [CI], 0.01–0.55 D; Table 2; Fig. 2). In a model further adjusted by including age, sex, ethnicity, baseline accommodative lag, and baseline near esophoria, in addition to baseline SER and prior SVL wear, the 3-year difference in progression between PAL and SVL subjects was 0.27 D (95% CI, 0.02–0.53 D). Table 2 also shows that the 3-year change in spherical equivalent myopia was at least −1.50 D in 13% (n = 7) of subjects in PALs versus 36% (n = 21) of subjects in SVLs.
Table 2.
Spherical Equivalent Refractive Error during Follow-up
| 1 Year |
2 Years |
3 Years |
||||
|---|---|---|---|---|---|---|
| SVL (n = 58) n (%) | PAL (n = 55) n (%) | SVL (n = 58) n (%) | PAL (n = 55) n (%) | SVL (n = 58) n (%) | PAL (n = 52) n (%) | |
| Change in Spherical Equivalent Refractive Error from Baseline, D* | ||||||
| Mean (SD) | −0.42 (0.37) | −0.29 (0.39) | −0.80 (0.61) | −0.58 (0.55) | −1.15 (0.75) | −0.87 (0.72) |
| Range | −1.38 to 0.70 | −1.56 to 0.82 | −2.26 to 0.84 | −2.16 to 0.78 | −2.97 to 0.38 | −3.13 to 0.22 |
| >0.00 (less myopia) | 6 (10) | 6 (11) | 5 (9) | 5 (9) | 3 (5) | 6 (12) |
| 0.00 to −0.49 | 27 (47) | 35 (64) | 11 (19) | 18 (33) | 8 (14) | 11 (21) |
| −0.50 to −0.99 | 21 (36) | 12 (22) | 19 (33) | 23 (42) | 13 (23) | 16 (31) |
| −1.00 to −1.49 | 4 (7) | 1 (2) | 17 (29) | 5 (9) | 13 (23) | 12 (23) |
| ≤−1.50 (more myopia) | 0 (0) | 1 (2) | 6 (10) | 4 (7) | 21 (36) | 7 (13) |
| Spherical Equivalent Refractive Error, D* | ||||||
| Mean (SD) | −1.88 (0.66) | −1.80 (0.63) | −2.26 (0.84) | −2.10 (0.81) | −2.60 (0.91) | −2.41 (0.95) |
| Range | −3.56 to −0.69 | −3.75 to −0.72 | −4.44 to 0.09 | −4.19 to −0.75 | −4.47 to −0.38 | −5.13 to −0.72 |
| Better than or equal to −0.49 | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
| −0.50 to −0.99 | 4 (7) | 4 (7) | 3 (5) | 3 (5) | 1 (2) | 2 (4) |
| −1.00 to −1.99 | 30 (52) | 34 (61) | 18 (31) | 25 (45) | 14 (24) | 18 (35) |
| −2.00 to −2.99 | 21 (36) | 16 (29) | 27 (47) | 19 (35) | 21 (36) | 20 (38) |
| −3.00 to −3.99 | 3 (5) | 2 (4) | 7 (12) | 6 (11) | 17 (29) | 8 (15) |
| Worse than or equal to −4.00 | 0 (0) | 0 (0) | 2 (3) | 2 (4) | 4 (7) | 4 (8) |
A negative value indicates that the myopia worsened over time; a positive value indicates that it improved over time. Rounded percentages may not sum to 100%.
For baseline and each time point, a spherical equivalent was calculated for each eye for each of the five readings of cycloplegic autorefraction, and the median for each eye was averaged to obtain the spherical equivalent used for analysis. The change in average spherical equivalent was calculated by subtracting the spherical equivalent at baseline from each time point.
Figure 2.
Mean change in spherical equivalent refractive error during follow-up. The mean difference between treatment groups (SVL − PAL) in the change in spherical equivalent refractive error from baseline to 1 year was 0.14 D (95% CI, −0.005–0.30 D), from baseline to 2 years was 0.23 D (95% CI, 0.02–0.45), and from baseline to the 3 years was 0.28 D (95% CI, 0.01–0.55).
