Segregation of intramuscular motor nerves indicates distinct superior and inferior zones within the horizontal but not vertical rectus extraocular muscles in humans and monkeys, supporting a potential functional role for differential innervation that might mediate oculorotary actions.
Abstract
Purpose.
It has been proposed that the lateral rectus (LR), like many skeletal and craniofacial muscles, comprises multiple neuromuscular compartments subserving different physiological functions. To explore the anatomic potential of compartmentalization in all four rectus extraocular muscles (EOMs), evidence was sought of possible regional selectivity in intramuscular innervation of all rectus EOMs.
Methods.
Whole orbits of two humans and one macaque monkey were serially sectioned at 10 μm thickness and stained with Masson's trichrome. Three-dimensional reconstruction was performed of the intramuscular courses of motor nerves from the deep orbit to the anterior extents of their arborizations within all four rectus EOMs in each orbit.
Results.
Findings concorded in monkey and human orbits. Externally to the global surface of the lateral (LR) and medial rectus (MR) EOMs, motor nerve trunks bifurcated into approximately equal-sized branches before entering the global layer and observing a segregation of subsequent arborization into superior zones that exhibited minimal overlap along the length of the LR and only modest overlap for MR. In contrast, intramuscular branches of the superior and the nasal portion of the inferior rectus were highly mixed.
Conclusions.
Consistent segregation of intramuscular motor nerve arborization suggests functionally distinct superior and inferior zones within the horizontal rectus EOMs in both humans and monkeys. Reduced or absent compartmentalization in vertical rectus EOMs supports a potential functional role for differential innervation in horizontal rectus zones that could mediate previously unrecognized vertical oculorotary actions.
Several skeletal muscles are composed of multiple neuromuscular compartments that can be controlled individually by corresponding motor neuron pools.1–3 The transversus abdominis is composed of distinct regions that contract differentially.3 Similarly, the human cricothyroid muscle has three bellies with distinct functions innervated by separate motor nerve branches.3 The triceps brachii is composed of multiple fascicles that may be considered as distinct muscles with completely independent motoneuron subnucleus innervation.4
There exist more extraocular muscle (EOM) fibers and motor neurons than apparently required by conventionally recognized mechanisms of ocular motility.5,6 Could this be because individual EOMs are compartmentalized to implement multiple functions? One such example is the active pulley hypothesis that proposes that orbital layers of EOMs insert in connective tissue rings, called pulleys, through which pass the global layer fibers that in turn insert on the sclera to rotate the eye. Consequently, during EOM contraction, shifts in pulley positions influence EOM pulling directions.7–10 This laminar aspect of compartmentalization is evident in all oculorotary EOMs.
Peng et al.11 traced the intramuscular arborization of the abducens nerve (CN6) within the lateral rectus (LR) muscles of two macaque monkeys and two humans, demonstrating that CN6 bifurcates externally to the EOM into two major trunks whose arborizations remain segregated into superior versus inferior zones throughout the EOM's length. Based on this finding, Peng et al.11 proposed that selective neural control of the two LR zones could execute significant torsional and vertical actions that are not classically recognized. This neuroanatomical finding of Peng et al.11 is consistent with the LR's dual-headed origin in the deep orbit.12,13 Some aspects of the LR are unique among EOMs, however, so that compartmentalization of LR innervation might be a developmental artifact rather than neural control strategy. Older studies of EOM embryology claimed that the LR originates from two different myotomes.14,15 More recent studies in birds demonstrate that the LR arises from somitomeres 4 and 5,16,17 and that CN6 arises from both rhombomeres 5 and 6.16 Separation of CN6 into two parallel nerve trunks is not rare. Indeed, 8–15% of CN6 in humans are split in this way.18–21 The LR can also exhibit longitudinal splitting, most prominently in congenital disorders of cranial nerve development such as processes involving the EOM or cranial nerve development such as congenital fibrosis of the extraocular muscles type 1,22 congenital oculomotor palsy,23 congenital trochlear palsy,23 and Duane syndrome.24–26
The possibility of selective activation of compartments requires topographical projection of motor nerves within segregated EOM regions. If selective control of rectus EOM compartments is a general neural strategy, evidence of compartmentalization should be evident in the intramuscular innervation of other rectus EOMs besides the LR. This study aimed at confirming, in additional specimens, Peng et al.'s report of compartmentalized LR innervation11 and extending study by tridimensional reconstruction of nerve arborizations, to the inferior (IR), medial (MR), and superior rectus (SR) muscles of humans and monkeys.