The 3-year change in SER is shown stratified by baseline characteristics in Table 3. The treatment group difference between PALs and SVLs was 0.35 D among subjects with baseline SER from −0.75 to −1.49 D and was 0.17 D among subjects with baseline SER from −11.50 to −2.50 D (P for continuous interaction = 0.24). In subjects with accommodative lag of 1.50 D or worse, the treatment effect was 0.41 D, and in subjects with lag from 0.50 to 1.49 D, it was 0.24 D (P for continuous interaction = 0.23). In subjects with no history of previous SVL wear, the treatment group difference was 0.42 D, whereas in subjects with previous SVL, wear it was 0.22 D (P for interaction = 0.48).
Table 3.
Three-Year Change in Spherical Equivalent Refractive Error According to Baseline Characteristics
| Baseline Characteristic | Change in Average Spherical Equivalent between Baseline and 3 Years (D) |
Estimated Difference, Adjusted for Baseline Spherical Equivalent | P for Interaction | |||
|---|---|---|---|---|---|---|
| SVL |
PAL |
|||||
| n | Mean | n | Mean | |||
| Age, y | 0.92* | |||||
| 8 to <9 | 13 | −1.39 | 8 | −1.23 | 0.18 | |
| 9 to <10 | 17 | −1.14 | 13 | −1.11 | 0.11 | |
| 10 to <11 | 14 | −1.19 | 15 | −0.71 | 0.48 | |
| 11 to <12 | 14 | −0.88 | 16 | −0.64 | 0.26 | |
| Sex | 0.52 | |||||
| Male | 31 | −1.09 | 19 | −0.95 | 0.20 | |
| Female | 27 | −1.21 | 33 | −0.82 | 0.39 | |
| Race/Ethnicity | 0.94 | |||||
| White | 30 | −1.10 | 26 | −0.79 | 0.32 | |
| Nonwhite | 28 | −1.19 | 26 | −0.94 | 0.30 | |
| Prior spectacle wear (SVLs) | 0.48 | |||||
| Currently or in past | 34 | −1.10 | 31 | −0.90 | 0.22 | |
| Never | 24 | −1.22 | 21 | −0.81 | 0.42 | |
| Spherical equivalent, D | 0.24/0.57† | |||||
| −0.75 to −1.49 | 33 | −1.20 | 25 | −0.79 | 0.35 | |
| −1.50 to −2.50 | 25 | −1.08 | 27 | −0.93 | 0.17 | |
| Accommodative lag, D | 0.23/0.55† | |||||
| 0.50 to 1.49 | 38 | −1.12 | 28 | −0.91 | 0.24 | |
| 1.50 or worse | 20 | −1.20 | 24 | −0.81 | 0.41 | |
| Near esophoria, PD | 0.56 | |||||
| 2 to 5 | 33 | −1.13 | 30 | −0.97 | 0.18 | |
| 6 to 10 | 21 | −1.09 | 18 | −0.66 | 0.46 | |
| >10 | 4 | −1.60 | 4 | −1.01 | 0.63 | |
| Parental history of myopia | 0.43 | |||||
| Neither parent | 5 | −0.72 | 9 | −0.83 | −0.10 | |
| One parent | 10 | −1.28 | 15 | −0.73 | 0.57 | |
| Both parents | 20 | −1.29 | 18 | −0.99 | 0.30 | |
Corresponds to the interaction between treatment and the continuous variable.
The first P value tests for the interaction between treatment and age as a continuous variable; the second P value tests for the interaction between treatment and the two-level categorical variable.
The mean change in SER from baseline to 1 year was −0.29 D in the PAL group versus −0.42 D in the SVL group (difference, 0.14; 95% CI, −0.005 to 0.28 D) and from baseline to 2 years was −0.58 D in the PAL group versus −0.80 D in the SVL group (difference, 0.23; 95% CI, 0.02–0.45 D; Table 2; Fig. 2).
Results of analyses (1) using the last-observation-carried-forward method, (2) using only data from subjects who completed the 3-year visit, and (3) limited to the 104 subjects with accommodative lag ≥1.00 D were similar to the results of the primary analysis.
Effect of Treatment on Change in Astigmatism
The change in astigmatism was small and did not differ by lens group. The mean change in J0 (main axis astigmatism) from baseline to 3 years was 0.07 D in the PAL group versus 0.11 D in the SVL group (difference, −0.04 D; 95% CI, −0.11 to 0.03 D). The mean change in J45 (oblique astigmatism) from baseline to 3 years was 0.04 D in the PAL group versus 0.02 D in the SVL group (difference, 0.03 D; 95% CI, −0.006 to 0.06 D).
Treatment Compliance
As shown in Table 4, the percentages of PAL and SVL subjects estimated at every follow-up visit to have been wearing their study spectacles either always (score of 5) or often (score of 4), were 72% and 90% during school, 60% and 76% after school, and 60% and 71% on weekends, respectively. Treatment compliance appeared to be worse in the PAL group than in the SVL group, particularly among subjects with lower amounts of baseline myopia (−0.75 to −1.49 D; Table 4). There was no evidence that poor treatment compliance was related to increased progression (data not shown).
Table 4.
Spectacle Compliance*
| Period | All Subjects |
Baseline Spherical Equivalent −0.75 D to −1.49 D |
Baseline Spherical Equivalent −1.50 D to −2.50 D |
|||
|---|---|---|---|---|---|---|
| SVL (n = 58) | PAL (n = 58) | SVL (n = 33) | PAL (n = 30) | SVL (n = 25) | PAL (n = 28) | |
| During school | ||||||
| Mean compliance score | 4.80 | 4.60 | 4.76 | 4.42 | 4.85 | 4.79 |
| Excellent compliance, %† | 90 | 72 | 88 | 60 | 92 | 86 |
| After school | ||||||
| Mean compliance score | 4.61 | 4.37 | 4.49 | 4.10 | 4.76 | 4.65 |
| Excellent compliance, %† | 76 | 60 | 70 | 47 | 84 | 75 |
| Weekends | ||||||
| Mean compliance score | 4.55 | 4.33 | 4.44 | 4.06 | 4.69 | 4.61 |
| Excellent compliance, %† | 71 | 60 | 63 | 50 | 80 | 71 |
Compliance was assessed on a five-point Likert scale: always, 5; often, 4; sometimes, 3; rarely, 2; and never, 1.
Excellent compliance indicates that for the specified period, spectacles were estimated at all visits to have been worn either always or often. Two subjects had no follow-up visits (one SVL, one PAL).
Two cases of treatment crossover are known. A PAL group subject wore SVLs for 6 months after SVLs were ordered by mistake when the distance correction was changed. This same subject later obtained contact lenses outside the study and wore them approximately 5 days a week for the last year of the study, although usually only for a few hours a day after school. Another PAL group subject reported having worn the PALs for 1.5 years before switching to wearing SVLs obtained outside the study for the remainder of the study.
Adverse Events
No serious treatment-related adverse events were reported during the 3 years of follow-up. Of the three PAL group subjects seen by a consulting clinician for a problem, one reported distance blur and was prescribed a change in distance correction, one had conjunctivitis, and one reported dizziness, particularly during gymnastics (the subject was advised not to wear spectacles during this activity). Of the four SVL subjects seen by a consulting clinician for a problem, two had reduced visual acuity and were prescribed a change in distance correction, one reported eye pain in addition to reduced distance visual acuity and was prescribed a change in distance correction (the eye pain later resolved), and one had a floater and was referred to a retina specialist who detected a retinal hole not associated with the treatment.