Materials and Methods
Source and Preparation of Specimen
A whole orbit was obtained through tissue sharing from a 7-year-old, nonstrabismic, male macaque monkey (M3) that had participated in behavioral studies of eye movements by means of surgically implanted scleral magnetic search coils. All experiments had been performed in compliance with the ARVO resolution on the use of animals in research. At the conclusion of behavioral studies, the monkey had been terminally anesthetized and perfused via the left ventricle with 10% neutral buffered formalin. The brain was removed, and the head was immersed in 10% neutral phosphate buffered formalin. Magnetic search coils were removed by minimal dissection from very anterior locations on the sclera that would have had no effect on EOM innervation.
Orbits were obtained from 4-year-old (H6) and 17-month-old (H7) male human cadavers that had been donated to a tissue bank (IIAM, Scranton, PA). All procedures were performed in conformity with legal requirements and had the approval of the UCLA Ethics Committee. Heads were frozen by the tissue bank to −78°C within 24 hours of death and then slowly thawed in 10% neutral buffered formalin for 1 week before orbit extraction.
Monkey and human orbits were removed en bloc with periorbita. After a 24-hour decalcification in 0.003 M EDTA and 1.35 N HCl, orbits were dehydrated in alcohol and xylenes and placed in a vacuum chamber for paraffin embedding.
Histologic Processing
After the formalin fixation and paraffin embedding, orbits were sectioned in the quasi-coronal plane (perpendicular to the long axis of the orbit) at 10 μm thickness, mounted on 50 × 75 mm glass slides, stained with Masson's trichrome (MT),27 and examined microscopically. Masson's trichrome stains EOM fibers red and nerves purple. Typical human orbits required approximately 4000 sections, while monkey orbits required approximately 3000 sections; approximately every tenth section was stained and examined. Staining of sections was performed at smaller intervals in regions of motor nerve divisions and junctions.
Microscopy
Digital photographs were taken using a light microscope (Eclipse E800, Nikon, Tokyo, Japan) fitted with a digital camera (Nikon D1X) using ×0.5–40 objectives. Images were processed using commercial imaging software (Photoshop CS4, Adobe Systems, San Jose, CA).