Discussion
In this randomized trial of 118 subjects aged 8 to <12 years with low myopia, high accommodative lag, and near esophoria, the PALs were found to slow the progression of myopia by 0.28 D (95% CI, 0.01–0.55 D) over 3 years. This result, while small in magnitude, is statistically significant and, combined with findings in other studies, suggests a true biological effect of slowed eye growth in children wearing PALs.14
The most prominent hypothesis for the development and progression of myopia is that children with insufficient accommodation when engaged in near work activities may experience retinal defocus that may lead to axial elongation and myopia.21,22 Because PALs would be expected to provide clearer vision across a range of viewing distances, one would expect that they would reduce defocus and might lead to slower eye growth.
This hypothesis was in part the rationale for the original COMET and its exploratory analyses of accommodative lag, which showed that the 3-year treatment benefit of PALs on progression of myopia was larger in subgroups of children with higher accommodative lag and near esophoria (0.64 D; 95% CI, 0.08–1.19 D; n = 76) and in children with higher accommodative lag and lower baseline myopia (0.48 D; 95% CI, 0.02–0.93 D; n = 104) than in the overall cohort (0.20 D; 95% CI, 0.06–0.33 D; n = 469).13,14 Analysis of data from a smaller subgroup of 35 COMET subjects meeting the COMET2 eligibility criteria for age, baseline myopia, accommodative lag, and near esophoria showed a 0.71-D (95% CI, 0.22–1.20 D) 3-year treatment effect of PALs (COMET, unpublished data, 1997–2001). The treatment effect found in COMET2 is not significantly different from that found in each of these three subgroup analyses from the original COMET study14 (P = 0.25, 0.46, and 0.12 for Cochrane's Q tests for heterogeneity comparing COMET2 results versus the results in each COMET subgroup, respectively).
Two other recent clinical trials of spectacle lenses for myopia control also reported statistically significant treatment effects in subgroups of children with high accommodative lag.15,16 Hasebe et al.,15 in a study of myopic Japanese children randomized to PALs or SVLs for 18 months, reported that a subgroup of 36 children with accommodative lag of at least 1.80 D had an 18-month treatment effect of 0.61 D (95% CI, 0.30–0.92 D), with myopia in the PAL group progressing by 0.87 D and in the SVL group by 1.48 D. The use of standard bifocals versus SVLs in a study by Cheng et al.16 of Chinese Canadian children with high rates of previous myopic progression significantly reduced progression over 2 years (by 0.88 D; 95% CI, 0.45–1.31 D) in a subgroup of 43 children with lags of accommodation of at least 1.00 D (progression in the standard bifocal group was 0.88 D and in the SVL group was 1.76 D). For comparison, in the present study the treatment effect of PALs was 0.17 D (95% CI, 0.02–0.32 D) at 18 months and 0.23 D (95% CI, 0.02–0.45 D) at 2 years. The treatment effect found in COMET2 is significantly smaller than those found in the subgroup analyses in Cheng et al.16 and Hasebe et al.15 at the same time points (P = 0.01 for Cochrane's Q tests for both comparisons).
Methodological differences among all the studies may have affected the results. First, the lens type used was different in each study: the Ellipse in the present study, the Varilux Comfort in COMET (Essilor of America),13 the Sola MCLens (Sola International, San Diego, CA) in Hasebe et al.,15 and Essilor Executive bifocals (Essilor of America) in Cheng et al.16 While many details of the lens designs are proprietary, it is known that the Varilux Comfort lens has a wider distance and intermediate zone and more induced astigmatism than does the Ellipse lens.23 In these lenses, the widths of the near zones vary greatly with fitting height; the Comfort's near zone is narrower than the Ellipse's for a fitting height of 14 mm, which is the minimum recommended for the Ellipse.23 Also, the present study and the original COMET study13 used a +2.00-D near addition, whereas Cheng et al.16 and Hasebe et al.15 used a +1.50-D addition. These lens variations may have produced differences in peripheral aberrations and in the power of the addition used for near work, both of which could affect progression.24,25 Second, high accommodative lag was defined as 1.00 D or more in the current study and in Cheng et al.,16 greater than 0.43 D in the original COMET study,13 and 1.80 D or more in Hasebe et al.15 Third, the other studies had higher rates of myopia progression, most likely because they included children who were younger15 and/or Asian,15,16 factors that are associated with increased progression.26,27 Also, Cheng et al.16 was limited to children who had previously demonstrated fast progression. It might be expected that treatments would work better when myopia is still progressing rather than when it is close to reaching a plateau.