Reconstruction
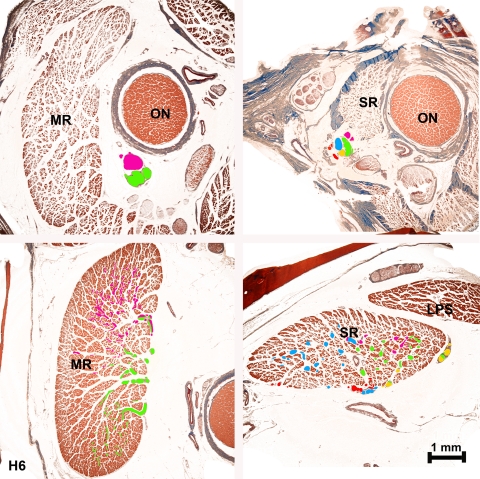
Histologic sections were analyzed sequentially as previously published.11 Nerves were traced from the posterior point, external to each EOM, in which the main nerve trunk directed to that muscle first bifurcated. Although this method potentially permits tracing of intramuscular nerves both anterior and posteriorly, intramuscular motor nerve branches large enough to trace did not travel posteriorly from the entry point. The main trunk of CN6 and CN3 was identified deep in the orbits. From this point, the two primary bifurcations of CN6 were overlayed in separate image layers with two different colors in serial sections (using Photoshop; Adobe Systems). The same process was performed with CN3 from the point that the branch to each individual EOM separated from the main CN3 trunk. If a given nerve developed more than two main branches at its initial division, these branches were color overlayed with the maximum effort to segregate them by similarity using two main colors. In one human superior rectus (SR), the CN3 trunk directed to the EOM had already divided into several branches in the initial section obtained at the orbital apex. In this case, multiple different colors were used to overlay each branch uniquely (Fig. 1). Using serial sections from this location, these secondary nerve trunks and all their daughter branches were highlighted to maintain colors corresponding to their lineage as far anteriorly as identifiable nerve bundles were present. When an anastomosis occurred between two different nerve divisions that had been overlaid with different colors, subsequent branches were colored with the two colors mixed proportionally (Fig. 1). After color overlay had been obtained in each serial section in the orbit, the colored nerve sections alone were reconstructed in three dimensions using the program ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) running on an 8-processor computer (Mac Pro, Apple Computer, Cupertino, CA) with 32 Gb random access memory.
Figure 1.
Photomicrographs from human H6 medial (MR, left panels) and superior (SR, right panels) rectus muscles showing the proximal divisions of the motor nerves deep in the orbit (upper panels) and their respective branches in anterior sections (lower panels). Note the mixed colors in nerves resulting from anastomoses between branches in SR.
Results
Lateral Rectus
Findings were consistent in the monkey and in both human orbits studied. The intramuscular distribution of CN6 in the LR of human orbit H7 was segregated into distinct superior and inferior zones with minimal overlap, confirming and extending in additional orbit H7 Peng et al.'s report in two other human orbits (Fig. 2).11 The main trunk of CN6 bifurcated in the posterior orbit into distinct superior and inferior divisions that entered the LR on its global surface and coursed anteriorly through the EOM with little or no overlap between them. The intramuscular course of subsequent CN6 branches followed almost parallel, separate courses, remaining within superior or inferior LR zones with no turns and at most rare anastomoses, even between branches of the same main trunk.
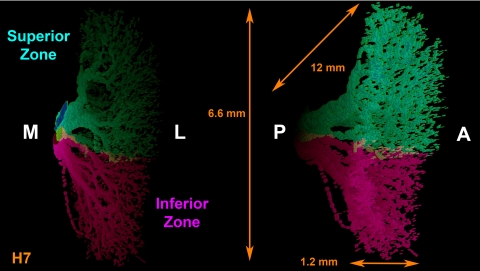
Figure 2.
Tridimensional reconstruction of human CN6 within the LR muscle, in two oblique perspectives of its 12 mm total anterior (A) to posterior (P), 6.6 mm inferior to superior, and 1.2 mm medial (M) to lateral (L) extent showing segregated superior and inferior zones. Note that there is minimal overlap between the two zones. Dimensions determined precisely from orthotropic projections may appear altered in oblique projections shown.
Medial Rectus
Although the MR did not show the same discrete two-zone innervational pattern found in CN6, the intramuscular branches of CN3 innervating the MR divided externally to the EOM and subsequently distributed within it in an organized, topographic pattern that was maintained along the length of the MR. Anastomoses between branches either from the same or from different main trunks of intramuscular CN3, although present, were less frequent than in the SR and IR (Fig. 3). Occasional CN3 branches in the MR took a recurrent course from anterior to posterior, mainly in the initial divisions of the nerve inside the EOM.
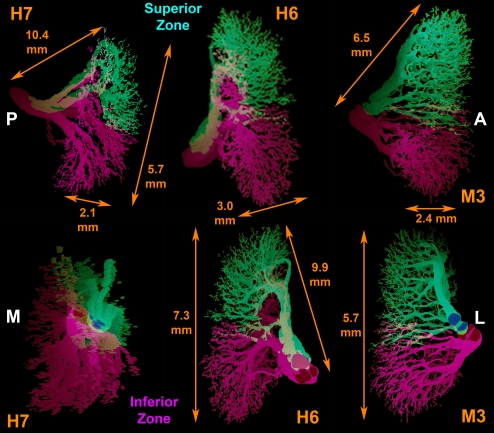
Figure 3.
Tridimensional reconstruction in two oblique perspectives each of CN3 trunk directed to the medial rectus muscle in human specimens H6 and H7 and monkey M3. Note the presence of superior and inferior zones showing greater overlap than in lateral rectus (Fig. 2). Dimensions determined precisely from orthotropic projections appear altered in oblique projections are shown. A, anterior; L, lateral; M, medial; P, posterior.
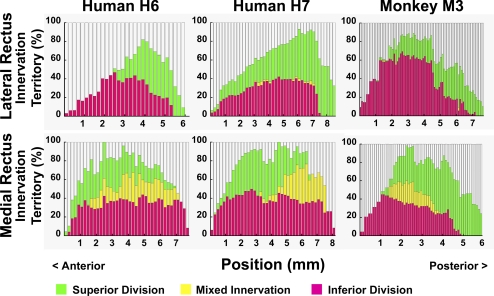
A quantitative comparison of nerve segregation within superior and inferior zones of the LR and MR was obtained by comparing, in transverse sections all along the length of each EOM where intramuscular nerves could be traced, the percentage of transverse EOM width occupied by exclusively segregated nerve projections, as well as the proportion containing overlapping projections (Fig. 4). These three percentages do not sum to 100% because traceable nerve fibers were not identified in all portions of each EOM cross section. As seen in Figure 4 (top), 1.7–4.1% of LR width contained nerve fibers from both the superior and inferior divisions of CN6, while the bulk of the LR contained nerve arborizations from either the superior or inferior division in both humans and monkey. By contrast, Figure 4 (bottom) illustrates that, in both humans and monkey, 2–41% of MR width contains mixed branches of both the superior and inferior divisions of the MR branch of CN3; the remaining 20–40% of MR width received segregated innervation from either superior or inferior division, but not both.
Figure 4.
Percentage muscle territory in which superior (green) and inferior (magenta) abducens nerve divisions arborize within the lateral (LR) and medial rectus (MR) muscles, as measured along the entire long axis of each reconstructed arborization, and extending posteriorly to the entry point into the muscle of the most posterior nerve division. Zero on the abscissa references the most anterior point where motor nerves could be traced. Yellow zone indicating overlap of territories is larger in the MR than LR. Human muscles were longer than monkey muscles.
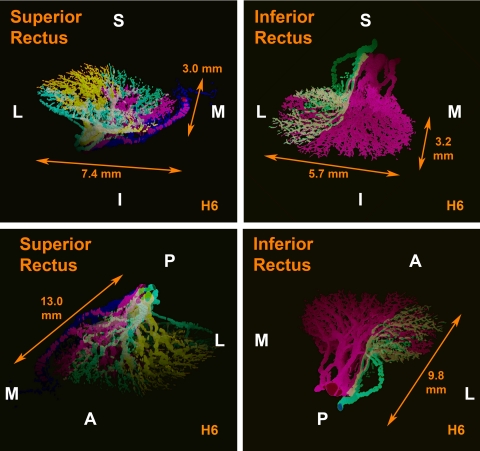
Superior Rectus
The intramuscular nerve distribution in the SR was thoroughly “mixed,” with a large number of anastomoses between branches from the same trunk and from different trunks within the EOM. In human orbit H6, the superior division of CN3 was already divided in several branches in the orbital apex in the deepest serial section examined. Therefore, we followed these branches with multiple different color overlay schemes seeking any possible pattern of segregation, but no pattern was found (Fig. 5). In the SR, many nerve branches showed a recurrent course, turning posteriorly after running anteriorly in the deep orbit.