We found no statistically significant associations between baseline factors and treatment effect; however, our study was underpowered to detect these types of associations unless they were very strong. Although not statistically significant, there may be some evidence in our data that children with lower myopia might benefit more from PALs. This finding is consistent with those in the COMET which showed greater treatment benefit among subjects with baseline myopia of −1.25 to −2.24 D compared with those with −2.25 to −4.50 D, both in the overall cohort and in the subgroup of subjects with higher accommodative lag.14 In addition, although COMET2 enrolled only subjects with larger lags of accommodation, there is a suggestion that, within this group, the children with higher lag (≥1.50 D) had an increased treatment effect, consistent with the COMET study's finding that higher lag was associated with increased treatment effect across a wider range of lags.
Strengths of the present study include the use of a double-masked design with random assignment to treatment groups and training and certification of study investigators. In addition, missing data did not appear to influence the results. Another strength is an adequately powered sample size and a high rate of follow-up overall, although a limitation is that there was higher loss to follow-up in the PAL (n = 7) group than in the SVL (n = 1) group. Another limitation is that compliance with lens wear was reported to be worse in the PAL group, a factor that could have diluted the treatment effect of the PALs. However, this difference in compliance did not appear to affect progression. In the present study, as in other studies of PALs or bifocals for control of myopia,15,16 we prescribed a consistent near addition power for all children, +2.00 D. It is possible that if this power were customized for individual children based on the level of myopia, near accommodative response, and phoria, then the treatment benefit of the lenses might be enhanced. In addition, although we collected estimates of compliance with spectacle wear, we do not have data on what part of the lens the children were actually looking through during near work, and so it is possible that the full benefit of the near addition was not realized.
The treatment effect in this study increased over the 3 years rather than maintaining the same level as found in the first year, the latter of which is a more typical finding with various lens treatments.17 The pattern in the present study is similar to that in the subgroup of COMET subjects with higher lag,13,14 but different from that in the overall COMET cohort.13 Given the current result, a longer trial might find a greater treatment effect of PALs.
The consistent presence of an effect of PALs or bifocals in different studies suggests that the visual environment plays a definite, but perhaps modest, role in myopia progression, although it is not completely clear what in the environment is being manipulated with the lenses. COMET2 was designed to examine the effects of high accommodative lag and the resultant central hyperopic defocus. Recent work28 indicates that an important additional mechanism may be peripheral hyperopic defocus, which was reduced in the lower visual field only with the PALs, in which case the effect might be stronger if defocus was reduced in the entire visual periphery. However, a recent study evaluating three different spectacle lens designs for reducing peripheral hyperopic defocus versus SVLs found no statistically significant differences in the 1-year progression of myopia.29 These results suggest that there is much more to be learned about the mechanisms of eye growth. It is hoped that when the mechanisms are better understood, more powerful, clinically useful lens treatments for slowing the progression of myopia may be developed.
In conclusion, the PALs used in this study were found to have a statistically, but not clinically significant effect on slowing myopia progression in children with high accommodative lag and near esophoria.
Appendix
The Correction of Myopia Evaluation Trial 2 (COMET2) Study Group
WRITING COMMITTEE: Lead Authors: Jane Gwiazda and Danielle L. Chandler. Additional Authors: Susan A. Cotter, Donald F. Everett, Leslie Hyman, Brett M. Kaminski, Marjean T. Kulp, Don W. Lyon, Ruth E. Manny, Wendy L. Marsh-Tootle, Noelle S. Matta, B. Michele Melia, Thomas T. Norton, Mitchell M. Scheiman, David I. Silbert, and Erik M. Weissberg.