Figure 5.
Tridimensional reconstruction in two perspectives of intramuscular innervation to human superior (SR, left panels) and inferior rectus (IR, right panels) muscles, showing mixing of intramuscular innervation. Note that in SR there is a mix of most colors used in different nerve branches with no mediolateral segregation between the colors. There is mixing in the lateral portion of the IR, but the medial portion is innervated by only one intramuscular nerve trunk. Dimensions determined precisely from orthotropic projections appear altered in oblique projections shown. A, anterior; I, inferior; L, lateral; M, medial; P, posterior; S, superior.
Inferior Rectus
Similarly to the SR, motor nerves within the IR were thoroughly “mixed” in the lateral portion of the EOM; however, the medial portion was exclusively innervated by one the medial division of the motor nerve (Fig. 5). Additionally, even after the anteriorly coursing main nerve trunk to the IR had already divided before entering the EOM, some progeny bundles anastomosed with the motor nerve directed to the MR. In addition, some nerve branches that innervated the IR came directly from a large, distinct nerve bundle that provided all innervation to the inferior oblique muscle. Although anastomoses between different motor nerve branches were less frequent than for nerves innervating the SR, anastamoses were still common within progeny of the same trunk and between different trunks innervating the IR. Occasional recurrent nerve branches were present.
Discussion
Using serial histologic sections from the whole human and monkey orbits, it was possible to reconstruct the tridimensional pattern of the larger intramuscular motor nerve distributions within all four rectus EOMs in macaque monkey and humans. Findings were similar in both species. Results in an additional human orbit confirm and extend an earlier report that, externally to the LR, CN6 bifurcates into two major divisions that separately innervate correspondingly distinct superior and inferior zones of the LR muscle.11 Progeny branches derived from these two divisions distributed to separate, minimally overlapping zones of the LR whose fibers run mutually parallel over a long anteroposterior extent consistent with reported broad distribution of motor endplates in the middle two thirds of the EOM (Fig. 2).28 This highly compartmentalized pattern was approximated to a slightly lesser degree by intramuscular innervation by the CN3 branch to the MR muscle, albeit with an intermediate region between the superior and inferior zones in which innervation was overlapping from the two major motor nerve trunks (Fig. 3). It may thus be concluded that an anatomic basis also exists for possible selective neural control of the superior versus inferior zone of the MR, as has been postulated for the LR.11 The distribution of CN3 to the vertical rectus EOM did not, in humans or monkey, show a clear two-zone pattern with significant segregation (Fig. 5). There was nevertheless some inhomogeneity in the IR, where the medial portion was exclusively innervated by one motor nerve branch, and the lateral IR portion was innervated by an overlapping mix of both major nerve branches. No topographic segregation could be discerned at all in the SR. The specificity of compartmental segregation of relatively large intramuscular motor nerves to horizontal but not to the SR, and only medially in the IR, argues for a functional correlation. Based on findings in rabbit, it is assumed that finer terminal branches of the motor nerves continue within their compartments to neuromuscular junctions that are widely distributed throughout each rectus EOM.29
Compartmentalization is not uncommon in skeletal muscles; different regions can act as if they were independent muscles and frequently are innervated by discrete motor subnuclei.3,4,30 Among cranial nerves, it is known that facial nerve fibers directed to the superior orbicularis oris exhibit a somatotopic organization, reflecting a potential compartmentalization of function of the rostral, middle, and caudal segments of the muscle.31 Although the horizontal EOMs exhibit compartmental innervation, it is not known if the distinct EOM zones are innervated by independent motor subnuclei. The CN3 nucleus is composed of a complex of subnuclei within the midbrain, each