Clinical Sites that Participated in the Protocol
Sites are listed in order by number of subjects enrolled in the study. Personnel are coded according their roles: I, Investigator; C, Coordinator; O, Optician; CC, Consulting Clinician.
University of Alabama at Birmingham School of Optometry, Birmingham, Alabama (25): Wendy L. Marsh-Tootle (I), Michelle L. Anderson (I), Marcela Frazier (I), Kristine T. Hopkins (I), Katherine K. Weise (I), Cathy Baldwin (C), and Michael Hill (C).
University of Houston College of Optometry, Houston, Texas (19): Ruth E. Manny (I), Heather A. Anderson (I), Soyung A. Kim (I), Karen D. Fern (CC), Gabynely Solis (C), and Andy Ketcham (O).
New England College of Optometry, Boston, Massachusetts (16): Erik M. Weissberg (I), Elise N. Harb (I), Sally Bittinger (C), and Robert Owens (C).
Family Eye Group, Lancaster, Pennsylvania (15): David I. Silbert (I), Don D. Blackburn (I), Troy J. Hosey (I), Eric L. Singman (I), Noelle S. Matta (C), Kourosh A. Dastgheib (CC), and Michael R. Pavlica (CC).
Indiana University School of Optometry, Indianapolis, Indiana (15): Don. W. Lyon (I), Kathryn Gray (I), Kristy Dunlap (C), Sara Long (C), Julia Wilhite (C), and Danielle F. Warren (CC).
Pennsylvania College of Optometry, Philadelphia, Pennsylvania (13): Mitchell M. Scheiman (I), Karen Pollack (C), and Brandy J. Scombordi (CC).
The Ohio State University, Columbus, Ohio (9): Marjean T. Kulp (I), Mark A. Bullimore (I), Jeffrey J. Walline (I), Nancy Stevens (C), Gilbert E. Pierce (CC), and Freda Dallas (O).
Southern California College of Optometry, Fullerton, California (6): Susan A. Cotter (I), Carmen N. Barnhardt (I), Kristine Huang (I), Monique M. Nguyen (I), Catherine Heyman (C), Sue M. Parker (C), and Michael W. Rouse (CC).
PEDIG Coordinating Center
Raymond T. Kraker, Roy W. Beck, Danielle L. Chandler, Elise R. Diamond, Quayleen Donahue, Heidi Gillespie, Brett M. Kaminski, Stephanie V. Lee, B. Michele Melia, and Pamela S. Moke.
National Eye Institute, Bethesda, Maryland
Donald F. Everett
COMET2 Steering Committee
Jane E. Gwiazda (Chair), Danielle L. Chandler, Susan A. Cotter, Donald F. Everett, Jonathan M. Holmes (2005–2009), Leslie Hyman, Marjean T. Kulp, Don W. Lyon, Ruth E. Manny, Wendy L. Marsh-Tootle, Noelle S. Matta, B. Michele Melia, Thomas T. Norton, Michael X. Repka (2005–2009), Mitchell M. Scheiman, David I. Silbert, and Erik M. Weissberg.
PEDIG Executive Committee
Jonathan M. Holmes, Darron A. Bacal (2009), Roy W. Beck, Eileen E. Birch, Stephen P. Christiansen (2007 to present), Susan A. Cotter (2005–2006, 2008–present), Sean Donahue (2005–2006), Donald F. Everett, Darren L. Hoover (2007–2009), Pamela A. Huston (2007, 2009–present), Raymond T. Kraker, Katherine A. Lee (2008–2009), Noelle S. Matta (2008–2009), David G. Morrison (2008–2009), Michael X. Repka, Robert P. Rutstein (2009–present), Nicholas A. Sala (2006, 2009–present), Mitchell M. Scheiman (2007–2008), and David K. Wallace (2006–present).
PEDIG Data and Safety Monitoring Committee
Marie Diener-West (2006–present), John D. Baker (2008–present), William Barlow (2005), Edward G. Buckley (2005–2007), Barry Davis, Velma Dobson, Donald F. Everett, Dale L. Phelps, Stephen Poff, Richard A. Saunders, and Lawrence Tychsen (2007–present).