command a specific EOM and so well defined topographically that a small lesion in the one subnucleus can cause an isolated palsy of the corresponding EOM.32,33 The MR subnucleus projects to the contralateral CN6 nucleus through the medial longitudinal fasciculus, which facilitates coordinated horizontal movements. In fact, single unit recording studies has shown that both versional and vergence signals are present in MR and LR motoneurons34; however, these motoneurons do not behave uniformly during different ocular movements. In both nuclei, most cells have the same firing rate for a given eye position regardless of vergence angle, but a minority of cells exist in which the firing rate depends on both eye position and vergence state.35 It has been commonly presumed that overall horizontal rectus EOM behavior reflects a simple population average of motor neurons, but this assumption might not apply under at least some physiological conditions. It seems possible that these different types of motoneurons might contribute differently to different tasks, and that CN6 innervation to the superior and inferior LR zones might be segregated at the motor nucleus and motor nerve levels. Indeed, 8–15% of CN6 in humans are grossly duplicated, with segregation throughout the nerve.20,21 Functional evidence for differential compartmental activation of the human LR is now available from magnetic resonance imaging during ocular counter-rolling induced by head tilt. The inferior but not superior LR zone demonstrated contractile changes due to head tilt in both normal humans and in the uninvolved orbit of patients with superior oblique (SO) palsy, while neither LR zone exhibited contractile changes due to head tilt in orbits affected by SO palsy.36
Peng et al.11 used a computational simulation (Orbit 1.8; Eidactics, San Francisco, CA) to infer that selective LR compartmental activation could impart vertical action ±13–15% of total LR tendon force and torsional action ±16–22% of total LR tendon force.37 This simulation assumed that differential activation shifts the effective point of LR insertion on the sclera by 2.5 mm, without shifting the effective origin at the pulley. If EOM fibers in the superior zone can slide independently of those in the inferior zone, then it may be more realistic to assume that the centroid of LR force also shifts within its pulley by the same direction and amount as the effective shift in scleral insertion. Such an assumption in the computational simulation (Orbit 1.8; Eidactics)37 reduces the torsional effect of differential activation of the two LR compartments to 0–4% of total LR force but increases vertical action to 22–24% of total LR force. Simulation of analogous shift in effective MR insertion and MR centroid at the pulley yields 5–7% torsional and 20–21% vertical action of total MR force. Simulation of simultaneous activation of the superior zones of both the MR and LR in one eye, with inhibition of the inferior zones, predicts a 6° supraduction relative to a central target, with torsion <0.5°; this vertical rotation exceeds by some sixfold the amplitude of normal vertical fusional vergence. Differential compartmental activation of the horizontal rectus EOMs is therefore a viable mechanism for vertical vergence eye movements associated with little or no torsion, potentially confounding efforts to identify motor mechanisms based on classical mechanical actions of the cyclovertical EOMs.38 Pathologic derangements of compartmental activation could perhaps be a cause of vertical strabismus. Evidence of a possible role of the horizontal rectus EOMs in vertical eye movements is the existence of projections to the CN6 nucleus derived from cell groups in vertical eye movement pathways, including the superior vestibular nucleus, interstitial nucleus of Cajal, and rostral interstitial nucleus of the medial longitudinal fasciculus36. Similar studies using different tracers in the distal superior and inferior zones of the EOMs would be of value to determine whether this compartmentalization has a topographic representation in the brain stem. Since the motor nerve divisions bifurcate externally to the horizontal rectus EOMs, possible behavioral effects of selective topographic lesions, nerve fiber recording, or electrical stimulation might be explored experimentally.