Footnotes
Supported by National Institutes of Health Grant EY011751 (PEDIG), Department of Health and Human Services. The funding organization had no role in the design of the study. It provided external oversight through an independent data and safety monitoring committee. Essilor of America and Eyewear Designs provided spectacles at a reduced cost.
Disclosure: None
References
- 1. Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127(12):1632–1639 [DOI] [PubMed] [Google Scholar]
- 2. Lin LL, Shih YF, Tsai CB, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci. 1999;76(5):275–281 [DOI] [PubMed] [Google Scholar]
- 3. Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from Shunyi District, China. Am J Ophthalmol. 2000;129(4):427–435 [DOI] [PubMed] [Google Scholar]
- 4. Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States. 1999–2004. Arch Ophthalmol. 2008;126(8):1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24(1):1–38 [DOI] [PubMed] [Google Scholar]
- 6. Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391 [DOI] [PubMed] [Google Scholar]
- 7. Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010–2015 [DOI] [PubMed] [Google Scholar]
- 8. Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111(1):62–69 [DOI] [PubMed] [Google Scholar]
- 9. Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111(1):53–61 [DOI] [PubMed] [Google Scholar]
- 10. Leung JT, Brown B. Progression of myopia in Hong Kong Chinese schoolchildren is slowed by wearing progressive lenses. Optom Vis Sci. 1999;76(6):346–354 [DOI] [PubMed] [Google Scholar]
- 11. Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77(8):395–401 [DOI] [PubMed] [Google Scholar]
- 12. Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43(9):2852–2858 [PubMed] [Google Scholar]
- 13. Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492–500 [DOI] [PubMed] [Google Scholar]
- 14. Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004;45(7):2143–51 [DOI] [PubMed] [Google Scholar]
- 15. Hasebe S, Ohtsuki H, Nonaka T, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49(7):2781–2789 [DOI] [PubMed] [Google Scholar]
- 16. Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Arch Ophthalmol. 2010;128(1):12–19 [DOI] [PubMed] [Google Scholar]
- 17. Gwiazda J. Treatment options for myopia. Optom Vis Sci. 2009;86(6):624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Little RJA, Rubin DB. The model-based approach to nonresponse: multiple imputation: statistical analysis with missing data. In. New York: Wiley;1987:255–259 [Google Scholar]
- 19. Thibos LN, Wheeler W, Horner D. Power Vectors: An application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74(6):367–375 [DOI] [PubMed] [Google Scholar]
- 20. Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group Accommodative lag by autorefraction and two dynamic retinoscopy methods. Optom Vis Sci 2009;86(3):233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goss DA. Clinical accommodation and heterophoria findings preceding juvenile onset of myopia. Optom Vis Sci. 1991;68(2):110–116 [DOI] [PubMed] [Google Scholar]
- 22. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34(3):690–694 [PubMed] [Google Scholar]
- 23. Sheedy J, Hardy RF, Hayes JR. Progressive addition lenses–measurements and ratings. Optometry. 2006;77:23–39 [DOI] [PubMed] [Google Scholar]
- 24. Charman W. Aberrations and myopia. Ophthal Physiol Opt. 2005;25(4):285–301 [DOI] [PubMed] [Google Scholar]
- 25. Lundstrom L, Mira-Agudelo A, Artal P. Peripheral optical errors and their change with accommodation differ between emmetropic and myopic eyes. J Vis. 2009;9(6):17, 1–11 [DOI] [PubMed] [Google Scholar]
- 26. Hyman L, Gwiazda J, Hussein M, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123(7):977–987 [DOI] [PubMed] [Google Scholar]
- 27. Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51–57 [DOI] [PubMed] [Google Scholar]
- 28. Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49(19):2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sankaridurg P, Donovan L, Varnas S, et al. Spectacle Lenses Designed to Reduce Progression of Myopia: 12-Month Results. Optom Vis Sci. 2010;87(9):631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]