Mean CN6 motor neuron firing rates are higher during convergent than conjugate adduction. In other words, relative to primary position, the decrease in firing rate of a right CN6 motor neuron during convergent adduction is only 50–62% of the decrease in firing rate observed during levoversion (conjugate adduction).39 This would suggest that there must be an increase in LR force during convergence with concomitant MR co-contraction. Surprisingly, it has been shown that during convergence there is a decrease of both LR and MR force, greater in LR, as measured across the insertional tendon.39 Compartmentalization in horizontal rectus EOMs might resolve this paradox. Based on the current observations, we propose that the higher firing rate recorded in the CN6 motoneurons might be directed to a specific LR compartment contributing a smaller value when this force is measured as an average across the whole tendon at the insertion. One possibility might be that an increase in motor neuron activity innervating the superior zone of one horizontal rectus EOM (for example, the MR) might be accompanied by a corresponding decrease in the inferior zone, leading to overall reduction in force at the scleral insertion; if this behavior were accompanied in the antagonist EOM (for example, the LR) by a decrease in innervation to the superior zone and a smaller increase in the inferior zone leading again to a reduction in force at the scleral insertion, horizontal and vertical eye position might remain unchanged.
Considering the close relationship between the MR and LR in coordination of horizontal movements, and the compartmental pattern found so prominently in these two rectus EOMs, we suppose that there might exist similar topographic organization of the subnuclei of CN3 and CN6 innervating the horizontal rectus EOMs. The more limited compartmental pattern of intramuscular innervation in the IR might also be associated with some topographical organization of its subnucleus. Although there as yet exists no direct evidence supporting the foregoing suppositions, neuroanatomical and neurophysiological studies of the selective intramuscular innervation zones are warranted to determine whether this segregation is maintained within the brain stem.
Footnotes
Supported by the U.S. Public Health Service; National Eye Institute Grants No. EY08313 and EY00331; and Research to Prevent Blindness.
Disclosure: R.M. Costa, None; J. Kung, None; V. Poukens, None; L. Yoo, None; L. Tychsen, None; J.L. Demer, None
References
- 1. English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys. Ther. 1993;73:857–867 [DOI] [PubMed] [Google Scholar]
- 2. Holtermann A, Roeleveld K, Mork PJ, et al. Selective activation of neuromuscular compartments within the human trapezius muscle. J Electromyogr Kinesiol. 2009;29:896–902 [DOI] [PubMed] [Google Scholar]
- 3. Urquhart DM, Hodges PW. Differential activity of regions of transversus abdominis during trunk rotation. Eur Spine. 2005;14:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lucas-Osma AM, Collazos-Castro JE. Compartmentalization in the triceps brachii motoneuron nucleus and its relation to muscle architecture. J Comp Neurol. 2009;516:226–239 [DOI] [PubMed] [Google Scholar]
- 5. Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve. 1997;20:1229–1235 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290 [PubMed] [Google Scholar]
- 8. Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738 [DOI] [PubMed] [Google Scholar]
- 9. Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng M, Poukens V, da Silva Costa RM, Yoo L, Tychsen L, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010;51:4612–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Govsa F, Kayalioglu G, Erturk M, Ozgur T. The superior orbital fissure and its contents. J Surg Radiol Anat. 1999;21:181–185 [DOI] [PubMed] [Google Scholar]
- 13. Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80 [DOI] [PubMed] [Google Scholar]
- 14. Gilbert PW. The origin and development of the extrinsic ocular muscles in the domestic cat. Contrib Embryol. 1957;36:61–78 [PubMed] [Google Scholar]
- 15. Neal HV. The history of the eye muscles. J Morphol. 1918;30:433–453 [Google Scholar]
- 16. Wahl CM, Noden DM, Baker R. Developmental relations between sixth nerve motor neurons and their targets in the chick embryo. Dev Dynam. 1994;20:191–202 [DOI] [PubMed] [Google Scholar]
- 17. Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dynam. 2006;235:1310–1325 [DOI] [PubMed] [Google Scholar]
- 18. Nathan H, Ouaknine G, Kosary IZ. The abducens nerve: anatomical variations in its course. J Neurosurg. 1974;42:561–566 [DOI] [PubMed] [Google Scholar]
- 19. Jain KK. Aberrant roots of the abducens nerve. J Neurosurg. 1964;21:349–351 [DOI] [PubMed] [Google Scholar]
- 20. Iaconetta G, Tessitore E, Samii M. Duplicated abducent nerve and its course: microanatomical study and surgery-related considerations. J Neurosurg. 2001;95:853–858 [DOI] [PubMed] [Google Scholar]
- 21. Ozeren MF, Sam B, Akdemir I, Lalkan A, Tekdemir I, Deda H. Duplication of the abducens nerve at the petroclival region: an anatomic study. Neurosurgery. 2003;52:645–651 [DOI] [PubMed] [Google Scholar]
- 22. Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539 [DOI] [PubMed] [Google Scholar]
- 23. Okanobu H, Kono R, Miyake K, Ohtsuki H. Splitting of the extraocular horizontal rectus muscle in congenital cranial dysinnervation disorders. Am J Ophthalmol. 2009;147:550–556 [DOI] [PubMed] [Google Scholar]
- 24. Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demer JL, Clark RA, Lim K-H, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okanobu H, Kono R, Ohtsuki H, Miyake K. Magnetic resonance imaging findings in Duane's retraction syndrome type III. Rinsho Ganka (Jpn Clin Ophthalmol). 2008;62:65–69 [Google Scholar]
- 27. Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. St. Louis: Mosby; 1973:95–116 [Google Scholar]
- 28. Mayr R, Gottschall J, Gruber H, Neuhuber W. Internal structure of cat extraocular muscle. Anat Embryol. 1975;148:25–34 [DOI] [PubMed] [Google Scholar]
- 29. Harrison AR, Anderson BC, Thompson LV, McLoon LK. Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles. Invest Ophthalmol Vis Sci. 2007;48:3594–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2007;23:21–28 [DOI] [PubMed] [Google Scholar]
- 31. Marshall CD, Hsu RH, Herring SW. Somatotopic organization of perioral musculature innervation within the pig facial motor nucleus. Brain Behav Evol. 2005;66:22–34 [DOI] [PubMed] [Google Scholar]
- 32. Chou TK, Demer JL. Isolated inferior rectus palsy caused by a metastasis to the oculomotor nucleus. Am J Ophthalmol. 1998;126:737–740 [DOI] [PubMed] [Google Scholar]
- 33. Castro OL, Johnson N, Mamourian AC. Isolated inferior oblique paresis from brain-stem infarction. Perspective on oculomotor fascicular organization in the ventral midbrain tegmentum. Arch Neurol. 1990;47:235–237 [DOI] [PubMed] [Google Scholar]
- 34. Robinson DA, Keller E. The behavior of eye movement motoneurons in the alert monkey. Bibl Ophthalmol. 1972;82:7–16 [PubMed] [Google Scholar]
- 35. Mays LE, Porter JD. Neural control of vergence eye movements: activity of abducens and oculomotor neurons. J Neurophysiol. 1984;52:743–761 [DOI] [PubMed] [Google Scholar]
- 36. Clark RA, Demer JL. Differential lateral rectus (LR) compartmental contraction: A novel mechanism accounts for impaired ocular counter-rolling (OCR) in superior oblique (SO) palsy. Abstr 37th Annual Mtg Am Ass'n Ped Ophthalmol Strab In press [Google Scholar]
- 37. Miller JM, Pavlovski DS, Shaemeva I. Orbit 1.8 Gaze Mechanics Simulation. San Francisco: Eidactics; 1999 [Google Scholar]
- 38. Mudgil AV, Walker M, Steffen H, Guyton DL, Zee DS. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS. 2002;6:145–153 [DOI] [PubMed] [Google Scholar]
- 39. Miller JM, Bockisch CJ, Pavlovski DS. Missing lateral rectus force and absence of medial rectus co-contraction in ocular convergence. J Neurophysiol. 2002;87:2421–2433 [DOI] [PubMed] [Google Scholar]